Bacterial Extracellular Vesicles and Antimicrobial Peptides: A Synergistic Approach to Overcome Antimicrobial Resistance
Abstract
:1. Introduction
Mechanisms of Antimicrobial Resistance
2. Bacterial Extracellular Vesicles
2.1. Role of BEVs in Antibiotic Resistance
2.2. Methods for BEV Extraction and Purification
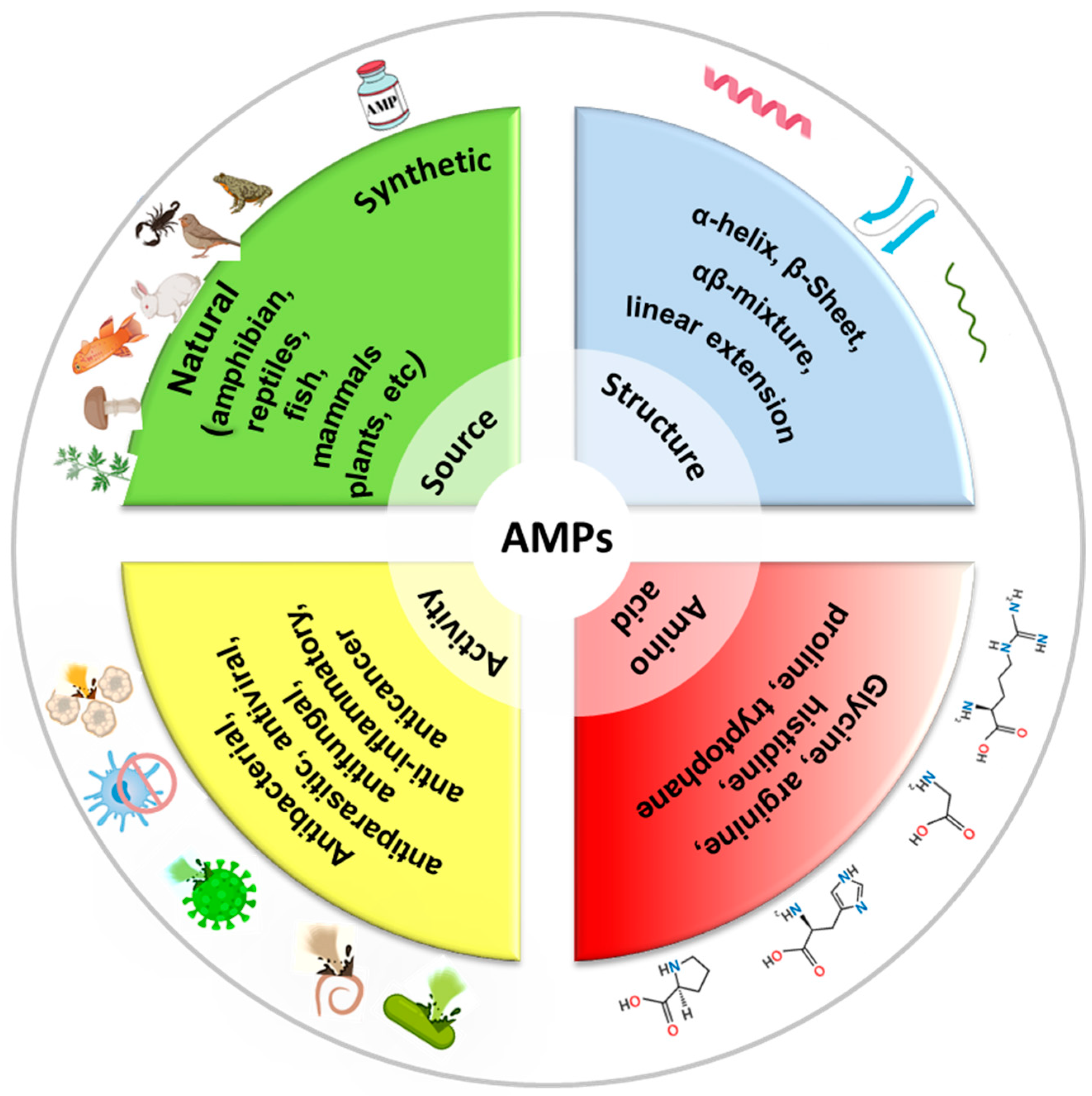
3. Antimicrobial Peptides
4. Synergistic Effects of AMPs and BEVs Against Antibiotic Resistance
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Huemer, M.; Shambat, S.M.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial Peptides for Combating Drug-Resistant Bacterial Infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial Peptides: Opportunities and Challenges in Overcoming Resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Hsu, H.-C.; Saito, H.; Wakatake, H.; Yoshida, H.; Umekawa, S.; Tsutsumi, K.; Yoshida, T.; Masui, Y.; Taira, Y.; et al. CTX-M Group Distribution and Positivity of Extended-Spectrum β-Lactamase (ESBL)-Producing Enterobacteriaceae in Urinary Tract Infections in a Tertiary Metropolitan Hospital in Japan. J. St. Marian. Univ. 2020, 11, 133–141. [Google Scholar] [CrossRef]
- Doi, Y.; Arakawa, Y. 16S Ribosomal RNA Methylation: Emerging Resistance Mechanism against Aminoglycosides. Clin. Infect. Dis. 2007, 45, 88–94. [Google Scholar] [CrossRef]
- Kleanthous, C.; Shaw, W.V. Analysis of the Mechanism of Chloramphenicol Acetyltransferase by Steady-State Kinetics. Evidence for a Ternary-Complex Mechanism. Biochem. J. 1984, 223, 211–220. [Google Scholar] [CrossRef]
- Zieliński, M.; Park, J.; Sleno, B.; Berghuis, A.M. Structural and Functional Insights into Esterase-Mediated Macrolide Resistance. Nat. Commun. 2021, 12, 1732. [Google Scholar] [CrossRef]
- Baysarowich, J.; Koteva, K.; Hughes, D.W.; Ejim, L.; Griffiths, E.; Zhang, K.; Junop, M.; Wright, G.D. Rifamycin Antibiotic Resistance by Adp-Ribosylation: Structure and Diversity of Arr. Proc. Natl. Acad. Sci. USA 2008, 105, 4886–4891. [Google Scholar] [CrossRef]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial Resistance among Streptococcus pneumoniae. In Antimicrobial Resistance in the 21st Century; Fong, I.W., Shlaes, D., Drlica, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 13–38. [Google Scholar]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial Resistance in Biofilm-Associated Bacteria. Futur. Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Swick, M.C.; Morgan-Linnell, S.K.; Carlson, K.M.; Zechiedrich, L. Expression of Multidrug Efflux Pump Genes Acrab-Tolc, Mdfa, and Nore in Escherichia Coli Clinical Isolates as a Function of Fluoroquinolone and Multidrug Resistance. Antimicrob. Agents Chemother. 2011, 55, 921–924. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Z.; Wang, Q.; Wang, M.; Ming, Y.; Chen, W.; Huang, Y.; Tang, Z.; Huang, M.; Liu, H.; et al. Opportunities and Challenges of Bacterial Extracellular Vesicles in Regenerative Medicine. J. Nanobiotechnol. 2025, 23, 4. [Google Scholar] [CrossRef]
- Muñoz-Echeverri, L.M.; Benavides-López, S.; Geiger, O.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A. Bacterial Extracellular Vesicles: Biotechnological Perspective for Enhanced Productivity. World J. Microbiol. Biotechnol. 2024, 40, 174. [Google Scholar] [CrossRef]
- Rima, M.; Dakramanji, M.; El Hayek, E.; El Khoury, T.; Fajloun, Z.; Rima, M. Unveiling the Wonders of Bacteria-Derived Extracellular Vesicles: From Fundamental Functions to Beneficial Applications. Heliyon 2025, 11, e42509. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-Positive Bacteria Produce Membrane Vesicles: Proteomics-Based Characterization of Staphylococcus Aureus-Derived Membrane Vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef]
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704. [Google Scholar] [CrossRef]
- Bitto, N.J.; Chapman, R.; Pidot, S.; Costin, A.; Lo, C.; Choi, J.; D’cruze, T.; Reynolds, E.C.; Dashper, S.G.; Turnbull, L.; et al. Bacterial Membrane Vesicles Transport Their DNA Cargo into Host Cells. Sci. Rep. 2017, 7, 7072. [Google Scholar] [CrossRef] [PubMed]
- Palomino, R.A.Ñ.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota–Host Communications: Bacterial Extracellular Vesicles as a Common Language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef]
- Henriquez, T.; Falciani, C. Extracellular Vesicles of Pseudomonas: Friends and Foes. Antibiotics 2023, 12, 703. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, M.J.; Park, Y.; Chung, J.; Kweon, H.-S.; Kang, N.-G.; Hwang, S.J.; Youn, S.H.; Hwang, B.K.; Kim, D. Visualizing Extracellular Vesicle Biogenesis in Gram-Positive Bacteria Using Super-Resolution Microscopy. BMC Biol. 2022, 20, 270. [Google Scholar] [CrossRef]
- Pathirana, R.D.; Kaparakis-Liaskos, M. Bacterial Membrane Vesicles: Biogenesis, Immune Regulation and Pathogenesis. Cell Microbiol 2016, 18, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Beveridge, T.J.; Kadurugamuwa, J.; Walther-Rasmussen, J.; Høiby, N. Chromosomal Beta-Lactamase is Packaged into Membrane Vesicles and Secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000, 45, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Najar Peerayeh, S.; Mahabadi, R.P.; Toupkanlou, S.P.; Siadat, S.D. Diversity of Beta-Lactamases Produced by Imipenem Resistant, Pseudomonas Aeruginosa Isolates from the Bloodstream. Burns 2014, 40, 1360–1364. [Google Scholar] [CrossRef]
- Jiang, B.; Lai, Y.; Xiao, W.; Zhong, T.; Liu, F.; Gong, J.; Huang, J. Microbial Extracellular Vesicles Contribute to Antimicrobial Resistance. PLoS Pathog. 2024, 20, e1012143. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, J.-S.; Bin Park, S.; Lee, A.R.; Lee, J.S.; Jung, J.W.; Chun, J.H.; Lazarte, J.M.S.; Kim, J.; Kim, J.-H.; et al. Significant Increase in the Secretion of Extracellular Vesicles and Antibiotics Resistance from Methicillin-Resistant Staphylococcus aureus Induced by ampicillin stress. Sci. Rep. 2020, 10, 21066. [Google Scholar] [CrossRef]
- Saad, M.G.; Beyenal, H.; Dong, W.-J. Dual Roles of the Conditional Extracellular Vesicles Derived from Pseudomonas aeruginosa Biofilms: Promoting and Inhibiting Bacterial Biofilm Growth. Biofilm 2024, 7, 100183. [Google Scholar] [CrossRef]
- Kraus, S.; Fletcher, M.L.; Łapińska, U.; Chawla, K.; Baker, E.; Attrill, E.L.; O’neill, P.; Farbos, A.; Jeffries, A.; Galyov, E.E.; et al. Phage-Induced Efflux Down-Regulation Boosts Antibiotic Efficacy. PLoS Pathog. 2024, 20, e1012361. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Billón, M.; Llambías-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macià, M.D. Mechanisms of Antibiotic Resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.J.; Wylam, M.E. Methicillin-Resistant Staphylococcus Aureus Infection and Treatment Options. Methods Mol. Biol. 2020, 2069, 229–251. [Google Scholar]
- Acevedo, R.; Zayas, C.; Norheim, G.; Fernández, S.; Cedré, B.; Aranguren, Y.; Cuello, M.; Rodriguez, Y.; González, H.; Mandiarote, A.; et al. Outer Membrane Vesicles Extracted from Neisseria Meningitidis Serogroup X for Prevention of Meningococcal Disease in Africa. Pharmacol. Res. 2017, 121, 194–201. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Luo, H.; Xie, Y.; Cao, H.; Mao, L.; Liu, T.; Yue, Y.; Qian, H. Extracellular Vesicles in Helicobacter Pylori-Mediated Diseases: Mechanisms and Therapeutic Potential. Cell Commun. Signal. 2025, 23, 79. [Google Scholar] [CrossRef]
- Bawali, P.; Brahma, A.; Rana, S.R.; Pal, A.; Bhattacharyya, A. Helicobacter Pylori Infection and Inflammatory Events: The Extracellular Vesicle-Connect in Driving Gastrointestinal Tract Cancers. Front. Med. 2024, 11, 1444242. [Google Scholar] [CrossRef]
- Goman, A.; Ize, B.; Jeannot, K.; Pin, C.; Payros, D.; Goursat, C.; Ravon-Katossky, L.; Murase, K.; Chagneau, C.V.; Revillet, H.; et al. Uncovering a New Family of Conserved Virulence Factors That Promote the Production of Host-Damaging Outer Membrane Vesicles in Gram-Negative Bacteria. J. Extracell. Vesicles 2025, 14, e270032. [Google Scholar] [CrossRef]
- Abbasnia, S.; Asnaashari, A.M.H.; Sharebiani, H.; Soleimanpour, S.; Mosavat, A.; Rezaee, S.A. Mycobacterium Tuberculosis and Host Interactions in the Manifestation of Tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 36, 100458. [Google Scholar] [CrossRef]
- Chatterjee, D.; Chaudhuri, K. Association of Cholera Toxin with Vibrio cholerae Outer Membrane Vesicles Which are Internalized by Human Intestinal Epithelial Cells. FEBS Lett. 2011, 585, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Minarini, L.A.d.R. Exploring Bacterial Extracellular Vesicles: Focus on Who Critical Priority Pathogens. Curr. Top. Membr. 2024, 94, 225–246. [Google Scholar]
- Vicente-Gil, S.; Nuñez-Ortiz, N.; Morel, E.; Serra, C.R.; Docando, F.; Díaz-Rosales, P.; Tafalla, C. Immunomodulatory Properties of Bacillus Subtilis Extracellular Vesicles on Rainbow Trout Intestinal Cells and Splenic Leukocytes. Front. Immunol. 2024, 15, 1394501. [Google Scholar] [CrossRef]
- Singorenko, P.D.; Chang, V.; Whitcombe, A.; Simonov, D.; Hong, J.; Phillips, A.; Swift, S.; Blenkiron, C. Isolation of Membrane Vesicles from Prokaryotes: A Technical and Biological Comparison Reveals Heterogeneity. J. Extracell. Vesicles 2017, 6, 1324731. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-Speed Centrifugation Induces Aggregation of Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Le, L.H.M.; Steele, J.R.; Ying, L.; Schittenhelm, R.B.; Ferrero, R.L. A New Isolation Method for Bacterial Extracellular Vesicles Providing Greater Purity and Improved Proteomic Detection of Vesicle Proteins. J. Extracell. Biol. 2023, 2, e84. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Dauros-Singorenko, P.; Whitcombe, A.; Payne, L.; Blenkiron, C.; Phillips, A.; Swift, S. Analysis of the Escherichia coli Extracellular Vesicle Proteome Identifies Markers of Purity and Culture Conditions. J. Extracell. Vesicles 2019, 8, 1632099. [Google Scholar] [CrossRef]
- Reimer, S.L.; Beniac, D.R.; Hiebert, S.L.; Booth, T.F.; Chong, P.M.; Westmacott, G.R.; Zhanel, G.G.; Bay, D.C. Comparative Analysis of Outer Membrane Vesicle Isolation Methods With an Escherichia coli Tola Mutant Reveals a Hypervesiculating Phenotype With Outer-Inner Membrane Vesicle Content. Front. Microbiol. 2021, 12, 628801. [Google Scholar] [CrossRef]
- Alves, N.J.; Turner, K.B.; DiVito, K.A.; Daniele, M.A.; Walper, S.A. Affinity Purification of Bacterial Outer Membrane Vesicles (Omvs) Utilizing a His-Tag Mutant. Res. Microbiol. 2017, 168, 139–146. [Google Scholar] [CrossRef]
- Ciobanasu, C.; Rzeszutek, A.; Kubitscheck, U.; Willumeit, R. Nkcs, a Mutant of the Nk-2 Peptide, Causes Severe Distortions and Perforations in Bacterial, but Not Human Model Lipid Membranes. Molecules 2015, 20, 6941–6958. [Google Scholar] [CrossRef]
- Michira, B.B.; Wang, Y.; Mwangi, J.; Wang, K.; Asmamaw, D.; Tadese, D.A.; Gao, J.; Khalid, M.; Lu, Q.-M.; Lai, R.; et al. A Tachyplesin Antimicrobial Peptide from Theraphosidae Spiders with Potent Antifungal Activity against Cryptococcus neoformans. Microorganisms 2024, 12, 2648. [Google Scholar] [CrossRef]
- Narula, P.; Kiruthika, S.; Chowdhari, S.; Vivekanandan, P.; Chugh, A. Inhibition of Hepatitis B Virus (Hbv) by Tachyplesin, a Marine Antimicrobial Cell-Penetrating Peptide. Pharmaceutics 2023, 15, 672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chugh, A. Peptide-Mediated Leishmaniasis Management Strategy: Tachyplesin Emerges as an Effective Anti-leishmanial Peptide against Leishmania Donovani. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183629. [Google Scholar] [CrossRef]
- Huang, H.W. Molecular Mechanism of Antimicrobial Peptides: The Origin of Cooperativity. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E.; Miller, I.R.; Biggin, P.C.; Sansom, M.S.; Shai, Y. Structure and Orientation of the Mammalian Antibacterial Peptide Cecropin P1 within Phospholipid Membranes. J. Mol. Biol. 1996, 258, 860–870. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides With Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-Membrane Permeabilizing Modes of Action of Antimicrobial Peptides on Bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Sitaram, N. Mechanism of Antimicrobial Action of Indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The Host Antimicrobial Peptide Bac71-35 Binds to Bacterial Ribosomal Proteins and Inhibits Protein Synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef]
- Shi, J.; Ganz, T. The Role of Protegrins and Other Elastase-Activated Polypeptides in the Bactericidal Properties of Porcine Inflammatory Fluids. Infect. Immun. 1998, 66, 3611–3617. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, J.S.; Lee, D.G. Periplanetasin-4, a Novel Antimicrobial Peptide from the Cockroach, Inhibits Communications between Mitochondria and Vacuoles. Biochem. J. 2019, 476, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Segev-Zarko, L.-A.; Saar-Dover, R.; Brumfeld, V.; Mangoni, M.L.; Shai, Y. Mechanisms of Biofilm Inhibition and Degradation by Antimicrobial Peptides. Biochem. J. 2015, 468, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Jaber, R.H.; Beahm, N.P. Daptomycin for the Treatment of Acute Bacterial Meningitis: A Narrative Review. Int. J. Antimicrob. Agents 2023, 61, 106770. [Google Scholar] [CrossRef] [PubMed]
- Ledger, E.V.K.; Sabnis, A.; Edwards, A.M. Polymyxin and Lipopeptide Antibiotics: Membrane-Targeting Drugs of Last Resort. Microbiology 2022, 168, 001136. [Google Scholar] [CrossRef]
- Swierstra, J.; Kapoerchan, V.; Knijnenburg, A.; van Belkum, A.; Overhand, M. Structure, Toxicity and Antibiotic Activity of Gramicidin S and Derivatives. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 763–769. [Google Scholar] [CrossRef]
- Aydemir, H.; Akduman, D.; Piskin, N.; Comert, F.; Horuz, E.; Terzi, A.; Kokturk, F.; Ornek, T.; Celebi, G. Colistin vs. The Combination of Colistin and Rifampicin for the Treatment of Carbapenem-Resistantacinetobacter Baumanniiventilator-Associated Pneumonia. Epidemiol. Infect. 2012, 141, 1214–1222. [Google Scholar] [CrossRef]
- Campos, J.V.; de Pontes, J.T.C.; Canales, C.S.C.; Roque-Borda, C.A.; Pavan, F.R. Advancing Nanotechnology: Targeting Biofilm-Forming Bacteria with Antimicrobial Peptides. BME Front. 2025, 6, 0104. [Google Scholar] [CrossRef]
- Yu, S.; Pan, J.; Xu, M.; Chen, Y.; Li, P.; Hu, H. Antibacterial Activity and Mechanism of Colistin-Loaded Polymeric Nanoparticles for Combating Multidrug-Resistant Pseudomonas aeruginosa biofilms: A synergistic approach. Int. J. Biol. Macromol. 2024, 282, 136757. [Google Scholar] [CrossRef]
- Pogue, J.; Ortwine, J.; Kaye, K. Clinical Considerations for Optimal Use of the Polymyxins: A Focus on Agent Selection and Dosing. Clin. Microbiol. Infect. 2017, 23, 229–233. [Google Scholar] [CrossRef]
- Lange, A.; Thunberg, U.; Söderquist, B. Ototoxicity Associated with Extended Dalbavancin Treatment for a Shoulder Prosthetic Joint Infection. BMC Infect. Dis. 2023, 23, 706. [Google Scholar] [CrossRef]
- Tegethoff, J.I.; Teitelbaum, I.; Kiser, T.H. Rapid and Effective Treatment of Peritonitis in Peritoneal Dialysis Patients with Intravenous Dalbavancin. Am. J. Case Rep. 2023, 25, e942755. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Yigin, A.; Demir, C. Efficacy of Antimicrobial Peptide Ll-37 against Biofilm Forming Staphylococcus Aureus Strains Obtained from Chronic Wound Infections. Microb. Pathog. 2021, 162, 105368. [Google Scholar] [CrossRef]
- Araujo, J.B.; de Souza, G.S.; Lorenzon, E.N. Indolicidin Revisited: Biological Activity, Potential Applications and Perspectives of an Antimicrobial Peptide not yet Fully Explored. World J. Microbiol. Biotechnol. 2022, 38, 39. [Google Scholar]
- Takada, Y.; Itoh, H.; Paudel, A.; Panthee, S.; Hamamoto, H.; Sekimizu, K.; Inoue, M. Discovery of Gramicidin a Analogues with Altered Activities by Multidimensional Screening of a One-Bead-One-Compound Library. Nat. Commun. 2020, 11, 4935. [Google Scholar] [CrossRef] [PubMed]
- Gottler, L.M.; Ramamoorthy, A. Structure, Membrane Orientation, Mechanism, and Function of Pexiganan--a Highly Potent Antimicrobial Peptide Designed from Magainin. Biochim Biophys Acta 2009, 1788, 1680–1686. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Rolff, J. Chapter 2—Skin Delivery of Antimicrobial Peptides. In Emerging Nanotechnologies in Immunology; Shegokar, R., Souto, E.B., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 23–45. [Google Scholar]
- Deshayes, C.; Arafath, N.; Apaire-Marchais, V.; Roger, E. Drug Delivery Systems for the Oral Administration of Antimicrobial Peptides: Promising Tools to Treat Infectious Diseases. Front. Med. Technol. 2022, 3, 778645. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Zanetti, M.; Litteri, L.; Gennaro, R.; Horstmann, H.; Romeo, D. Bactenecins, Defense Polypeptides of Bovine Neutrophils, are Generated from Precursor Molecules Stored in the Large Granules. J. Cell Biol. 1990, 111, 1363–1371. [Google Scholar] [CrossRef]
- Dutta, P.; Das, S. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2015, 16, 99–129. [Google Scholar] [CrossRef]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Higginbotham, J.N.; Liu, L.; Zhao, G.; Acra, S.A.; Peek, R.M.; Polk, D.B.; Li, H.; Yan, F. Production of a Functional Factor, p40, by Lactobacillus rhamnosus GG Is Promoted by Intestinal Epithelial Cell-Secreted Extracellular Vesicles. Infect. Immun. 2019, 87, e00113-19. [Google Scholar] [CrossRef]
- Liu, C.; Yazdani, N.; Moran, C.S.; Salomon, C.; Seneviratne, C.J.; Ivanovski, S.; Han, P. Unveiling Clinical Applications of Bacterial Extracellular Vesicles as Natural Nanomaterials in Disease Diagnosis and Therapeutics. Acta Biomater. 2024, 180, 18–45. [Google Scholar] [CrossRef]
- Sonallya, T.; Juhasz, T.; Szigyarto, I.C.; Ilyes, K.; Singh, P.; Khamari, D.; Buzas, E.I.; Varga, Z.; Beke-Somfai, T. Categorizing Interaction Modes of Antimicrobial Peptides with Extracellular Vesicles: Disruption, Membrane Trespassing, and Clearance of the Protein Corona. J. Colloid Interface Sci. 2025, 679, 496–509. [Google Scholar] [CrossRef]
- Ciobanasu, C. Peptides-Based Therapy and Diagnosis. Strategies for Non-Invasive Therapies in Cancer. J. Drug Target. 2021, 29, 1063–1079. [Google Scholar] [PubMed]
- Li, Z.; Clarke, A.J.; Beveridge, T.J. Gram-Negative Bacteria Produce Membrane Vesicles Which Are Capable of Killing Other Bacteria. J. Bacteriol. 1998, 180, 5478–5483. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Goes, A.; Garcia, R.; Panter, F.; Koch, M.; Müller, R.; Fuhrmann, K.; Fuhrmann, G. Biocompatible Bacteria-Derived Vesicles Show Inherent Antimicrobial Activity. J. Control. Release 2018, 290, 46–55. [Google Scholar] [CrossRef]
- Sharpe, S.W.; Kuehn, M.J.; Mason, K.M. Elicitation of Epithelial Cell-Derived Immune Effectors by Outer Membrane Vesicles of Nontypeable Haemophilus Influenzae. Infect. Immun. 2011, 79, 4361–4369. [Google Scholar] [CrossRef]
- Kaparakis, M.; Turnbull, L.; Carneiro, L.; Firth, S.; Coleman, H.A.; Parkington, H.C.; Le Bourhis, L.; Karrar, A.; Viala, J.; Mak, J.; et al. Bacterial Membrane Vesicles Deliver Peptidoglycan to NOD1 in Epithelial cells. Cell. Microbiol. 2010, 12, 372–385. [Google Scholar] [CrossRef]
- Elmi, A.; Watson, E.; Sandu, P.; Gundogdu, O.; Mills, D.C.; Inglis, N.F.; Manson, E.; Imrie, L.; Bajaj-Elliott, M.; Wren, B.W.; et al. Campylobacter Jejuni Outer Membrane Vesicles Play an Important Role in Bacterial Interactions with Human Intestinal Epithelial Cells. Infect. Immun. 2012, 80, 4089–4098. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome mdr in Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Z.; Cuvillier, L.; Dobhal, G.; Goreham, R.V. Electroporation of Outer Membrane Vesicles Derived from Pseudomonas aeruginosa with Gold Nanoparticles. SN Appl. Sci. 2019, 1, 1600. [Google Scholar] [CrossRef]
- Ibrahim, U.H.; Gafar, M.A.; Khan, R.; Tageldin, A.; Govender, T.; Mackraj, I. Engineered Extracellular Vesicles Coated with an Antimicrobial Peptide for Advanced Control of Bacterial Sepsis. J. Extracell. Biol. 2024, 3, e70000. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of Novel Nanoantibiotics Using an Outer Membrane Vesicle-Based Drug Efflux Mechanism. J. Control. Release 2020, 317, 1–22. [Google Scholar] [CrossRef]
- Bril’kov, M.S.; Stenbakk, V.; Jakubec, M.; Vasskog, T.; Kristoffersen, T.; Cavanagh, J.P.; Ericson, J.U.; Isaksson, J.; Flaten, G.E. Bacterial Extracellular Vesicles: Towards Realistic Models for Bacterial Membranes in Molecular Interaction Studies by Surface Plasmon Resonance. Front. Mol. Biosci. 2023, 10, 1277963. [Google Scholar] [CrossRef]
- Masignani, V.; Pizza, M.; Moxon, E.R. The Development of a Vaccine against Meningococcus B Using Reverse Vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef]
- Rappazzo, C.G.; Watkins, H.C.; Guarino, C.M.; Chau, A.; Lopez, J.L.; DeLisa, M.P.; Leifer, C.A.; Whittaker, G.R.; Putnam, D. Recombinant M2e Outer Membrane Vesicle Vaccines Protect against Lethal Influenza a Challenge in BALB/c Mice. Vaccine 2016, 34, 1252–1258. [Google Scholar] [CrossRef]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef]
- Velimirov, B.; Velimirov, B.A. Immune Responses Elicited by Outer Membrane Vesicles of Gram-Negative Bacteria: Important Players in Vaccine Development. Life 2024, 14, 1584. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Giuliani, M.M.; Biolchi, A.; Pizza, M.; Beebeejaun, K.; Lucidarme, J.; Findlow, J.; Ramsay, M.E.; Borrow, R. Effectiveness of Meningococcal B Vaccine against Endemic Hypervirulent Neisseria Meningitidis W Strain, England. Emerg. Infect. Dis. 2016, 22, 309–311. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Liang, X.; Qin, K.; Zhang, Y.; Wang, J.; Wu, Q.; Gupta, T.B.; Ding, Y. Bacterial Extracellular Vesicle: A Non-Negligible Component in Biofilm Life Cycle and Challenges in Biofilm Treatments. Biofilm 2024, 8, 100216. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-J.; Khan, F.; Tabassum, N.; Cho, K.-J.; Kim, Y.-M. Bacterial Extracellular Vesicles: Modulation of Biofilm and Virulence Properties. Acta Biomater. 2024, 178, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide Ll-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed]

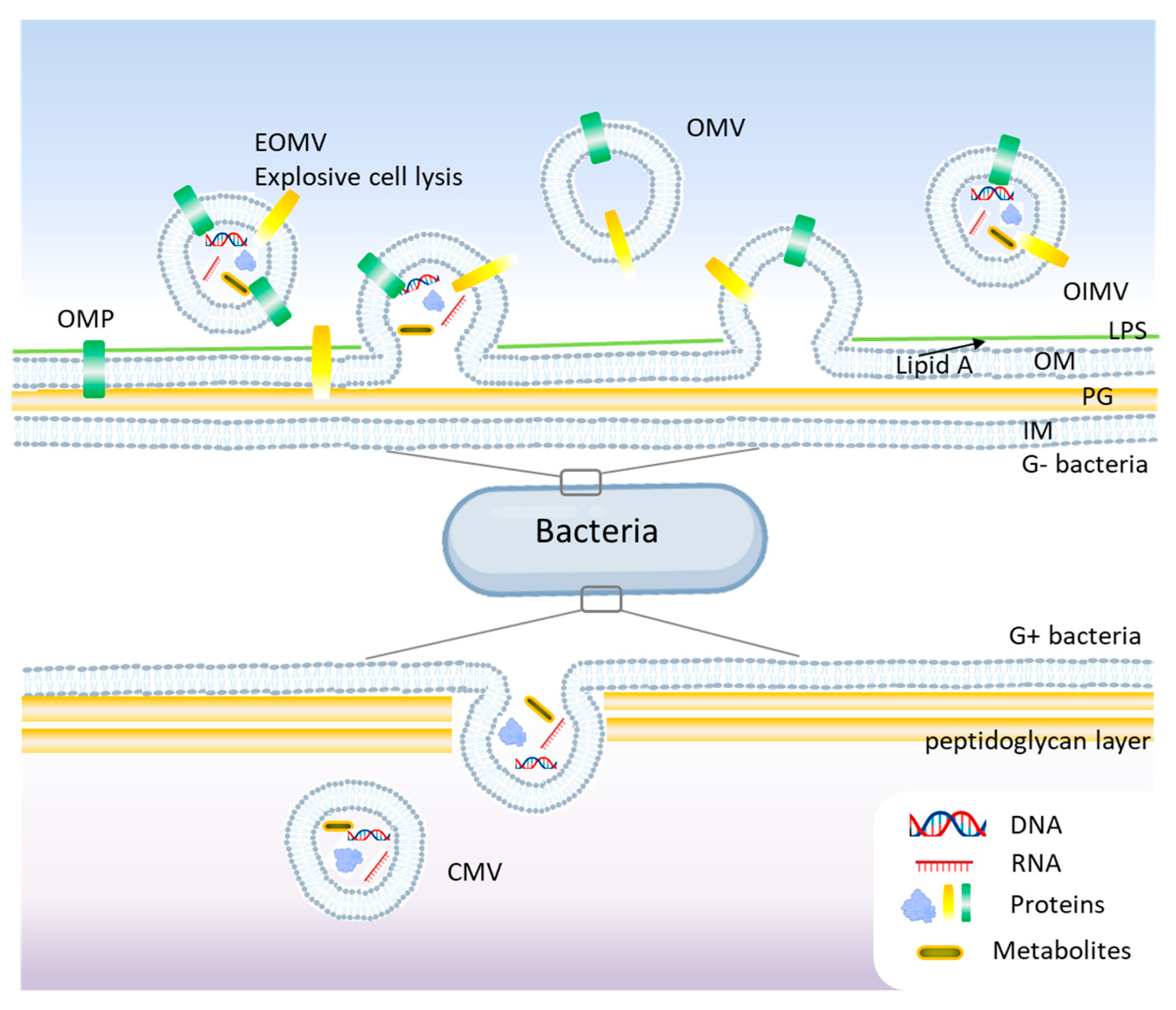
| Bacterial Strain | Type of BEV | Composition | Role in Antibiotic Resistance | Pathogenicity Role | Ref. |
|---|---|---|---|---|---|
| Escherichia coli | OMVs | Lipopolysaccharides (LPS), heat-labile toxin, heat-stable toxin, virulence factors, small RNAs | Carries β-lactamase, aiding resistance to β-lactam antibiotics | Promotes intestinal infections, urinary tract infections (UTIs) | [27] |
| Pseudomonas aeruginosa | OMVs, Explosive Vesicles | Quorum-sensing molecules, phospholipids, alkaline protease, elastase, efflux pump proteins | Carries enzymes that degrade antibiotics, enhances biofilm formation | Facilitates lung infections (CF patients), immune evasion | [19,28] |
| Staphylococcus aureus | CMVs | α-hemolysin, peptidoglycan, toxins, adhesins | Transfers methicillin-resistant genes (MRSA), promotes biofilm integrity | Causes skin infections, pneumonia, sepsis | [29] |
| Neisseria meningitidis | OMVs | Lipooligosaccharides (LOS), outer membrane proteins, adhesins | Helps evade immune system, limited role in antibiotic resistance | Causes meningitis, septicemia | [30] |
| Helicobacter pylori | OMVs | CagA, VacA toxin, LPS, adhesins | Alters host immune response but limited antibiotic resistance | Promotes gastric ulcers and gastric cancer | [31,32] |
| Acinetobacter baumannii | OMVs | OmpA, phospholipids, proteases, outer membrane proteins | Transfers resistance genes (carbapenemase, aminoglycoside resistance) | Causes multidrug-resistant (MDR) infections in hospitals | [33] |
| Mycobacterium tuberculosis | MVs | Mycolic acids, lipoproteins, glycolipids, DNA | Protects against antibiotics, modulates host immune response | Enhances survival in host macrophages | [34] |
| Vibrio cholerae | OMVs | Cholera toxin, outer membrane vesicle proteins, quorum-sensing molecules | Helps bacteria resist phage attacks but not majorly involved in antibiotic resistance | Contributes to cholera toxin delivery and infection spread | [35] |
| Klebsiella pneumoniae | OMVs | Capsular polysaccharides, lipoproteins, LPS | Facilitates β-lactam resistance, carries carbapenemase | Major cause of nosocomial infections, pneumonia, and sepsis | [36] |
| Bacillus subtilis | MVs | Peptidoglycan, proteins, signaling molecules | Provides defense against antibiotics in soil environments | Beneficial for plant growth and biocontrol rather than pathogenic | [37] |
| Method | Purity | Yield | Time Required | Cost | Best Used For | Ref |
|---|---|---|---|---|---|---|
| Differential Centrifugation | Medium, May contain contaminants like protein aggregates and cell debris | Medium | Medium | Medium, requires minimal equipment and reagents. | For initial screening of BEVs, where purity is not the primary concern | [44,49] |
| Density Gradient Ultracentrifugation | High, with minimal contamination from other cellular components. | Medium | Long | Medium, specific equipment for preparing and processing density gradients. | High-purity BEVs | [45] |
| Size-Exclusion Chromatography | High, preserves the integrity of the BEVs and their cargo. | Medium | Long | High | Specific vesicle subtypes based on size | [46] |
| Ultrafiltration | Medium, difficult to remove soluble protein contaminants | High | Fast | Medium | Large-scale prep | [47] |
| Immunoaffinity Capture | High | Low | Very Long | High | Specific BEV isolation | [48] |
| AMP | Applications | Challenges | Potential Solutions | Ref. |
|---|---|---|---|---|
| Colistin (Polymyxin E) | antibiotic for MDR Gram-negative bacteria (Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii). | Nephrotoxicity, neurotoxicity, resistance development (mcr-1 gene). | Liposomal formulations, combination therapy with rifampin, polymyxin derivatives with reduced toxicity. | [69,70] |
| Daptomycin | Effective against MRSA, VRE, and drug-resistant Enterococcus species. | Reduced activity in lung surfactants (not effective for pneumonia). | Novel formulations, nanoparticle delivery systems. | [71,72] |
| LL-37 (Human Cathelicidin) | Prevents biofilm formation (Pseudomonas aeruginosa), immunomodulatory, wound healing. | Susceptible to enzymatic degradation in vivo. | Chemical modifications (D-amino acids, cyclization), nanoparticle-based delivery. | [73] |
| Indolicidin | Inhibits DNA synthesis in E. coli, prevents biofilms. | Cytotoxicity at high concentrations. | Peptide engineering for improved selectivity. | [74] |
| Gramicidin | Used in topical antibiotics (eye and skin infections). | Hemolytic toxicity limits systemic use. | Liposomal encapsulation to reduce toxicity. | [75] |
| Magainins (Frog-Derived AMPs) | Potential use in antiviral and antifungal therapies, broad antimicrobial spectrum. | Poor stability in the bloodstream. | PEGylation (PEG-modified peptides), hybrid peptides. | [76,77] |
| Defensins | Found in human neutrophils, effective against Gram-positive and Gram-negative bacteria. | Limited large-scale production. | Recombinant peptide production (synthetic biology). | [78,79] |
| Bactenecins | Inhibits Gram-negative pathogens, anti-biofilm properties. | Rapid degradation in the bloodstream. | Protease-resistant peptide analogs. | [80] |
| Protegrins | Used in oral care, shows activity against drug-resistant Pseudomonas strains. | Toxicity in mammalian cells. | Sequence modifications to enhance selectivity. | [81] |
| Histatins | Antifungal AMPs (active against Candida albicans), used in oral care products. | Enzymatic degradation in saliva. | Hybrid peptide engineering. | [82] |
| Mechanism | Role of BEVs | Role of AMPs | Synergistic Effect |
|---|---|---|---|
| Targeted Drug Delivery | BEVs act as natural nanocarriers, delivering AMPs directly to bacterial cells. | AMPs attack bacterial membranes, disrupting integrity. | Enhanced local AMP concentration and specificity for resistant bacteria. |
| Membrane Permeabilization | BEVs fuse with bacterial membranes, increasing permeability. | AMPs create pores in membranes, leading to bacterial lysis. | Stronger membrane disruption, causing rapid bacterial death. |
| Overcoming Efflux Pumps | BEVs bypass efflux pumps, preventing bacteria from expelling AMPs. | AMPs disrupt efflux pump proteins, making bacteria more vulnerable. | Increased retention of AMPs inside bacterial cells. |
| Biofilm Penetration | BEVs carry biofilm-degrading enzymes or AMPs to bacterial communities. | AMPs break down biofilm structures, increasing bacterial exposure. | Biofilm eradication, making bacteria more susceptible to treatment. |
| Gene Transfer and Regulation | BEVs carry regulatory RNA/proteins that modulate bacterial gene expression. | AMPs interfere with bacterial gene transcription and translation. | Disruption of resistance gene expression, reducing bacterial survival. |
| Immunomodulation | BEVs influence host immune responses by modulating inflammation. | AMPs act as immune activators, enhancing pathogen clearance. | Strengthened innate immune defense against infections. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanasu, C. Bacterial Extracellular Vesicles and Antimicrobial Peptides: A Synergistic Approach to Overcome Antimicrobial Resistance. Antibiotics 2025, 14, 414. https://doi.org/10.3390/antibiotics14040414
Ciobanasu C. Bacterial Extracellular Vesicles and Antimicrobial Peptides: A Synergistic Approach to Overcome Antimicrobial Resistance. Antibiotics. 2025; 14(4):414. https://doi.org/10.3390/antibiotics14040414
Chicago/Turabian StyleCiobanasu, Corina. 2025. "Bacterial Extracellular Vesicles and Antimicrobial Peptides: A Synergistic Approach to Overcome Antimicrobial Resistance" Antibiotics 14, no. 4: 414. https://doi.org/10.3390/antibiotics14040414
APA StyleCiobanasu, C. (2025). Bacterial Extracellular Vesicles and Antimicrobial Peptides: A Synergistic Approach to Overcome Antimicrobial Resistance. Antibiotics, 14(4), 414. https://doi.org/10.3390/antibiotics14040414






