Metabolic Stress Induced by Quercetin Enhances Dormancy and Persistence in Staphylococcus aureus
Abstract
1. Introduction
2. Results and Discussion
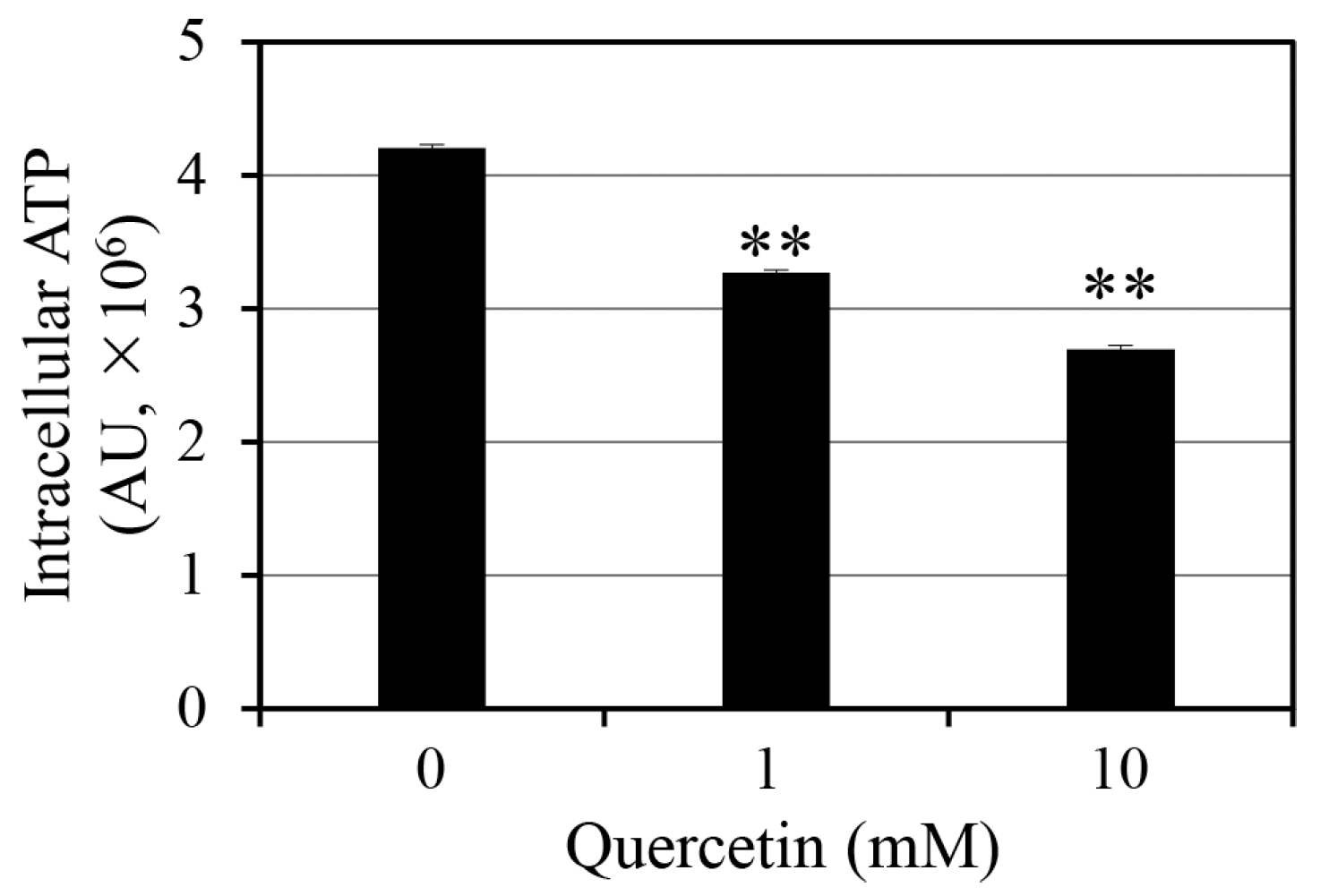
2.1. Effect of Quercetin on Intracellular ATP Levels
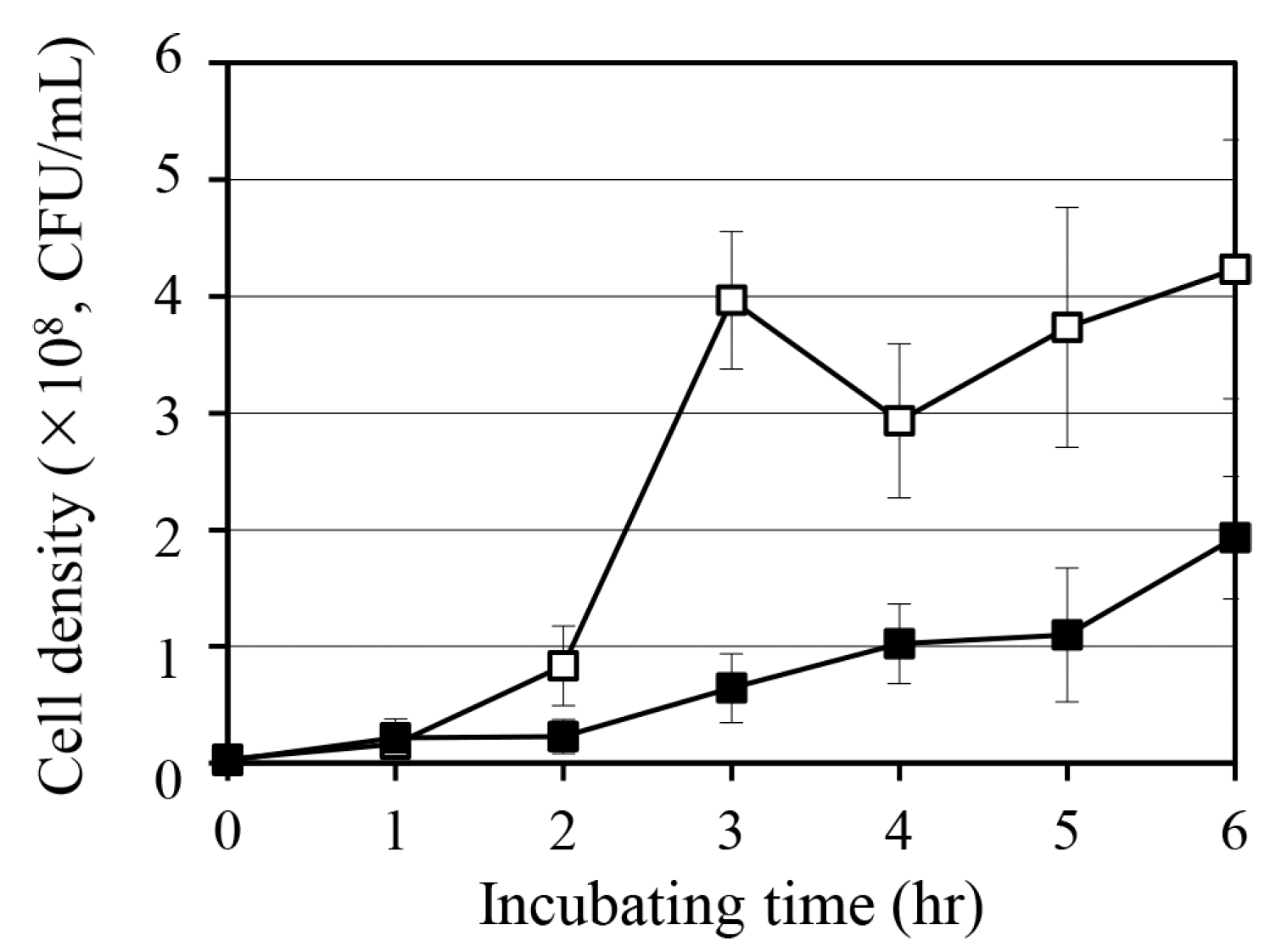
2.2. Effect of Quercetin on S. aureus Growth
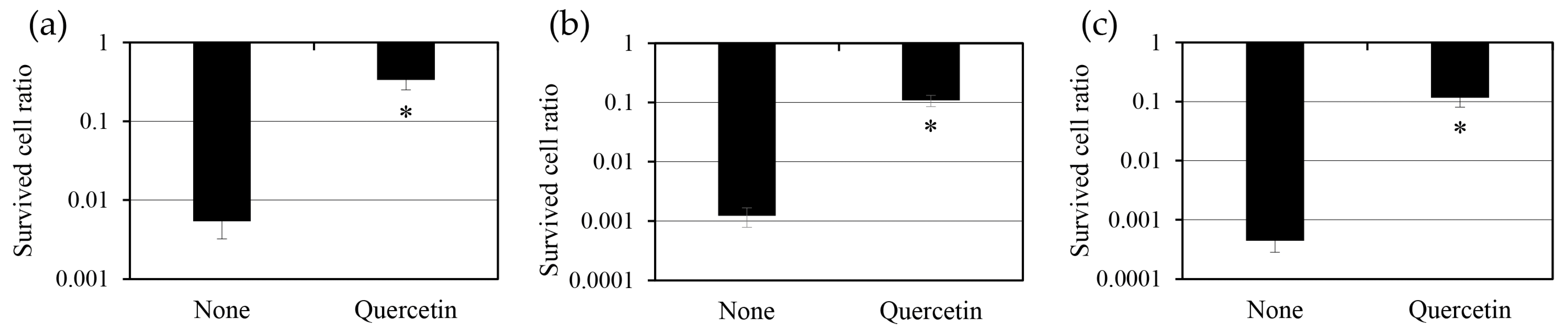
2.3. Effect of Quercetin on Persister Cell Formation
2.4. Effect of Quercetin Pre-Treatment on Persister Cells
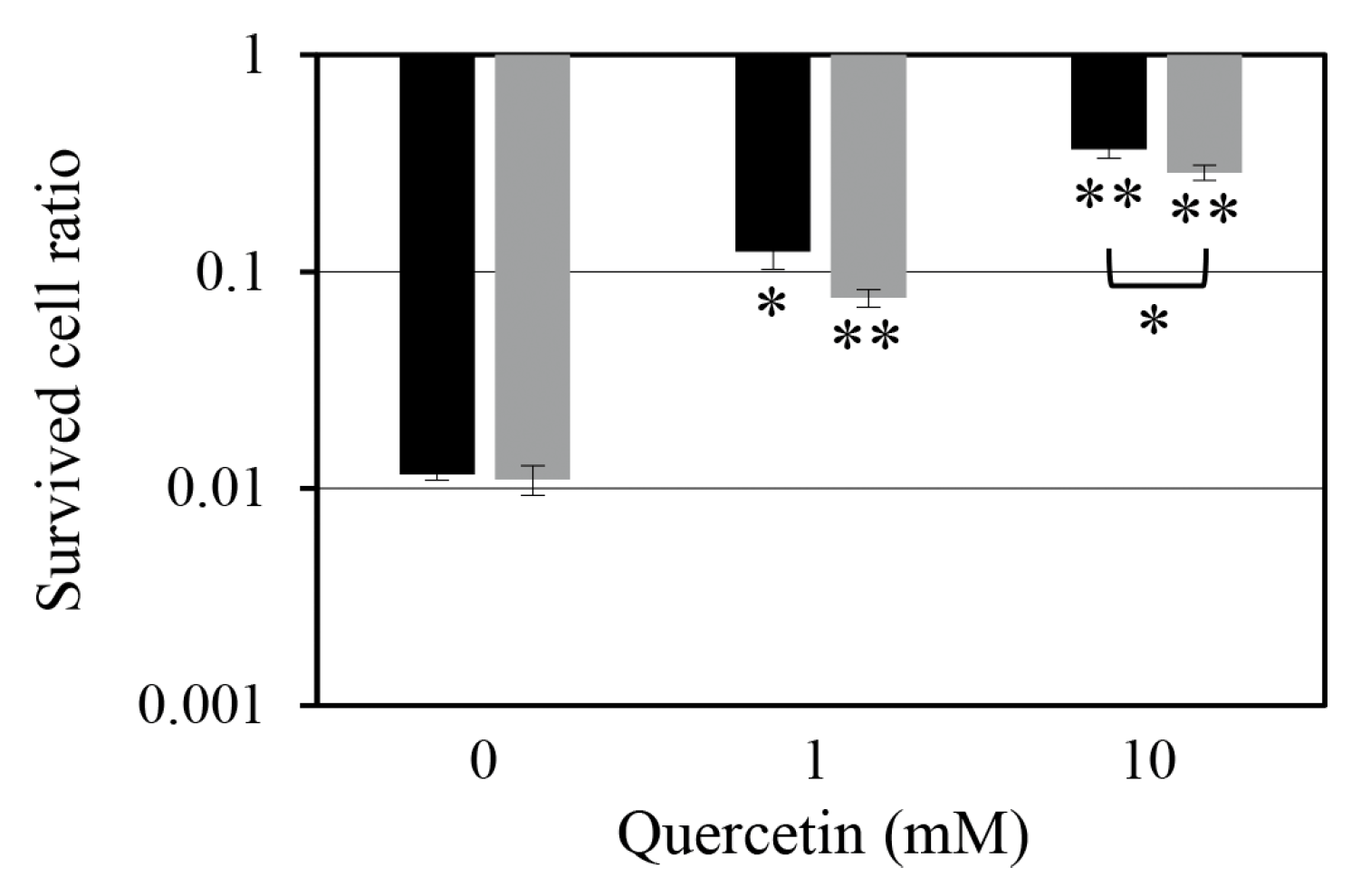
2.5. Effect of Quercetin Treatment After Antibiotic Exposure on the Survival of Persister Cells
2.6. Implications of Quercetin-Induced ATP Depletion on Bacterial Persistence
3. Materials and Methods
3.1. Strain and Chemicals
3.2. Measurement of Intracellular ATP Levels
3.3. Measurement of Growth Curve
3.4. Persister Cell Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AU | Arbitrary units |
| ATP | Adenosine triphosphate |
| CFU | Colony-forming units |
| DMSO | Dimethyl sulfoxide |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
References
- Lewis, K. Persister cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells. Bioessays 2014, 36, 991–996. [Google Scholar] [CrossRef]
- Kahl, B.C.; Becker, K.; Löffler, B. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti. Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.; Fauvart, M.; Michiels, J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef]
- Zalis, E.A.; Nuxoll, A.S.; Manuse, S.; Clair, G.; Radlinski, L.C.; Conlon, B.P.; Adkins, J.; Lewis, K. Stochastic variation in expression of the tricarboxylic acid cycle produces persister cells. mBio 2019, 10, e01930-19. [Google Scholar] [CrossRef]
- Shan, Y.; Gandt, A.B.; Rowe, S.E.; Deisinger, J.P.; Conlon, B.P.; Lewis, K. ATP-dependent persister formation in Escherichia coli. mBio 2017, 8, e02267-16. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Nešović, M.; Ćirić, I.; Tešić, Ž.; Pezo, L.; Tosti, T.; Gašić, U.; Dojčinović, B.; Lončar, B.; Meland, M. Polyphenolics and chemical profiles of domestic Norwegian apple (Malus × domestica Borkh.) cultivars. Front. Nutr. 2022, 9, 941487. [Google Scholar] [CrossRef]
- Behling, E.B.; Sendão, M.C.; Francescato, H.D.C.; Antunes, L.M.G.; Bianchi, M.d.L.P. Flavonoide quercetina: Aspectos gerais e acoes biologicas. Aliment. Nutr. 2004, 15, 285–292. [Google Scholar]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Ravera, S.; Tancreda, G.; Vezzulli, L.; Schito, A.M.; Panfoli, I. Cirsiliol and quercetin inhibit ATP synthesis and decrease the energy balance in methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE) strains isolated from patients. Molecules 2023, 28, 6183. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative In vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Salazar, C.; Jensen, E.; Ruiz, P.A.; Tiznado, W.; Quintanilla, R.A.; Barreto, M.; Elorza, A.A. Quercetin affects erythropoiesis and heart mitochondrial function in mice. Oxid. Med. Cell Longev. 2015, 2015, 836301. [Google Scholar] [CrossRef]
- Zymone, K.; Benetis, R.; Trumbeckas, D.; Baseviciene, I.; Trumbeckaite, S. Different effects of quercetin glycosides and quercetin on kidney mitochondrial function—Uncoupling, cytochrome C reducing and antioxidant activity. Molecules 2022, 27, 6377. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, H.C.; Hong, S.K.; Lee, M.O.; Cho, S.J.; Cha, H.J. Quercetin induced ROS production triggers mitochondrial cell death of human embryonic stem cells. Oncotarget 2016, 8, 64964–64973. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS metabolism in cancer cells: The potential role of quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, Y.; Bai, F.; Cheng, Z.; Wu, W.; Pan, X. Pseudomonas aeruginosa phosphate transporter PitA (PA4292) controls susceptibility to aminoglycoside antibiotics by regulating the proton motive force. Antimicrob. Agents Chemother. 2022, 66, e00992. [Google Scholar] [CrossRef]
- Yuan, H.; Xun, H.; Wang, J.; Wang, J.; Yao, X.; Tang, F. Integrated metabolomic and transcriptomic analysis reveals the underlying antibacterial mechanisms of the phytonutrient quercetin-induced fatty acids alteration in Staphylococcus aureus ATCC 27217. Molecules 2024, 29, 2266. [Google Scholar] [CrossRef] [PubMed]
- Audretsch, C.; Gratani, F.; Wolz, C.; Dandekar, T. Modeling of stringent-response reflects nutrient stress induced growth impairment and essential amino acids in different Staphylococcus aureus mutants. Sci. Rep. 2021, 11, 9651. [Google Scholar] [CrossRef]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268. [Google Scholar] [CrossRef]
- Trastoy, R.; Manso, T.; Fernández-García, L.; Blasco, L.; Ambroa, A.; del Molino, M.L.P.; Bou, G.; García-Contreras, R.; Wood, T.K.; Tomás, M. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin. Microbiol. Rev. 2018, 31, e00023-18. [Google Scholar] [CrossRef]
- Palazzotti, D.; Felicetti, T.; Sabatini, S.; Moro, S.; Barreca, M.L.; Sturlese, M.; Astolfi, A. Fighting antimicrobial resistance: Insights on how the Staphylococcus aureus NorA efflux pump recognizes 2-phenylquinoline inhibitors by supervised molecular dynamics (SuMD) and molecular docking simulations. J. Chem. Inf. Model. 2023, 63, 4875–4887. [Google Scholar] [CrossRef] [PubMed]
- Waditzer, M.; Bucar, F. Flavonoids as inhibitors of bacterial efflux pumps. Molecules 2021, 26, 6904. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, X.; Yang, J.Y.; Gao, S.; Bai, F. Intracellular ATP concentration is a key regulator of bacterial cell fate. J. Bacteriol. 2024, 206, e00208–e00224. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T.J. Coicis semen reduces Staphylococcus aureus persister cell formation by increasing membrane permeability. J. Korean Wood Sci. Technol. 2024, 52, 145–156. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Kim, T.-J. Metabolic Stress Induced by Quercetin Enhances Dormancy and Persistence in Staphylococcus aureus. Antibiotics 2025, 14, 424. https://doi.org/10.3390/antibiotics14050424
Kim D-Y, Kim T-J. Metabolic Stress Induced by Quercetin Enhances Dormancy and Persistence in Staphylococcus aureus. Antibiotics. 2025; 14(5):424. https://doi.org/10.3390/antibiotics14050424
Chicago/Turabian StyleKim, Dae-Youn, and Tae-Jong Kim. 2025. "Metabolic Stress Induced by Quercetin Enhances Dormancy and Persistence in Staphylococcus aureus" Antibiotics 14, no. 5: 424. https://doi.org/10.3390/antibiotics14050424
APA StyleKim, D.-Y., & Kim, T.-J. (2025). Metabolic Stress Induced by Quercetin Enhances Dormancy and Persistence in Staphylococcus aureus. Antibiotics, 14(5), 424. https://doi.org/10.3390/antibiotics14050424






