Suitability of Piperacillin–Tazobactam for Treating Dogs Infected with Extended-Spectrum β-Lactamase-Producing Enterobacterales: Pharmacokinetic and Pharmacodynamic Analysis
Abstract
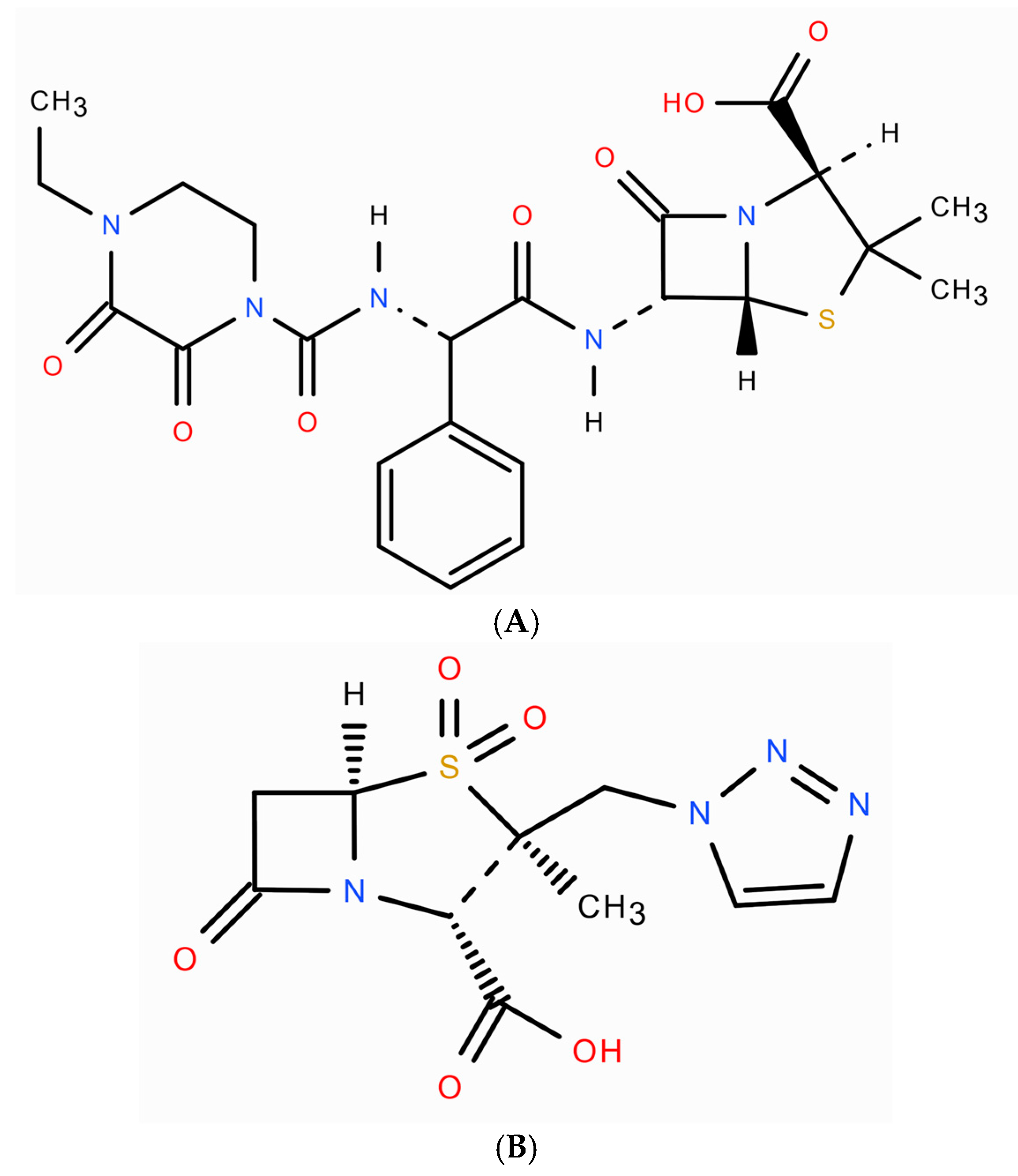
1. Introduction
2. Results and Discussion
| Bacterial Species 1 | No. of Isolates with TZP MIC (µg/mL) 1,2 | MIC50 4 (µg/mL) | MIC90 5 (µg/mL) | No. of Susceptible Isolates (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/4 | 2/4 | 4/4 | 8/4 | 16/4 | 32/4 | 64/4 | 128/4 | 256/4 | 512/4 | ||||
| E. coli [n = 62 (5)] 3 | 6 | 20 | 26 (1) | 4 (1) | 3 (2) | 1 | 0 | 1 (1) | 1 | 0 | 4/4 | 16/4 | 56 (90.3) |
| K. pneumoniae [n = 89 (8)] | 2 | 9 (1) | 26 (2) | 31 (4) | 15 | 3 (1) | 0 | 1 | 1 | 1 | 8/4 | 16/4 | 68 (76.4) |
| E. cloacae [n = 31 (7)] | 0 | 1 | 13 (1) | 8 (2) | 6 (2) | 2 (1) | 0 | 0 | 1 (1) | 0 | 8/4 | 16/4 | 22 (71.0) |
| Dosing Intervals | Cumulative Fraction of Response (%) | ||
|---|---|---|---|
| Escherichia coli | Klebsiella pneumoniae | Enterobacter cloacae | |
| q12h | 1.60 | 0.48 | 0.15 |
| q8h | 32.56 | 14.57 | 9.65 |
| q6h | 74.51 | 45.85 | 43.92 |
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing Escherichia coli in dogs and cats-a scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef]
- Kusumoto, M.; Jitsuiki, M.; Motegi, T.; Harada, K. Pharmacokinetic and pharmacodynamic analysis of the oxacephem antibiotic flomoxef against extended-spectrum β-lactamase-producing Enterobacterales in dogs. Int. J. Mol. Sci. 2024, 25, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Motegi, T.; Uno, H.; Yokono, M.; Harada, K. Pharmacokinetic-pharmacodynamic analysis of cefmetazole against extended-spectrum β-lactamase-producing Enterobacteriaceae in dogs using Monte Carlo Simulation. Front. Vet. Sci. 2023, 10, 1270137. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Narita, H.; Motegi, T.; Harada, K. Estimation of latamoxef (moxalactam) dosage regimens against β-lactamase-producing Enterobacterales in dogs: A pharmacokinetic and pharmacodynamic analysis using Monte Carlo simulation. J. Vet. Med. Sci. 2024, 86, 841–846. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yamamoto, M.; Nagao, M.; Tanaka, M.; Takakura, S.; Ichiyama, S. In vitro activities and detection performances of cefmetazole and flomoxef for extended-spectrum β-lactamase and plasmid-mediated AmpC β-lactamase-producing Entero-bacteriaceae. Diagn. Microbiol. Infect. Dis. 2016, 84, 322–327. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Mei, Y.; Wu, S.; Li, X.; Li, S.; Wang, J.; Gao, L.; Xu, H.; Tuo, Y. Population pharmacokinetics and dosing regimen optimization of latamoxef in Chinese children. Pharmaceutics 2022, 14, 1033. [Google Scholar] [CrossRef]
- Hayashi, Y.; Roberts, J.A.; Paterson, D.L.; Lipman, J. Pharmacokinetic evaluation of piperacillin-tazobactam. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1017–1031. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pea, F. Piperacillin-tazobactam vs. carbapenems for treating hospitalized patients with ESBL-producing Enterobacterales bloodstream infections: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2024, 39, 27–36. [Google Scholar] [CrossRef]
- Karaiskos, I.; Giamarellou, H. Carbapenem-sparing strategies for ESBL producers: When and how. Antibiotics 2020, 9, 61. [Google Scholar] [CrossRef]
- Tamma, P.D.; Rodriguez-Bano, J. The use of noncarbapenem β-lactams for the treatment of extended-spectrum β-lactamase infections. Clin. Infect. Dis. 2017, 64, 972–980. [Google Scholar] [CrossRef]
- Sykes, J.E.; Papich, M.G. 10: Antibacterial Drugs. In Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Sykes, J.E., Ed.; Elsevier: Cambridge, MA, USA, 2023; pp. 103–126. [Google Scholar]
- Shimizu, T.; Harada, K.; Tsuyuki, Y.; Kimura, Y.; Miyamoto, T.; Hatoya, S.; Hikasa, Y. In vitro efficacy of 16 antimicrobial drugs against a large collection of β-lactamase-producing isolates of extraintestinal pathogenic Escherichia coli from dogs and cats. J. Med. Microbiol. 2017, 66, 1085–1091. [Google Scholar] [CrossRef]
- Tebano, G.; Zaghi, I.; Cricca, M.; Cristini, F. Antibiotic treatment of infections caused by AmpC-producing Enterobacterales. Pharmacy 2024, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.T.M.; Mason, J.; Roberts, P.; Parry, C.M.; Corless, C.; van Aartsen, J.; Howard, A.; Bulgasim, I.; Fraser, A.J.; Adams, E.R.; et al. Piperacillin/tazobactam resistance in a clinical isolate of Escherichia coli due to IS26-mediated amplification of blaTEM-1B. Nat. Commun. 2020, 11, 4915. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Park, K.S.; Jeon, J.H.; Lee, J.K.; Lee, J.H.; Choi, E.H.; Lee, S.H. SHV hyperproduction as a mechanism for piperacil-lin-tazobactam resistance in extended-spectrum cephalosporin-susceptible Klebsiella pneumoniae. Microb. Drug Resist. 2020, 26, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Guérin, F.; Isnard, C.; Cattoir, V.; Giard, J.C. Complex regulation pathways of AmpC-mediated-lactam resistance in Enterobacter cloacae complex. Antimicrob. Agents Chemother. 2015, 59, 7753–7761. [Google Scholar] [CrossRef]
- Gill, C.M.; Nicolau, D.P.; ERACE-PA Global Study Group. Piperacillin/tazobactam dose optimization in the setting of pipe-racillin/tazobactam-susceptible, carbapenem-resistant Pseudomonas aeruginosa: Time to reconsider susceptible dose dependent. Clin. Ther. 2023, 45, 72–77. [Google Scholar] [CrossRef]
- Kim, M.K.; Quintiliani, R.; Nightingale, C.H.; Nicolau, D.P. Pharmacokinetic and pharmacodynamic profile of high dose extended-interval piperacillin-tazobactam. J. Antimicob. Chemother. 2001, 48, 259–267. [Google Scholar] [CrossRef]
- Shea, K.M.; Cheatham, S.C.; Wack, M.F.; Smith, D.W.; Sowinski, K.M.; Kays, M.B. Steady-state pharmacokinetics and phar-macodynamics of piperacillin/tazobactam administered by prolonged infusion in hospitalised patients. Int. J. Antimicrob. Agents 2009, 34, 429–433. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 7th ed.; CLSI Supplement VET01S; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Lappin, M.R.; Blondeau, J.; Boothe, D.; Breitschwerdt, E.B.; Guardabassi, L.; Lloyd, D.H.; Papich, M.G.; Rankin, S.C.; Sykes, J.E.; Turnidge, J.; et al. Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Anti-microbial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J. Vet. Intern. Med. 2017, 31, 279–294. [Google Scholar] [CrossRef]
- Hayashi, T.; Yada, H.; Blair, M.; Laughlin, K.A.; Blanchard, G.L.; Tucek, P.C.; Geil, R.G. A six-month intravenous repeated dose toxicity study of tazobactam/piperacillin and tazobactam in dogs. J. Toxicol. Sci. 1994, 19, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Alawyia, B.; Fathima, S.; Spernovasilis, N.; Alon-Ellenbogen, D. Continuous versus intermittent infusion of beta-lactam antibiotics: Where do we stand today? A narrative review. Germs 2024, 14, 162–178. [Google Scholar] [CrossRef]
- Gatti, M.; Cojuttim, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assess-ment of a PK/PD target of continuous infusion beta-lactams useful for preventing microbiological failure and/or resistance de-velopment in critically ill patients affected by documented Gram-negative infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Kanao, Y.; Narita, H.; Jitsuiki, M.; Iyori, K.; Tsunoi, M.; Tsuyuki, Y.; Torii, K.; Harada, K. In vitro efficacy of cephamycins against multiple extended-spectrum β-lactamase-producing Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae isolates from dogs and cats. J. Vet. Med. Sci. 2023, 85, 653–656. [Google Scholar] [CrossRef]
- López-Cerero, L.; Picón, E.; Morillo, C.; Hernández, J.R.; Decobo, F.; Pachón, J.; Rodríguez-Baño, J.; Pascual, A. Comparative assessment of inoculum effects on the antimicrobial activity of amoxycillin–clavulanate and piperacillin–tazobactam with extended-spectrum β-lactamase-producing and extended-spectrum β-lactamase-non-producing Escherichia coli isolates. Clin. Microbiol. Infect. 2010, 16, 132–136. [Google Scholar] [CrossRef]
- Zaghloul, I.; Kuck, N.; Yacobi, A. The effect of tazobactam on the pharmacokinetics and the antibacterial activity of piperacillin in dogs. Int. J. Pharm. 1997, 153, 115–121. [Google Scholar] [CrossRef]
- Komuro, M.; Maeda, T.; Kakuo, H.; Matsushita, H.; Shimada, J. Inhibition of the renal excretion of tazobactam by piperacillin. J. Antimicrob. Chemother. 1994, 34, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Frei, C.R.; Wiederhold, N.P.; Burgess, D.S. Antimicrobial breakpoints for Gram-negative aerobic bacteria based on pharmacokinetic–pharmacodynamic models with Monte Carlo simulation. J. Antimicrob. Cemother. 2008, 61, 621–628. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, L.; Yu, W.; Huang, C.; Ji, J.; Ying, C.; Liu, Z.; Chen, Y.; Xiao, Y. Evaluation of piperacillin/sulbactam, pipera-cillin/tazobactam and cefoperazone/sulbactam dosages in Gram-negative bacterial bloodstream infections by Monte Carlo Simulation. Antibiotics 2023, 12, 363. [Google Scholar] [CrossRef]
- Mouton, J.W.; Brown, D.F.; Apfalter, P.; Canton, R.; Giske, C.G.; Ivanova, M.; MacGowan, A.P.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: The EUCAST ap-proach. Clin. Microbiol. Infect. 2012, 18, E37–E45. [Google Scholar] [CrossRef]
- Landmesser, K.B.; Clark, J.A.; Burgess, D.S. Time above all else: Pharmacodynamic analysis of ß-lactams in critically ill patients. J. Clin. Pharmacol. 2022, 62, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Bousquet-Mélou, A. Plasma clearance. J. Vet. Pharmacol. Therap. 2004, 27, 415–425. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; Licence: CC BY-NC-SA 3.0 IGO; WHO: Geneva, Switzerland, 2024. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harada, K.; Harada, H.; Kanao, Y.; Kusumoto, M. Suitability of Piperacillin–Tazobactam for Treating Dogs Infected with Extended-Spectrum β-Lactamase-Producing Enterobacterales: Pharmacokinetic and Pharmacodynamic Analysis. Antibiotics 2025, 14, 425. https://doi.org/10.3390/antibiotics14050425
Harada K, Harada H, Kanao Y, Kusumoto M. Suitability of Piperacillin–Tazobactam for Treating Dogs Infected with Extended-Spectrum β-Lactamase-Producing Enterobacterales: Pharmacokinetic and Pharmacodynamic Analysis. Antibiotics. 2025; 14(5):425. https://doi.org/10.3390/antibiotics14050425
Chicago/Turabian StyleHarada, Kazuki, Hyo Harada, Yuka Kanao, and Mizuki Kusumoto. 2025. "Suitability of Piperacillin–Tazobactam for Treating Dogs Infected with Extended-Spectrum β-Lactamase-Producing Enterobacterales: Pharmacokinetic and Pharmacodynamic Analysis" Antibiotics 14, no. 5: 425. https://doi.org/10.3390/antibiotics14050425
APA StyleHarada, K., Harada, H., Kanao, Y., & Kusumoto, M. (2025). Suitability of Piperacillin–Tazobactam for Treating Dogs Infected with Extended-Spectrum β-Lactamase-Producing Enterobacterales: Pharmacokinetic and Pharmacodynamic Analysis. Antibiotics, 14(5), 425. https://doi.org/10.3390/antibiotics14050425




