Characterization of Pure Rutile Titania Nanoparticle Prepared by Feasible Method for Coatings and Visible Light-Driven Dye Removal Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Rutile TiO2 Hydrothermal Method
2.2. Material Characterization
2.3. Band Gap Evolution of Synthesized Rutile TiO2
2.4. Photocatalytic Study of Synthesized Rutile Titania Catalysts
3. Results
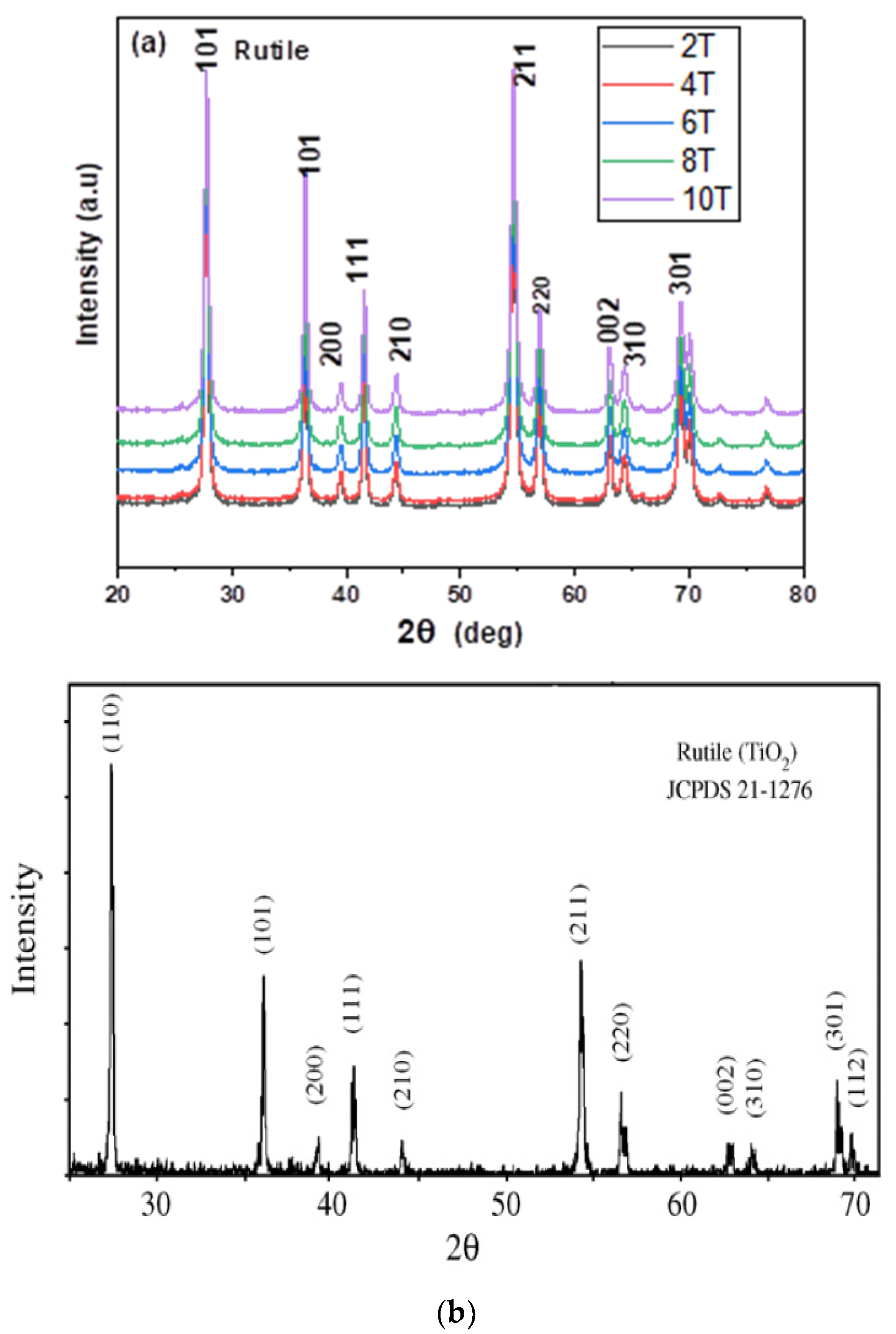
3.1. Crystallinity and Surface Area Characterization
3.2. Morphology and Optical Property Characterization
3.3. Photocatalytic Activity of Rutile TiO2 under Visible Light and Direct Sunlight Irradiation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, R.; Christensen, M.; Shen, Y.; Kibsgaard, J.; Elgh, B.; Vang, R.T.; Bechstein, R.; Wendt, S.; Palmqvist, A.; Iversen, B.B.; et al. Rapid synthesis of porous, mixed phase titania films with tailored orientation of rutile for enhanced photocatalytic performance. J. Phys. Chem. C 2013, 117, 27039–27046. [Google Scholar] [CrossRef]
- Veréb, G.; Manczinger, L.; Bozsó, G.; Sienkiewicz, A.; Forró, L.; Mogyorósi, K.; Hernadi, K.; Dombi, A. Comparison of the photocatalytic efficiencies of bare and doped rutile and anatase TiO2 photocatalysts under visible light for phenol degradation and E. coli inactivation. Appl. Catal. B: Environ. 2013, 129, 566–574. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Munirah D., A.; Al-Lohedan, H.A.; Prabhakarn, A.; Kandasamy, T.; Jimmy Nelson, A. Preparation, characterization and Morphologies insights of ZnO nanodisk–TiO2-coated SWCNT thin film composites for catalytic sensor application, surface and interface analysis. Surf. Interface Anal. 2021, 53, 395–405. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C.D.N. Neodymium doped TiO2 nanoparticles by sol-gel method for antibacterial and photocatalytic activity. Mater. Sci. Semicond. Process. 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Hao, Y.; Ma, J.; Li, W.; Cai, S. Preparation, characterization and photoelectrochemical behaviors of Fe(III)-doped TiO2 nanoparticles. J. Mater. Sci. 1999, 34, 3721–3729. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, S.; Sato, T. Synthesis of iron-containing nitrogen-doped titania by hydrothermal method and its photocatalytic activity. Res. Chem. Intermed. 2011, 37, 479–485. [Google Scholar] [CrossRef]
- Anju, K.; Thankapan, R.; Rajabathar, J.R.; Al-Lohedan, H.A. Hydrothermal synthesis of nanosized (Fe, Co, Ni)-TiO2 for enhanced visible light photosensitive applications. Optik 2018, 165, 408–415. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Shukla, A.K.; Kombaiah, K.; Vijaya, J.J.; Tawfeek, A.M. Synthesis, characterization and optical properties of sulfur and fluorine doped ZnO nanostructures for visible light utilized catalysis. Optik 2017, 148, 325–331. [Google Scholar] [CrossRef]
- Latha, P.; Prakash, K.; Karuthapandian, S. Effective photodegradation of CR & MO dyes by morphologically controlled cerium oxide nanocubes under visible light illumination. Optik 2018, 154, 242–250. [Google Scholar] [CrossRef]
- Da Silva, E.P.; Rubira, A.F.; Ferreira, O.P.; Silva, R.; Muniz, E.C. In situ growth of manganese oxide nanosheets over titanium dioxide nanofibers and their performance as active material for supercapacitor. J. Colloid Interface Sci. 2019, 555, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Lee, S.Y.; Yang, Y. Fabrication of morphology-controlled TiO2 photocatalyst nanoparticles and improvement of photocatalytic activities by modification of Fe compounds. Rare Met. 2015, 34, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Sun, M.; Gao, B.; Ding, W.; Zhang, Z.; Anandan, S.; Umar, A. Hydrothermally regulating phase composition of TiO2 nanocrystals toward high photocatalytic activity. J. Alloys Compd. 2021, 850, 156653. [Google Scholar] [CrossRef]
- George, J.; Gopalakrishnan, C.; Manikuttan, P.; Mukesh, K.; Sreenish, S. Preparation of multi-purpose TiO2 pigment with improved properties for coating applications. Powder Technol. 2021, 377, 269–273. [Google Scholar] [CrossRef]
- Kumari, K.; Reeshma, R.; Arunkumar, D.; Meti, S.; Rahman, M. Fabrication of Ag/PDMS-TiO2 flexible piezoresistive pressure sensor. Phys. B Condens. Matter 2020, 597, 412386. [Google Scholar] [CrossRef]
- Kumar, R.; El-Shishtawy, R.M.; Barakat, M.A. Synthesis and characterization of Ag-Ag2O/TiO2@polypyrrole heterojunction for enhanced photocatalytic degradation of methylene blue. Catalysts 2016, 6, 76. [Google Scholar] [CrossRef]
- Hamed, N.; Ahmad, M.; Hairom, N.H.H.; Faridah, A.; Mamat, M.; Mohamed, A.; Suriani, A.; Nafarizal, N.; Fazli, F.; Mokhtar, S.; et al. Dependence of photocatalysis on electron trapping in Ag-doped flowerlike rutile-phase TiO2 film by facile hydrothermal method. Appl. Surf. Sci. 2020, 534, 147571. [Google Scholar] [CrossRef]
- Suzuki, H.; Awa, K.; Naya, S.-I.; Tada, H. Heat treatment effect of a hybrid consisting of SnO2 nanorod and rutile TiO2 with heteroepitaxial junction on the photocatalytic activity. Catal. Commun. 2020, 147, 106148. [Google Scholar] [CrossRef]
- Ramalingam, R.J.; Radhika, T.; Ranjan, P.R.; Sayed, S.R.; Al-Lohedan, H.A.; Moydeen, A.M.; Al-Dhayan, D.M. Platinum nanoparticle decorated rutile titania synthesized by surfactant free hydrothermal method for visible light catalysis for dye degradation and hydrogen production study. Int. J. Hydrog. Energy 2019, 44, 23959–23968. [Google Scholar] [CrossRef]
- Morad, I.; Alshehri, A.; Mansour, A.; Wasfy, M.; El-Desoky, M. Facile synthesis and comparative study for the optical performance of different TiO2 phases doped PVA nanocomposite films. Phys. B Condens. Matter 2020, 597, 412415. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, Z.; Zhu, C.; Zhang, R.; Hu, X.; Zhang, X.; Zhu, H.; Lin, S.H. The electronic structure, optical absorption and photocatalytic water splitting of (Fe + Ni)-codoped TiO2: A DFT + U study. Int. J. Hydrog. Energy 2017, 42, 4966–4976. [Google Scholar] [CrossRef]
- Hamed, A.; Mahyar, M.; Razieh, K.; Sadrnezhaad, S.K. Sonochemical preparation of TiO2 nanoparticles. Mater. Lett. 2007, 61, 4561. [Google Scholar]
- Mousavi, D.-S.; Asen, P.; Shahrokhian, S.; Irajizad, A. Three-dimensional hybrid of iron–titanium mixed oxide/nitrogen-doped graphene on Ni foam as a superior electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2020, 563, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Shukla, A.; Choi, S.S.; Lee, S.-M. Surface modified nanostructured-TiO2 thin films for removal of Congo red. Korean J. Chem. Eng. 2018, 35, 2133–2137. [Google Scholar] [CrossRef]
- Park, H.-G.; Kim, J.I.; Kang, M.; Yeo, M.-K. The effect of metal-doped TiO2 nanoparticles on zebrafish embryogenesis. Mol. Cell. Toxicol. 2014, 10, 293–301. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, K.; Han, X.; Zhao, Q.; Wang, D.; Fu, F.; Liang, Y. Highly efficient visible-light-driven photo-Fenton catalytic performance over FeOOH/Bi2WO6 composite for organic pollutant degradation. J. Alloys Compd. 2020, 816, 152560. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, M.; Cerrato, G.; Morandi, S.; Gatto, S.; Oldani, V.; Di Michele, A.; Bianchi, C. Photoactive TiO2–montmorillonite composite for degradation of organic dyes in water. J. Photochem. Photobiol. A Chem. 2014, 295, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Ye, P.; Wu, M.M.; Wei, M.; Yang, Z.; Han, Q.F. Preparation, Characterization and Properties of BiOCl1-xIx and BiOBr1-xIx Solid Solution, Guang Pu Xue Yu Guang Pu Fen Xi/Spectroscopy Spectr. Analysts 2019, 39, 2443–2449. [Google Scholar] [CrossRef]











| S. No. | Concentration of HNO3 (M) | Temperature (K), Time (hours) | Sample Identification |
|---|---|---|---|
| 1. | 2 | 453, 2 | 2T |
| 2. | 4 | 453, 2 | 4T |
| 3. | 6 | 453, 2 | 6T |
| 4. | 8 | 453, 2 | 8T |
| 5. | 10 | 453, 2 | 10T |
| Sl. No. | Material | BET Surface Area (m2/g) | Particle Size (nm) |
|---|---|---|---|
| 1. | TiO2 (Commercial-P25) | ~50 | 26.5 |
| 2. | TiO2 (2T) | 110 | 14.9 |
| 3. | TiO2 (4T) | 78.3 | 20.3 |
| 4. | TiO2 (6T) | 49.0 | 32.5 |
| 5. | TiO2 (8T) | 43.5 | 57.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabathar, J.R.; Al-Lohedan, H.A.; Arokiyaraj, S.; Issa, Z.A.; Dash, C.S.; Murugesan, S.; Khadheer Pasha, S.K.; Al-dhayan, D.M.; Appaturi, J.N. Characterization of Pure Rutile Titania Nanoparticle Prepared by Feasible Method for Coatings and Visible Light-Driven Dye Removal Application. Coatings 2021, 11, 1150. https://doi.org/10.3390/coatings11101150
Rajabathar JR, Al-Lohedan HA, Arokiyaraj S, Issa ZA, Dash CS, Murugesan S, Khadheer Pasha SK, Al-dhayan DM, Appaturi JN. Characterization of Pure Rutile Titania Nanoparticle Prepared by Feasible Method for Coatings and Visible Light-Driven Dye Removal Application. Coatings. 2021; 11(10):1150. https://doi.org/10.3390/coatings11101150
Chicago/Turabian StyleRajabathar, Jothi Ramalingam, Hamad A. Al-Lohedan, Selvaraj Arokiyaraj, Zuheir A. Issa, Chandra Sekhar Dash, Sundararajan Murugesan, Shaik K. Khadheer Pasha, Dhaifallah M. Al-dhayan, and Jimmy Nelson Appaturi. 2021. "Characterization of Pure Rutile Titania Nanoparticle Prepared by Feasible Method for Coatings and Visible Light-Driven Dye Removal Application" Coatings 11, no. 10: 1150. https://doi.org/10.3390/coatings11101150








