Numerical Analysis of the Interactions between Plasma Jet and Powder Particles in PS-PVD Conditions
Abstract
:1. Introduction
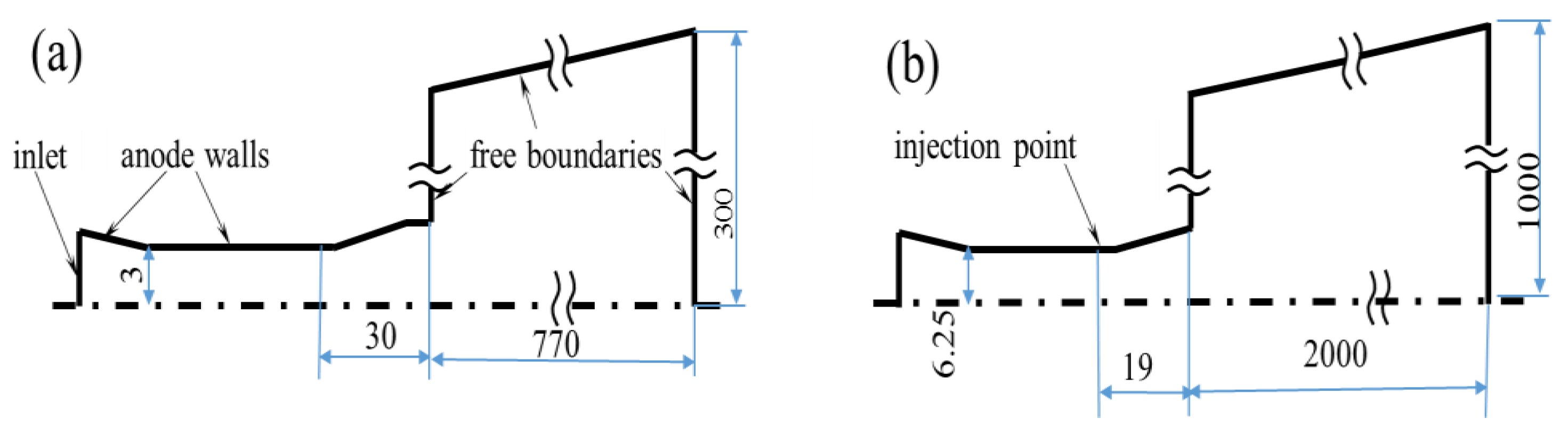
2. Operating Conditions
3. Numerical Model
3.1. Plasma Jet Modeling
3.2. Particle Dynamics and Heating
- (1)
- The powder particles are spherical; their size is much larger than the mean free path length of the plasma particles; the thickness of the plasma sheath around the particles is much less than their diameter;
- (2)
- The plasma consists of atoms, singly ionized ions, and electrons that follow a Maxwell velocity distribution function;
- (3)
- Electron emission and radiation from the powder particle surface are neglected;
- (4)
- Ions colliding with the particle surface recombine with electrons emitted by the surface and electrons colliding with the particle surface are absorbed by the surface;
- (5)
- Complete diffuse reflection is assumed for all atoms leaving the particle surface;
- (6)
- The effect of powder particle evaporation on the drag force exerted by the gas flow and plasma heat transfer is not taken into account;
- (7)
- The powder particle surface has a steady floating potential.
4. Results and Discussion
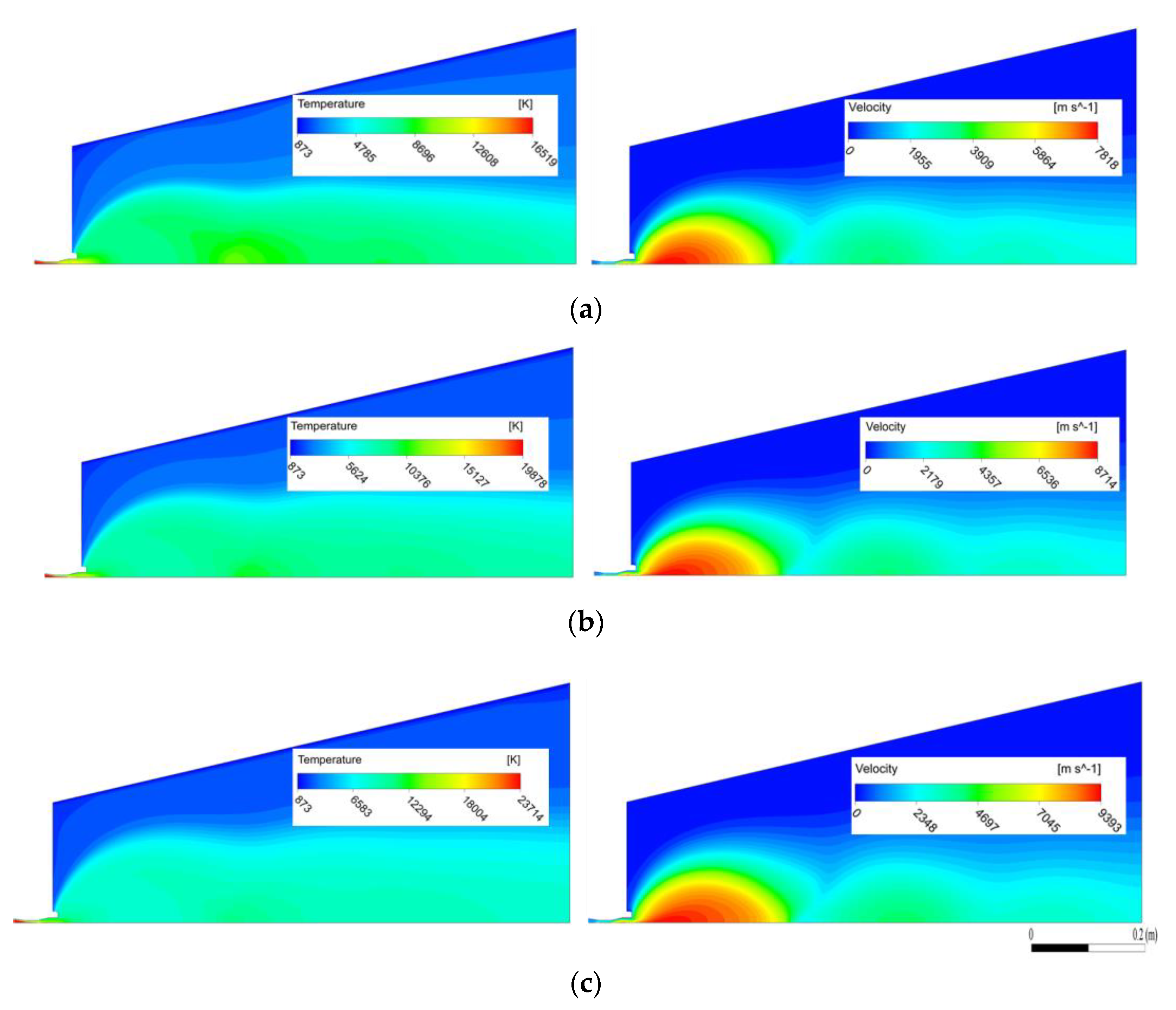
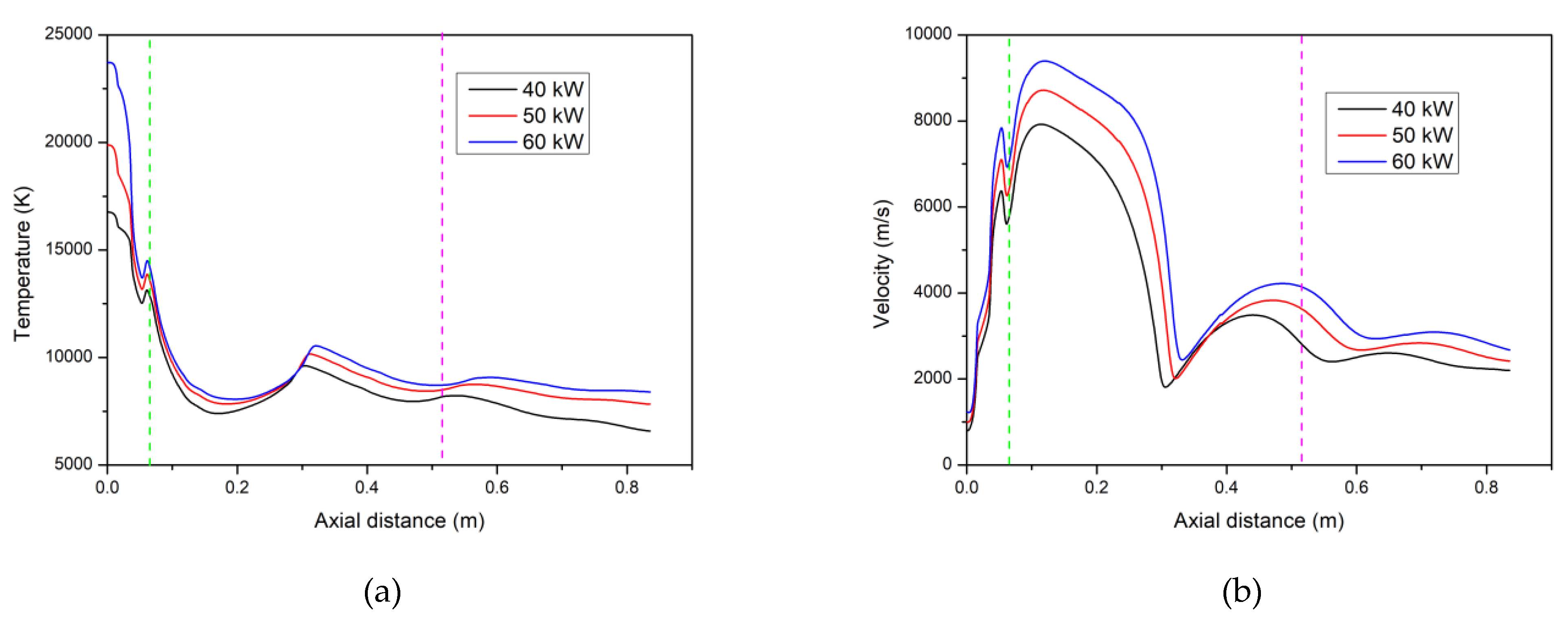
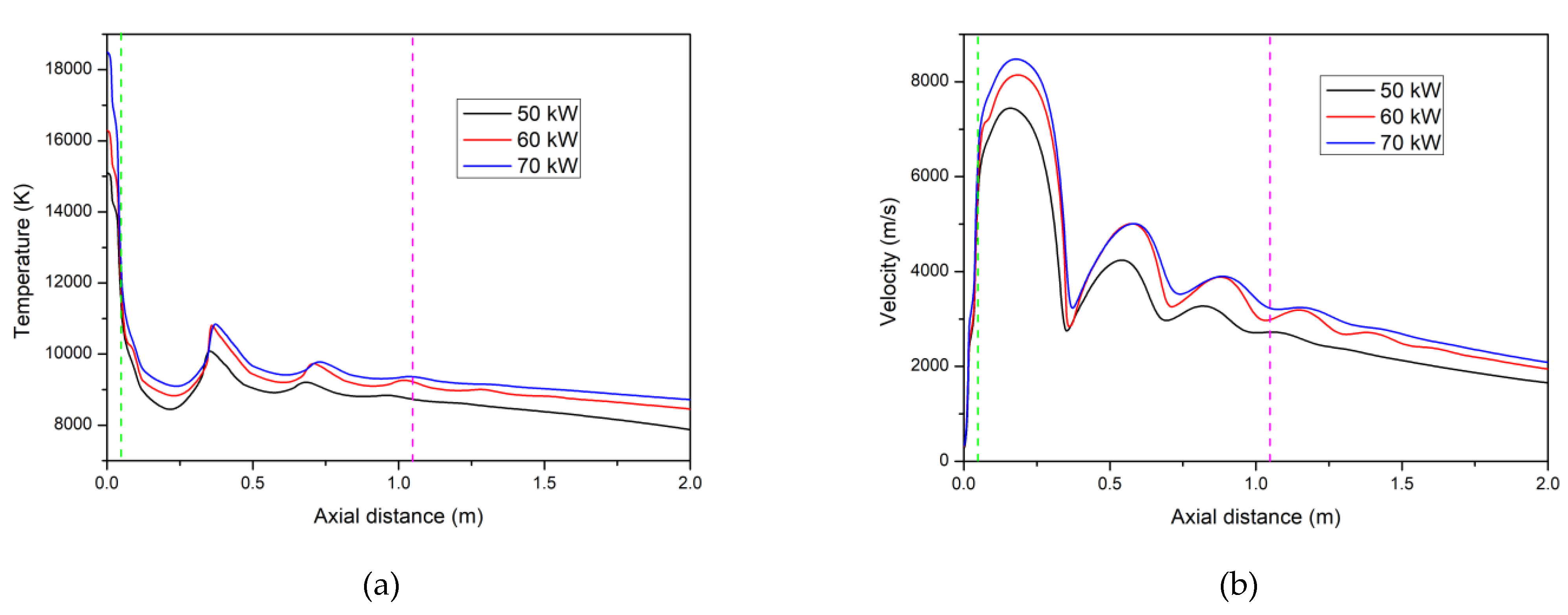
4.1. Temperature and Velocity Fields of the Ar–H2 Plasma Jet
4.2. The Temperature and Velocity Distribution of Ar–He Plasma Jet
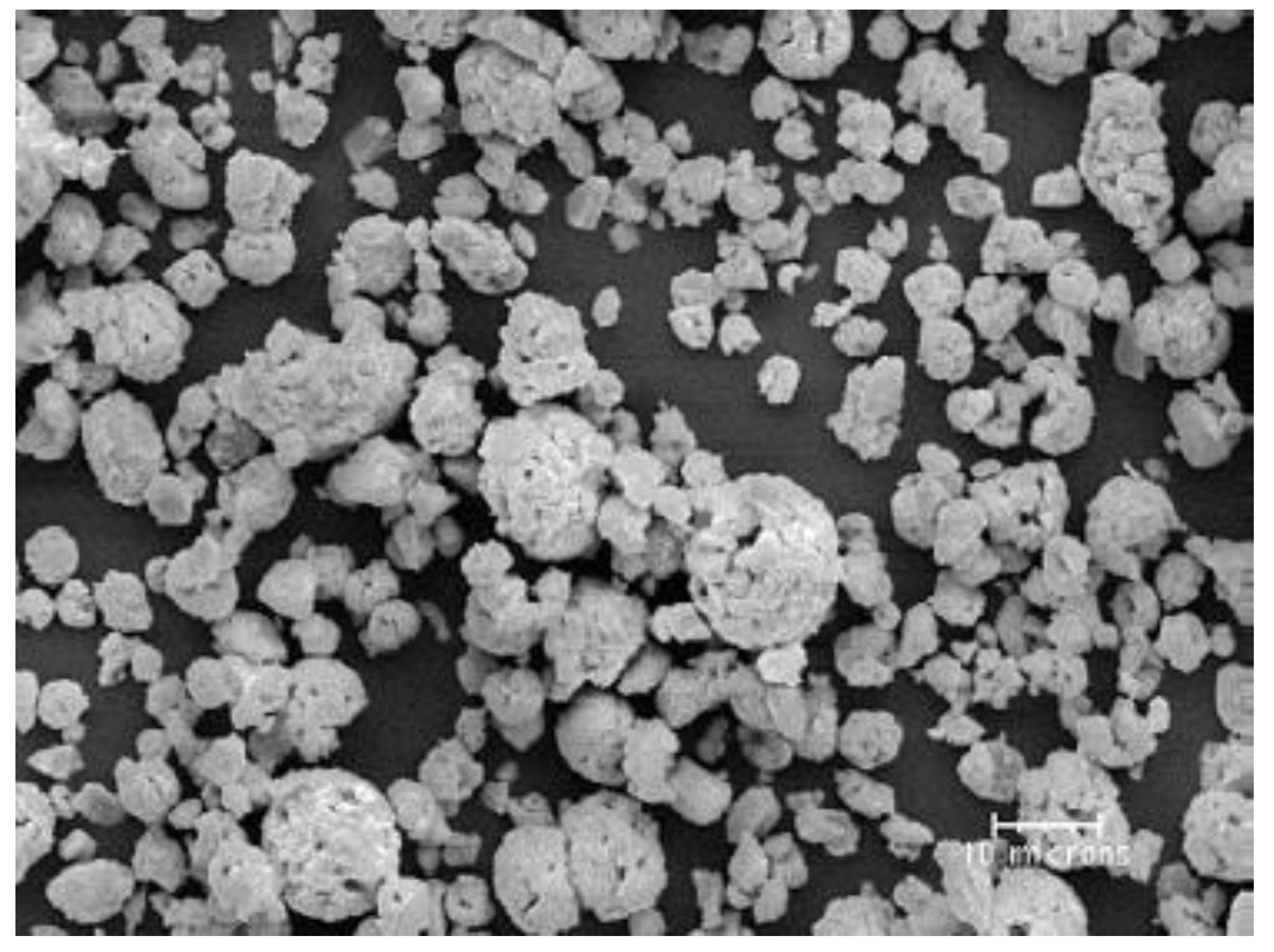
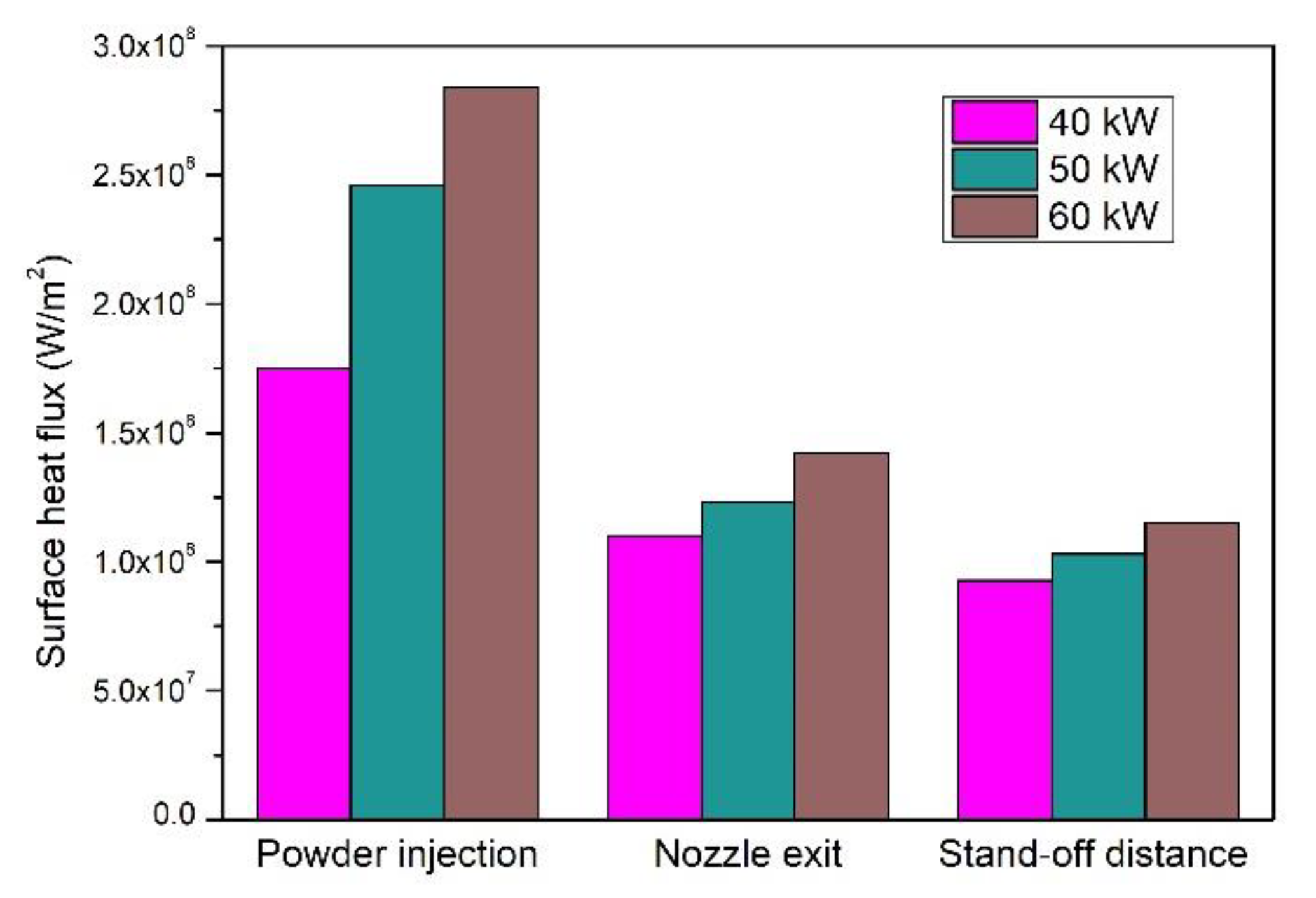
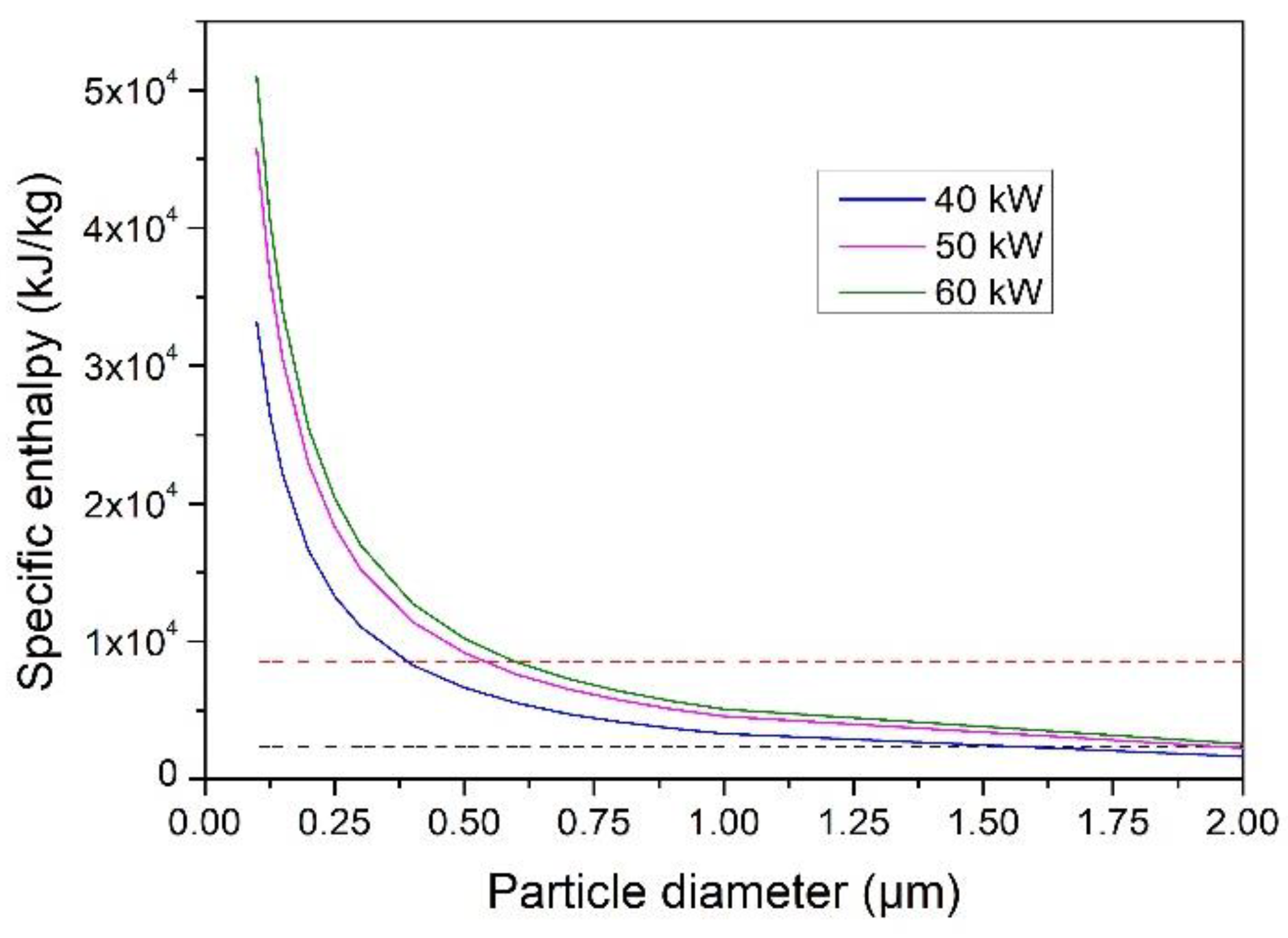
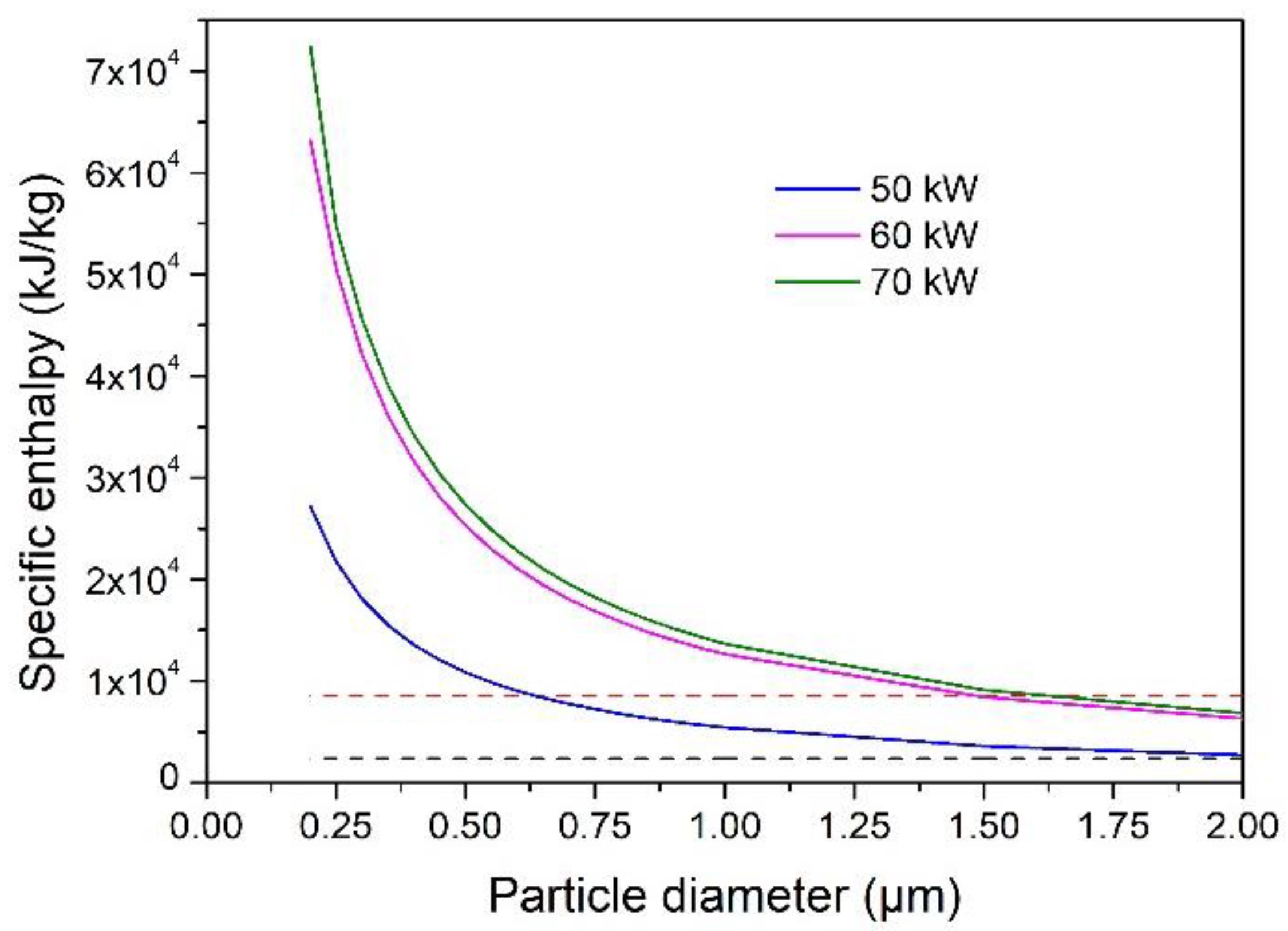
4.3. Evaporation Capacity of YSZ Particle Processed in an Ar–H2 Plasma Jet
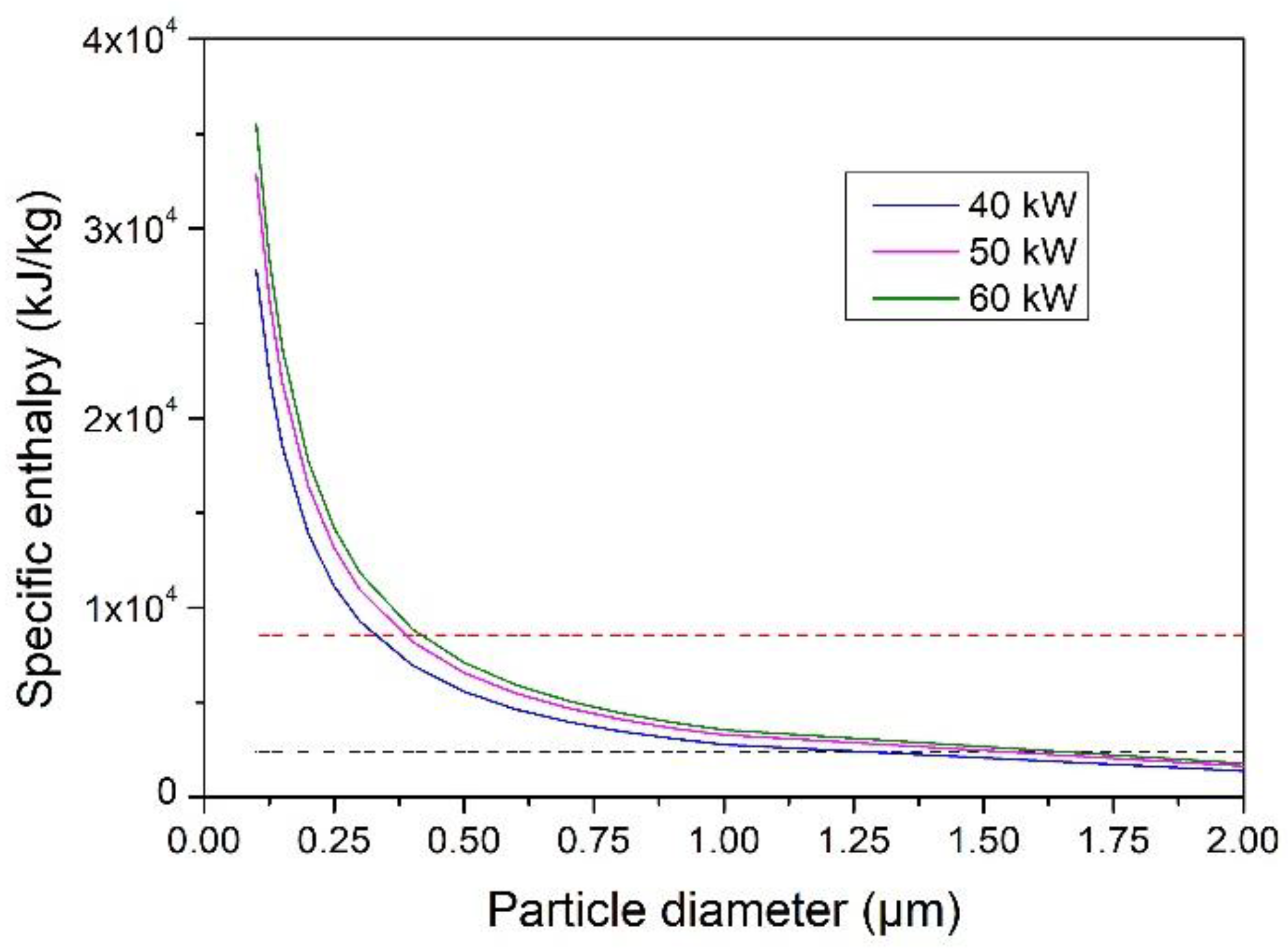
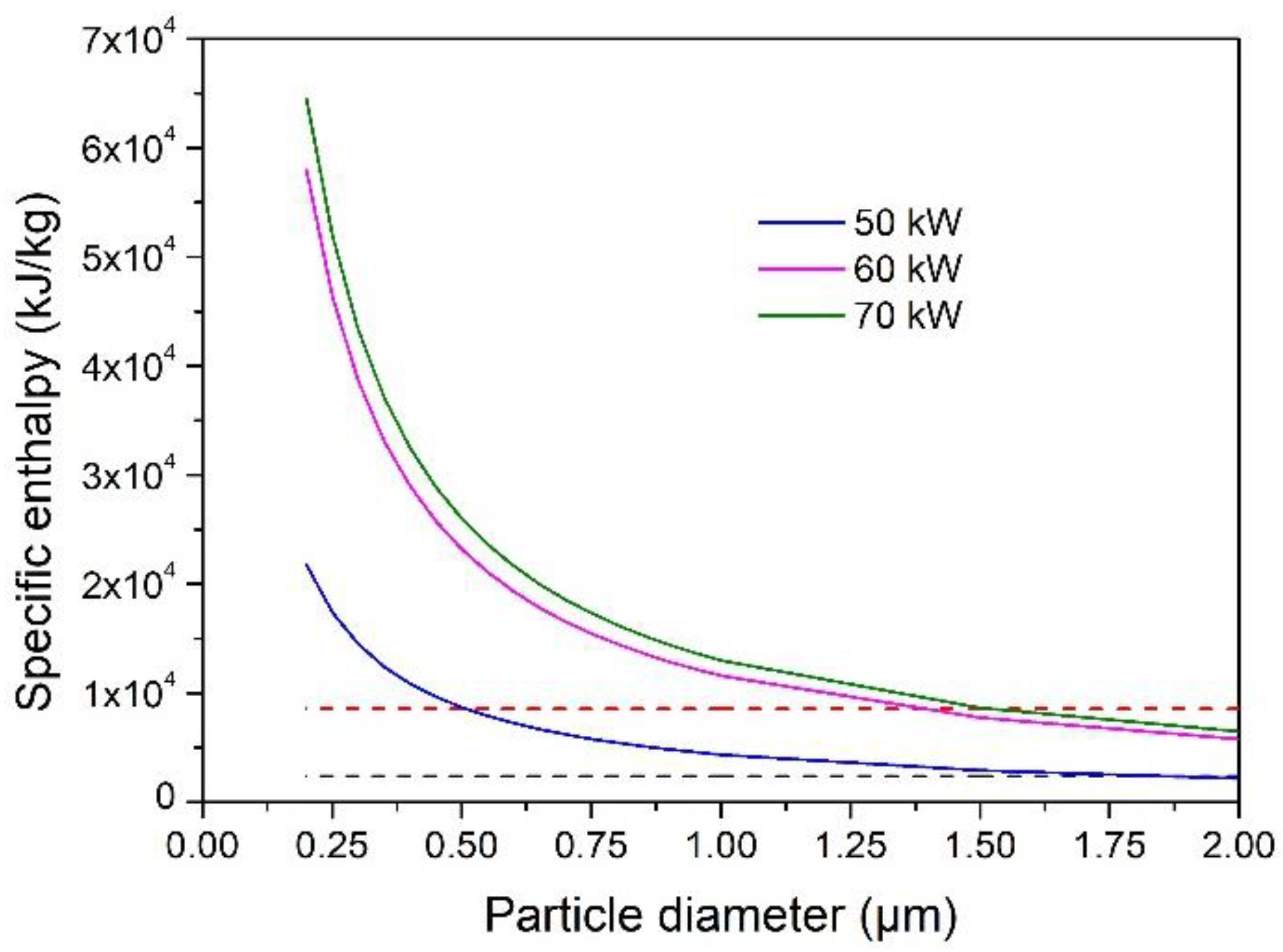
4.4. Evaporation Capacity of YSZ Particles Processed in an Ar–He Plasma Jet
5. Conclusions
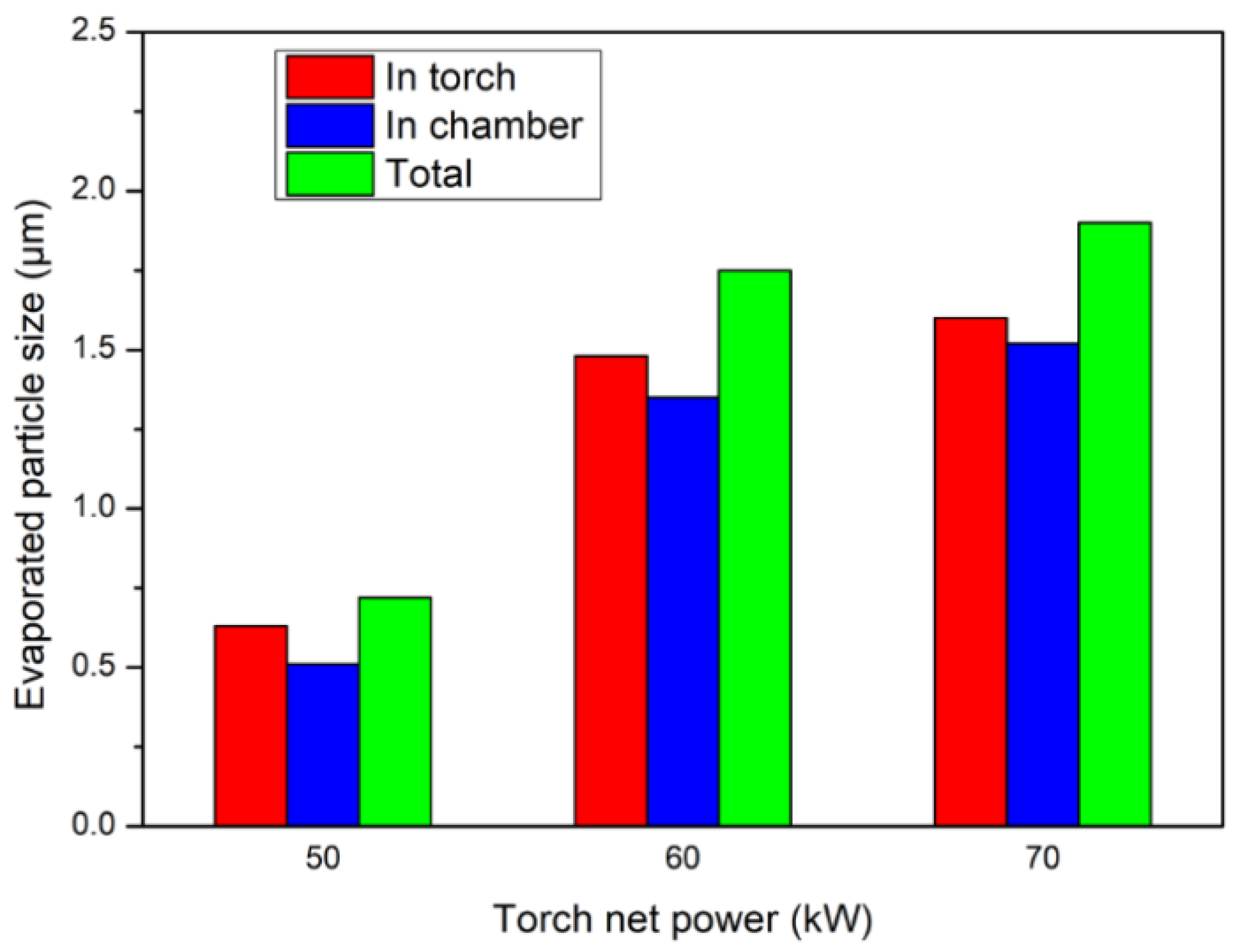
- The size of the particles fully evaporated inside the torch and chamber deposition was comparable under the Ar–H2 and Ar–He plasma conditions of this study. The particle residence time in the deposition chamber was much longer than that inside the torch nozzle due to a longer flying distance. The longer dwelling time in the chamber was favorable to the heat transfer from plasma to particles in spite of the Knudsen effect at the pressure of 100 Pa that prevailed in the deposition chamber.
- For a torch net power of 60 kW, the fully vaporized particles were significantly larger in the Ar–He plasma than that in the Ar–H2 plasma under the operating conditions of this study. This was explained by a twice-longer residence time of the particles in the deposition chamber and different characteristics of the plasma jets.
- An increase in the torch net power brought about an increase in the size of the particles fully vaporized in the Ar–H2 plasma jet. The maximum size was, however, less than 1 µm when the torch power was less than 60 kW.
- With Ar–He plasma jet, the size of the completely vaporized particles increased significantly when the torch power was increased from 50 to 60 kW, but only slightly when the torch power was increased up to 70 kW. The maximum evaporable was less than 2 µm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, M.F.; Hall, A.C.; Fleetwood, J.D.; Meyer, P. Very low pressure plasma spray—A review of an emerging technology in the thermal spray community. Coatings 2011, 1, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Gindrat, M.; Hohle, H.M.; von Niessen, K.; Guittienne, P.; Grange, D.; Hollenstein, C. Plasma spray-CVD: A New Thermal spray process to produce thin films from liquid or gaseous precursors. J. Therm. Spray Technol. 2011, 20, 882–887. [Google Scholar] [CrossRef]
- Salhi, Z.; Klein, D.; Gougeon, P.; Coddet, C. Development of coating by thermal plasma spraying under very low-pressure condition <1 mbar. Vacuum 2005, 77, 145–150. [Google Scholar]
- Gao, Y.; Yang, D.M.; Gao, J.Y. Characteristics of a plasma torch designed for very low pressure plasma spraying. J. Therm. Spray Technol. 2012, 21, 740–744. [Google Scholar] [CrossRef]
- von Niessen, K.; Gindrat, M.; Refke, A. Vapor phase deposition using plasma spray-PVD (TM). J. Therm. Spray Technol. 2010, 19, 502–509. [Google Scholar] [CrossRef]
- Vautherin, B.; Planche, M.P.; Bolot, R.; Quet, A.; Bianchi, L.; Montavon, G. Vapors and droplets mixture deposition of metallic coatings by very low pressure plasma spraying. J. Therm. Spray Technol. 2014, 23, 596–608. [Google Scholar] [CrossRef]
- Harder, B.J. Oxidation performance of Si–HfO2 environmental barrier coating bond coats deposited via plasma spray-physical vapor deposition. Surf. Coat. Technol. 2020, 384, 125311. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, Q.; Wei, L.L.; He, W.T.; Guo, H.B. Microstructures and phases of ytterbium silicate coatings prepared by plasma spray-physical vapor deposition. Materials 2020, 13, 1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goral, M.; Kotowski, S.; Nowotnik, A.; Pytel, M.; Drajewicz, M.; Sieniawski, J. PS-PVD deposition of thermal barrier coatings. Surf. Coat. Technol. 2013, 237, 51–55. [Google Scholar] [CrossRef]
- Schmitt, M.P.; Harder, B.J.; Wolfe, D.E. Process-structure-property relations for the erosion durability of plasma spray-physical vapor deposition (PS-PVD) thermal barrier coatings. Surf. Coat. Technol. 2016, 297, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Mauer, G. Plasma Characteristics and plasma-feedstock interaction under PS-PVD process conditions. Plasma Chem. Plasma P 2014, 34, 1171–1186. [Google Scholar] [CrossRef] [Green Version]
- Mauer, G.; Hospach, A.; Zotov, N.; Vassen, R. Process Conditions and Microstructures of Ceramic Coatings by Gas Phase Deposition Based on Plasma Spraying. J. Therm. Spray Technol. 2013, 22, 83–89. [Google Scholar] [CrossRef]
- Song, C.; Xie, S.-m.; Fan, X.-J.; He, P.-J.; Liu, M.; Zhou, K.-S.; Deng, C.-M.; Liao, H.-L. Very low-pressure plasma-sprayed dense yttria-stabilized zirconia coatings using an axial bi-cathode plasma torch. Surf. Coat. Technol. 2020, 402, 126281. [Google Scholar] [CrossRef]
- Mauer, G.; Vassen, R.; Stover, D. Thin and dense ceramic coatings by plasma spraying at very low pressure. J. Therm. Spray Technol. 2010, 19, 495–501. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Li, C.-X.; Zhao, J.-Z.; Yang, G.-J.; Li, C.-J. Microstructure of YSZ coatings deposited by PS-PVD using 45 kW shrouded plasma torch. Mater. Manuf. Process. 2015, 31, 1183–1191. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Peng, X.-Z.; Yang, G.-J.; Li, C.-X.; Li, C.-J. Characterization of plasma jet in plasma spray-physical vapor deposition of YSZ using a <80 kW shrouded torch based on optical emission spectroscopy. J. Therm. Spray Technol. 2015, 24, 1038–1045. [Google Scholar] [CrossRef]
- Mauer, G.; Jarligo, M.O.; Rezanka, S.; Hospach, A.; Vassen, R. Novel opportunities for thermal spray by PS-PVD. Surf. Coat. Technol. 2015, 268, 52–57. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Wang, Y.D. Heat-Transfer from a rarefied plasma-flow to a metallic particle with high surface-temperature. J. Phys. D Appl. Phys. 1994, 27, 1637–1645. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.M. Drag on a metallic or nonmetallic particle exposed to a rarefied plasma-flow. Plasma Chem. Plasma P 1989, 9, 387–408. [Google Scholar] [CrossRef]
- Chen, X.; Chyou, Y.P.; Lee, Y.C.; Pfender, E. Heat-transfer to a particle under plasma conditions with vapor contamination from the particle. Plasma Chem. Plasma Process. 1985, 5, 119–141. [Google Scholar] [CrossRef]
- Chen, X.; Pfender, E. Effect of pressure on heat-transfer to a particle exposed to a thermal plasma. J. Eng. Gas Turbines Power 1985, 107, 147–151. [Google Scholar] [CrossRef]
- Chen, X. The drag force on a spherical particle in a rarefied plasma flow with combined specular and diffuse reflection at the particle surface. J. Phys. D Appl. Phys. 1997, 30, 2561–2565. [Google Scholar] [CrossRef]
- Xi, C.; Ping, H. Heat-transfer from a rarefied plasma-flow to a metallic or nonmetallic particle. Plasma Chem. Plasma Process. 1986, 6, 313–333. [Google Scholar]
- Anwaar, A.; Wei, L.; Guo, H.; Zhang, B. Plasma–powder feedstock interaction during plasma spray–physical vapor deposition. J. Therm. Spray Technol. 2017, 26, 292–301. [Google Scholar] [CrossRef]
- Liu, M.-J.; Zhang, M.; Zhang, Q.; Yang, G.-J.; Li, C.-X.; Li, C.-J. Evaporation of droplets in plasma spray–physical vapor deposition based on energy compensation between self-cooling and plasma heat transfer. J. Therm. Spray Technol. 2017, 26, 1641–1650. [Google Scholar] [CrossRef]
- Ivchenko, D.; Zhang, T.; Mariaux, G.; Vardelle, A.; Goutier, S.; Itina, T.E. On the Validity of continuum computational fluid dynamics approach under very low-pressure plasma spray conditions. J. Therm. Spray Technol. 2018, 27, 3–13. [Google Scholar] [CrossRef]
- Zhang, T.; Mariaux, G.; Vardelle, A.; Li, C.-J. numerical simulation of plasma jet characteristics under very low-pressure plasma spray conditions. Coatings 2021, 11, 726. [Google Scholar] [CrossRef]
- Mauer, G. Numerical Study on Particle-Gas Interaction close to the substrates in thermal spray processes with high-kinetic and low-pressure conditions. J. Therm. Spray Technol. 2019, 28, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Vautherin, B.; Planche, M.P.; Montavon, G.; Lapostolle, F.; Quet, A.; Bianchi, L. Study of metallic powder behavior in very low pressure plasma spraying (VLPPS)—Application to the manufacturing of titanium-aluminum coatings. Surf. Coat. Technol. 2015, 275, 341–348. [Google Scholar] [CrossRef]
- Ivchenko, D. Modeling and Design of a Physical Vapor Deposition Process Assisted by Thermal Plasma (PS-PVD). Ph.D. Thesis, Université de Limoges, Limoges, France, 2018. [Google Scholar]
- Mariaux, G.; Vardelle, A. 3-D time-dependent modelling of the plasma spray process. Part 1: Flow modelling. Int. J. Sci. 2005, 44, 357–366. [Google Scholar] [CrossRef]
- Ivchenko, D.; Mariaux, G.; Vardelle, A.; Goutier, S.; Itina, T.E.; Andre, P. Feasibility study of an adaptive-pressure plasma coating process-Part 1: Model description. J. Therm. Spray Technol. 2020, 29, 25–37. [Google Scholar] [CrossRef]
- Lu, T. Micro and Nanoscale Characterization of Three Dimensional Surfaces: Basics and Applications; Napoca Star Publishing House: Cluj-Napoca, Romania, 2015. [Google Scholar]
- Dykhuizen, R.C.; Smith, M.F. Gas dynamic principles of cold spray. J. Therm. Spray Technol. 1998, 7, 205–212. [Google Scholar] [CrossRef]
- Dolmaire, A.; Hartikainen, E.; Goutier, S.; Béchade, E.; Vardelle, M.; Geffroy, P.-M.; Joulia, A. Benefits of hydrogen in a segmented-anode plasma torch in suspension plasma spraying. J. Therm. Spray Technol. 2021, 30, 236–250. [Google Scholar] [CrossRef]
- Anwaar, A.; Wei, L.; Guo, Q.; Zhang, B.; Guo, H. Novel Prospects for plasma spray–physical vapor deposition of columnar thermal barrier coatings. J. Therm. Spray Technol. 2017, 26, 1810–1822. [Google Scholar] [CrossRef]
- Vardelle, A.; Moreau, C.; Themelis, N.J.; Chazelas, C. A Perspective on plasma spray technology. Plasma Chem. Plasma Process. 2015, 35, 491–509. [Google Scholar] [CrossRef]



| Parameters | F4 | O3CP |
|---|---|---|
| Net power, kW | 40–50–60 | 50–60–70 |
| Plasma gas, SLPM | 40 Ar/8 H2 | 60 Ar/30 He |
| Nozzle diameter, mm | 6 | 12.5 |
| Chamber pressure, Pa | 100 | 100 |
| Spray distance, m | ~0.45 | ~1 |
| Mass flow rate, kg/s | 0.0012 | 0.00187 |
| Specific enthalpy, MJ/kg | 33.3–41.7–50 | 26.8–32.1–37.4 |
| Plasma Torch Type | Net Power (kW) | Residence Time of Particles (µs) | |
|---|---|---|---|
| In Torch | In Chamber | ||
| F4 | 40 | 40 | 160 |
| 50 | 37 | 155 | |
| 60 | 33 | 149 | |
| O3CP | 50 | 38 | 256 |
| 60 | 35 | 232 | |
| 70 | 30 | 214 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Mariaux, G.; Vardelle, A.; Li, C.-J. Numerical Analysis of the Interactions between Plasma Jet and Powder Particles in PS-PVD Conditions. Coatings 2021, 11, 1154. https://doi.org/10.3390/coatings11101154
Zhang T, Mariaux G, Vardelle A, Li C-J. Numerical Analysis of the Interactions between Plasma Jet and Powder Particles in PS-PVD Conditions. Coatings. 2021; 11(10):1154. https://doi.org/10.3390/coatings11101154
Chicago/Turabian StyleZhang, Tao, Gilles Mariaux, Armelle Vardelle, and Chang-Jiu Li. 2021. "Numerical Analysis of the Interactions between Plasma Jet and Powder Particles in PS-PVD Conditions" Coatings 11, no. 10: 1154. https://doi.org/10.3390/coatings11101154






