Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review
Abstract
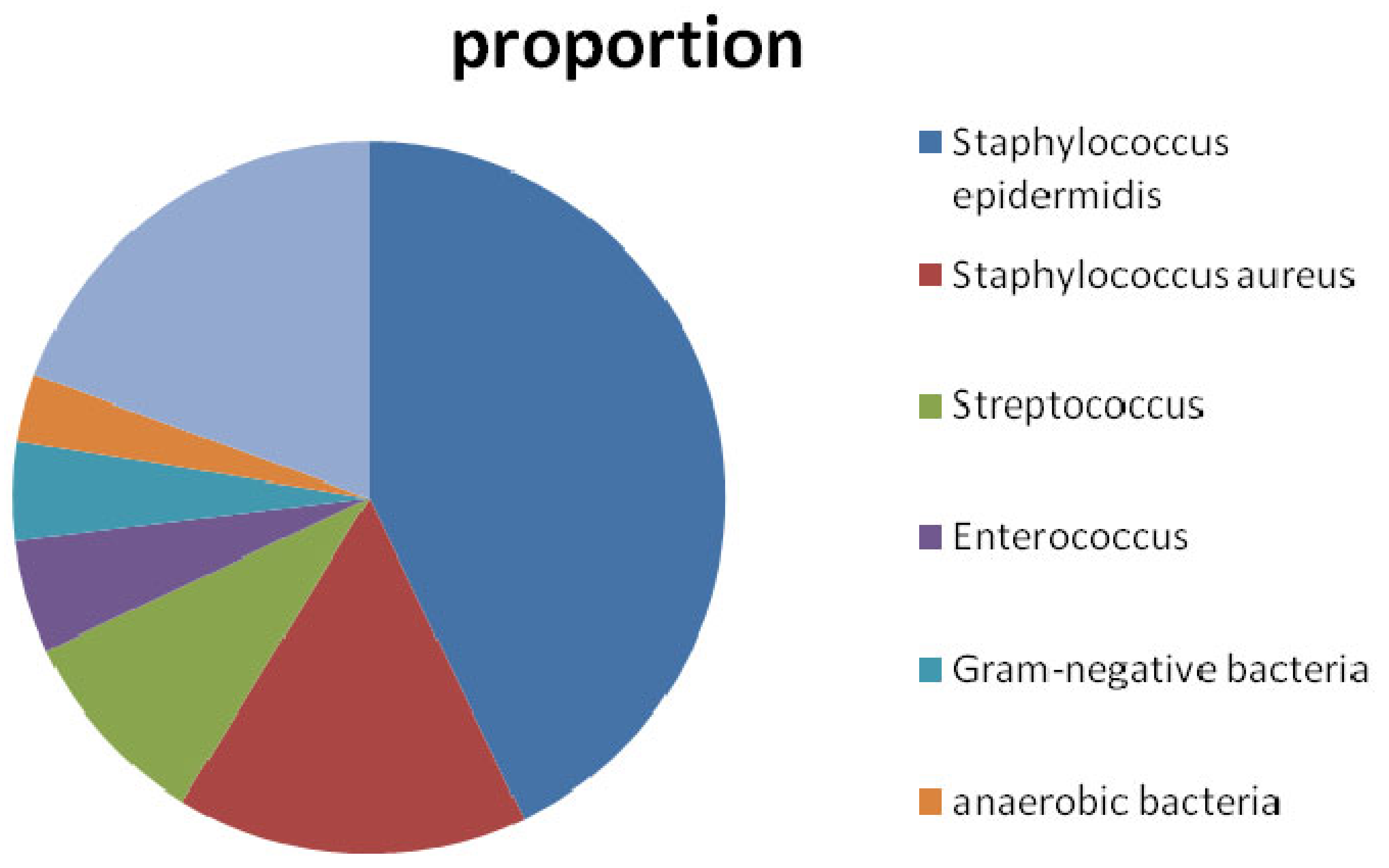
:1. Introduction
2. Coating Materials with Antibacterial Activity
2.1. Bactericidal Coating Materials
2.1.1. Chitosan
2.1.2. Quaternary Ammonium Compounds
2.1.3. Antibiotics
Gentamicin
Vancomycin
Rifampicin
2.1.4. Metals
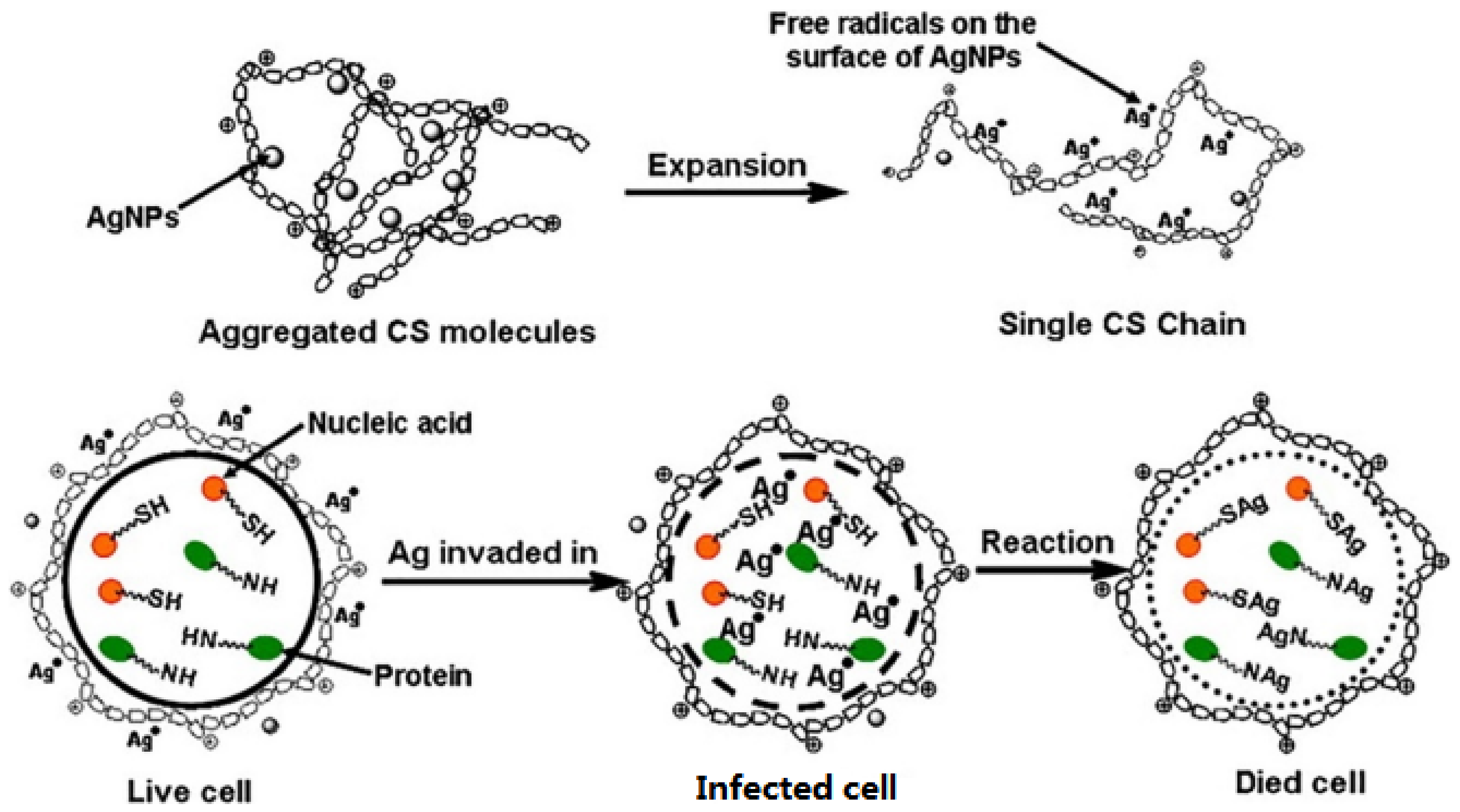
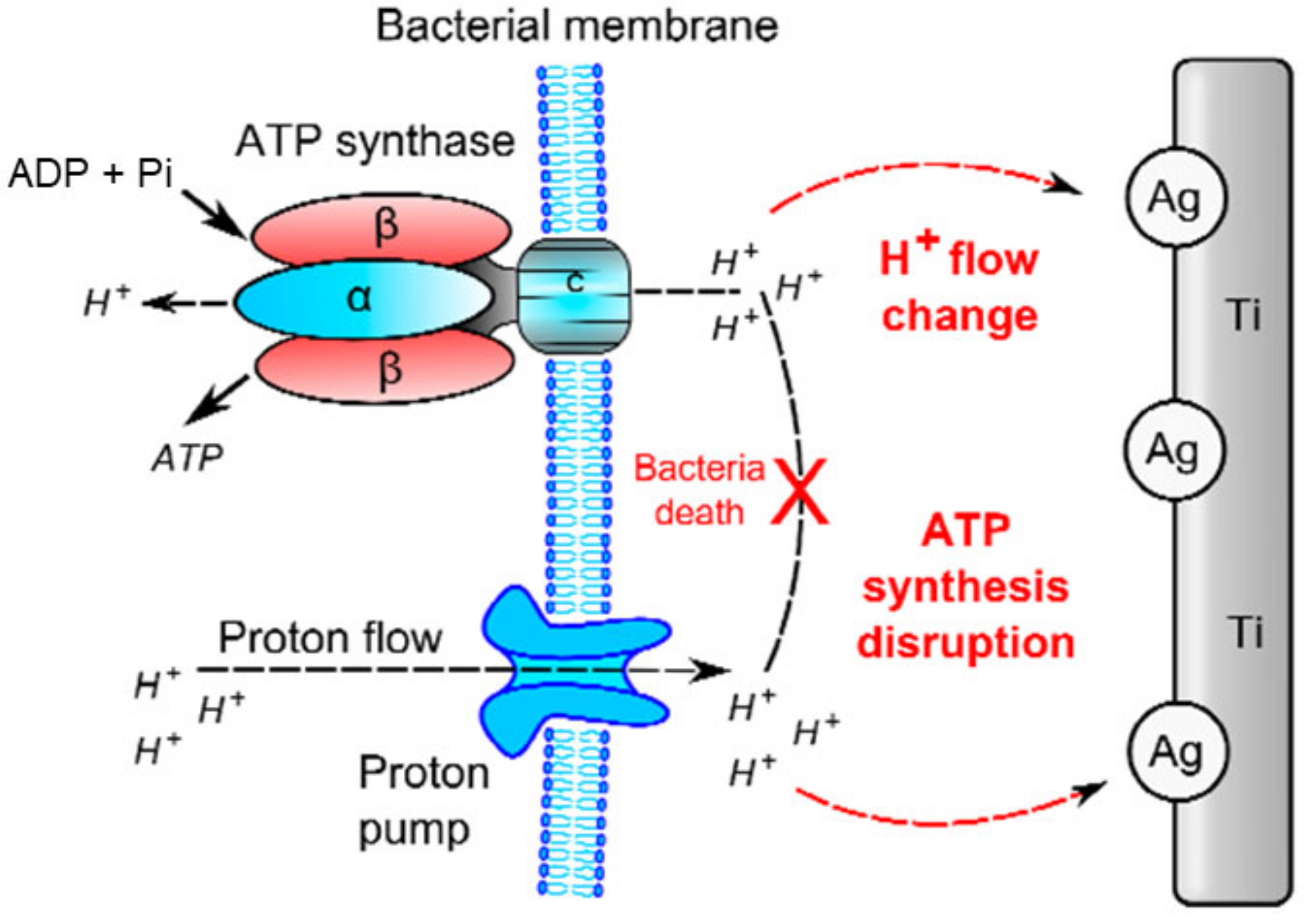
Ag and AgNPs
Gold Nanoparticles
Copper and Copper Nanoparticles
Selenium Nanoparticles
Zinc Nanoparticles
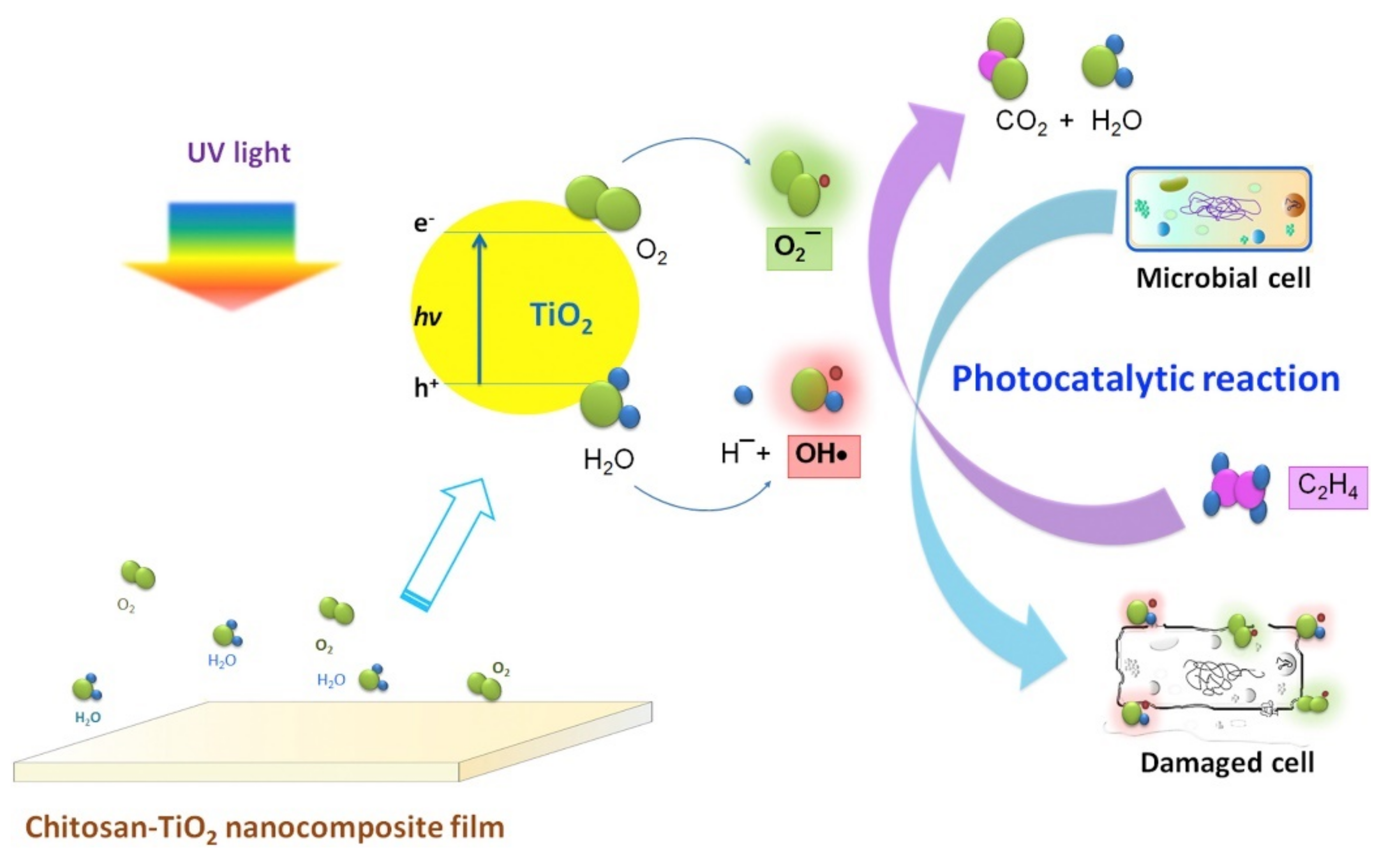
Ti and Ti-Oxide
2.2. Bacteriostatic Surface Coating Materials
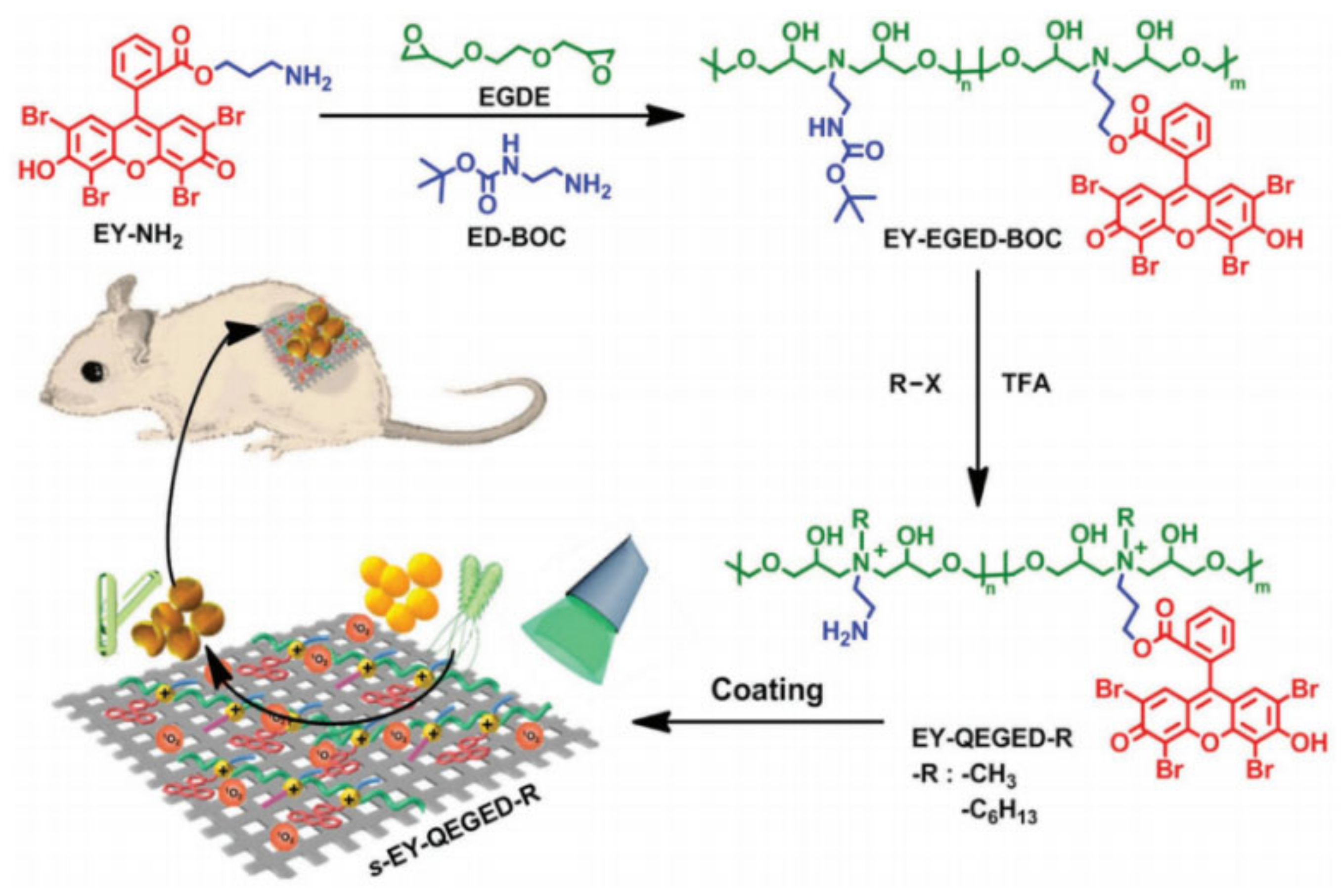
2.3. Antimicrobial Peptides with Bactericidal and Bacteriostatic Properties
3. Coating Materials with Anti-Inflammatory Activity
3.1. Anti-Inflammatory Materials Regulate Macrophage Polarization
3.2. Other Anti-Inflammatory Materials
4. Conclusions and Future Horizons
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | silver nanoparticles |
| Ag | silver |
| Ag-CuNPs | Ag/Cu nanoparticles |
| ALP | alkaline phosphatase |
| AMR | antimicrobial resistance |
| AMPs | antimicrobial peptides |
| ARG | arginine |
| Au | gold |
| AuNPs | gold nanoparticles |
| BMSC | bone marrow stem cells |
| CaO3 | calcium carbonate |
| CMS | chymotrypsin |
| CeO2 | cerium dioxide |
| CM | Coumarin |
| COX-2 | cyclooxygenase 2 |
| CS | Chitosan |
| CS-SeNPs | CS–se nanoparticles |
| CSZP | calcium–strontium–zinc–phosphate coating |
| CuAA | copper-catalyzed azide alkyne |
| Cu | copper |
| CuO | copper oxide |
| CuONPs | CuO nanoparticles |
| Cur | Curcumin |
| CurNPs | curcumin nanoparticles |
| DAIs | device-associated infections |
| DNA | deoxyribonucleic acid |
| DEX | dexamethasone |
| ELISA | enzyme-linked immunosorbent assay |
| EPS | extracellular polymeric substance |
| EY | eosin Y |
| GO | graphene oxide |
| H+ | positive hole |
| HA | hyaluronic acid |
| HAp | hydroxyapatite |
| IFN-γ | interferon-γ |
| IL-1β | interleukin-1β |
| IL-4 | interleukin 4 |
| IL-6 | interleukin-6 |
| IL-10 | interleukin 10 |
| INOS | inducible nitric oxide synthase |
| LPS | lipopolysaccharide |
| MAO | micro arc oxidation |
| mAbs | monoclonal antibodies |
| MIC | minimum inhibitory concentrations |
| MMP-3 | matrix metalloproteinase 3 |
| MMT | montmorillonite |
| mRNA | messenger ribonucleic acid |
| MRSA | Methicillin-resistant S.aureus |
| MRSE | methicillin-resistant S. epidermidis |
| MSSA | methicillin-susceptible S. aureus |
| N-CQDs | N-doped graphene quantum dots |
| NF-kß | nuclear factor kβ |
| n-HA/RES/CS | nano-hydroxyapatite/resveratrol/CS composite microspheres |
| NO | nitric oxide |
| NSAIDs | nonsteroidal anti-inflammatory and analgesic drugs |
| OH- | hydroxide ions |
| OH | hydroxyl radical |
| O2- | superoxide ions |
| OPG | osteoprotegerin |
| OPGL | osteoprotegerin ligand |
| ORIF | open reduction and internal fixation |
| PCL | poly (caprolactone) |
| PDA | polydopamine |
| PDT | photodynamic therapy |
| PEEKP | polyether ether ketone |
| PEG | poly(ethylene glycol) |
| PEO | poly(ethylene oxide) |
| PGE | prostaglandin E |
| PIII | plasma immersion ion implantation |
| PJI | periprosthetic Joint Infection |
| PLA | poly (lactic acid) |
| PLGA | poly-dl-lactic-co-glycolic |
| PLL | poly L-lysine |
| PLLA | poly (L-lactide) |
| PMM | poly(methyl methacrylate) |
| PMPC | poly(2-methacryloyloxyethyl phosphorylcholine) |
| PSBMA | polysulfobetaine methacrylate |
| PSL | phosphatidylserine |
| PTFEMA-r-SBMA | poly(trifluoroethyl methacrylate-random-sulfobetaine methacrylate) |
| QACs | quaternary ammonium compounds |
| QD | quantum dot |
| RCT | randomized controlled trial |
| RES | resveratrol |
| ROS | reactive oxygen species |
| RT-PCR | reverse-transcription polymerase chain reaction |
| SAM | self-assembled monolayer |
| S. aureus | Staphylococcus aureus |
| SB2VP | sulfobetaine-2-vinylpyridine |
| Se | selenium |
| SeNPs | Se nanoparticles |
| S. epidermidis | Staphylococcus epidermidis |
| SLA | sandblasted and acid-etched |
| TA | tannic acid |
| TA-CaO3NPs | TA-CaO3 nanomaterials |
| TGF-β3 | transforming growth factorβ3 |
| THA | total hip arthroplasty |
| Ti | titanium |
| TiO2 | titanium dioxide |
| TiO2-CuNPs | TiO2-Cu nanoparticles |
| TJA | total joint arthroplasty |
| TKA | total knee arthroplasty |
| TNF-α | tumor necrosis factor-α |
| TRP | tryptophan |
| UV | ultraviolet |
| VCM/nHAC/PLA | nano-HAp/collagen/poly(lactic acid) |
| VRSA | Vancomycin resistant S.aureus |
| ZnO | zinc oxide |
| Zn | zinc |
| ZnONPs | zinc oxide nanoparticles |
| Zn2+ | zinc ions |
| 4VP | 4-vinylpyridine |
| 4VPPS | 4-vinylpyridine propylsulfobetaine |
References
- Sharkey, P.F.; Lichstein, P.M.; Shen, C.; Tokarski, A.T.; Parvizi, J. Why are total knee arthroplasties failing today-has anything changed after 10 years? J. Arthroplast. 2014, 29, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Kenney, C.; Dick, S.; Lea, J.; Liu, J.; Ebraheim, N.A. A systematic review of the causes of failure of revision total hip arthroplasty. J. Orthop. 2019, 16, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Coraa-Huber, D.C.; Kreidl, L.; Steixner, S.; Hinz, M.; Fille, M. Identification and morphological characterization of biofilms formed by strains causing infection in orthopedic implants. Pathogens 2020, 9, 649. [Google Scholar] [CrossRef]
- Mcconoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Byun, H.; Perikamana, S.K.M.; Lee, S.; Shin, H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthc. Mater. 2019, 8, e1801106. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hallstoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Najafi-Hajivar, S.; Zakeri-Milani, P.; Mohammadi, H.; Niazi, M.; Soleymani-Goloujeh, M.; Baradaran, M.; Valizadeh, H. Overview on experimental models of interactions between nanoparticles and the immune system. Biomed. Pharmacother. 2016, 83, 1365–1378. [Google Scholar] [CrossRef]
- Koreny, T.; Tunyogi-Csapó, T.; Gál, I.; Vermes, C.; Glant, T.T. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheumatol. 2006, 54, 3221–3232. [Google Scholar] [CrossRef]
- Bauer, T.W. Particles and periimplant bone resorption. Clin. Orthop. Relat. Res. 2002, 405, 138–143. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Abidi, S.H.; Sherwani, S.K.; Siddiqui, T.R.; Bashir, A.; Kazmi, S.U. Drug resistance profile and biofilm forming potential of pseudomonas aeruginosa isolated from contact lenses in Karachi-Pakistan. BMC Ophthalmol. 2013, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afroz, M.M.; Kashem, M.N.H.; Piash, K.M.P.S.; Islam, N. Saccharomyces cerevisiae as an untapped source of fungal chitosan for antimicrobial action. Appl. Biochem. Biotechnol. 2021, 193, 3765–3786. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, R.; Lin, C.C.; Liu, Z.W.; Tang, T.T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.E.V.; Tovar, C.D.G.; Hernandez, J.H.M. The role of chitosan and graphene oxide in bioactive and antibacterial properties of acrylic bone cement. Biomolecules 2020, 10, 1616. [Google Scholar] [CrossRef]
- Chen, M.C.; Yeh, H.C.; Chiang, B.H. Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J. Food Process. Pres. 2010, 20, 379–390. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R.L.; Lee, C.P.; Hyun, J. Antimicrobial activity of native chitosan, degraded chitosan, and O-carboxymethylated chitosan. J. Food Prot. 2003, 66, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Iovinekobata, S.; Moreirateixeira, L.E.; Antunesfernandes, S.O.; Augusto, A. Prevention of bone infection after open fracture using a chitosan with ciprofloxacin implant in animal model. Acta Cir. Bras. 2020, 35, e202000803. [Google Scholar] [CrossRef]
- Shi, S.; Jia, J.; Guo, X.K.; Zhao, Y.; Zhang, X. Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 6499–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.H.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel asymmetric wettable AgNPs/Chitosan wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.B.; Chen, Z.; Yang, X.P.; Cen, L.; Zhang, X.; Gao, P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014, 42, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Jing, A.; Ji, Z.; Wang, D.; Luo, Q.; Li, X. Preparation and characterization of uniform-sized chitosan/silver microspheres with antibacterial activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 33–41. [Google Scholar] [CrossRef]
- Vaca-Cornejo, F.; Reyes, H.M.; Jiménez, D.; Velázquez, R.A.L.; Dueas Jiménez, J.M. Pilot study using a chitosan-hydroxyapatite implant for guided alveolar bone growth in patients with chronic periodontitis. J. Funct. Biomater. 2017, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Xiang, Y.; Yu, J.; Wang, Y.; Cui, W. Fabrication of antibacterial and antiwear hydroxyapatite coatings via In situ chitosan-mediated pulse electrochemical deposition. ACS Appl. Mater. Interfaces 2017, 9, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Z.; Zhang, Y.; Liu, X.; Xiang, Y.; Li, Z.; Wu, S. Synergistic antibacterial activity of multi components in lysozyme/chitosan/silver/hydroxyapatite hybrid coating. Mater. Des. 2018, 139, 351–362. [Google Scholar] [CrossRef]
- Hsu, L.H.; Kwaśniewska, D.; Wang, S.C.; Shen, T.L.; Chen, Y.L. Gemini quaternary ammonium compound PMT12-BF4 inhibits candida albicans via regulating iron homeostasis. Sci. Rep. 2020, 10, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Efstathia, K.; Maria, T.; Gavriil, V.; Georgia, L.; Nikos, K.; Denisa, D.; Georgios, B.; Apostolos, V.; Joannis, K. Evaluation of antimicrobial efficiency of new polymers comprised by covalently attached and/or electrostatically bound bacteriostatic species, based on quaternary ammonium compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef]
- Asri, L.A.T.W.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; Mei, H.C.V.D.; Loontjens, T.J.A.; Busscher, H.J. A shape-adaptive, antibacterial-coating of immobilized quaternary ammonium compounds tethered on hyperbranched polyurea and its mechanism of action. Adv. Funct. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, C.; Zhang, N.; Ding, X.; Yu, B.; Xu, F.J. Polycationic synergistic antibacterial agents with multiple functional components for efficient anti-infective therapy. Adv. Funct. Mater. 2018, 28, 1706–1709. [Google Scholar] [CrossRef]
- Liu, B.; Monro, S.; Jabed, M.A.; Cameron, C.G.; Colón, K.L.; Xu, W.; Kilina, S.; Mcfarland, S.A.; Sun, W. Neutral iridium(iii) complexes bearing BODIPY-substituted N-heterocyclic carbene (NHC) ligands: Synthesis, photophysics, in vitro theranostic photodynamic therapy, and antimicrobial activity. Photochem. Photobiol. Sci. 2019, 18, 2381–2396. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Wei, M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef]
- Silva, D.B.D.; Silva, C.L.D.; Davanzo, N.N.; Souza, R.D.S.; Pierre, M.B.R. Protoporphyrin IX (PpIX) loaded PLGA nanoparticles for topical photodynamic therapy on melanoma cells. Photodiagn. Photodyn. Ther. 2021, 35, 102317. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Uyama, H.; Kwon, O.K.; Kim, Y.J. Nitric oxide and reactive oxygen species-releasing polylactic acid monolith for enhanced photothermal therapy of osteosarcoma. J. Ind. Eng. Chem. 2021, 94, 498–506. [Google Scholar] [CrossRef]
- Briggs, T.; Blunn, G.; Hislop, S.; Ramalhete, R.; Bagley, C.; McKenna, D.; Coathup, M. Antimicrobial photodynamic therapy-a promising treatment for prosthetic joint infections. Lasers Med. Sci. 2018, 33, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inage, K.; Sakuma, Y.; Yamauchi, K.; Suganami, A.; Orita, S.; Kubota, G.; Oikawa, Y.; Sainoh, T.; Sato, J.; Fujimoto, K.; et al. Effect of photodynamic therapy on local muscle treatment in a rat muscle injury model: A controlled trial. J. Orthop. Surg. Res. 2015, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Meo, D.D.; Cannari, F.M.; Petriello, L.; Persiani, P.; Villani, C. Gentamicin-coated tibia nail in fractures and nonunion to reduce fracture-related infections: A systematic review. Molecules 2020, 25, 5471. [Google Scholar] [CrossRef] [PubMed]
- Bidossi, A.; Bottagisio, M.; Logoluso, N.; Vecchi, E.D. In vitro evaluation of gentamicin or vancomycin containing bone graft substitute in the prevention of orthopedic implant-related infections. Int. J. Mol. Sci. 2020, 21, 9250. [Google Scholar] [CrossRef] [PubMed]
- Butini, M.E.; Cabric, S.; Trampuz, A.J.; Luca, M.D. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf. B 2018, 161, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Akid, R. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated titanium. Bone Jt. J. 2021, 103-B(3), 522–529. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Kerstan, M.; Schwabe, P.; Südkamp, N.; Raschke, M. Clinical experiences in the use of a gentamicin-coated titanium nail in tibia fractures and revisions. Injury 2017, 48, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.; Bertolini, M.; Costa, R.C.; Nagay, B.E.; Baro, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2020, 24, 102008. [Google Scholar] [CrossRef]
- Freischmidt, H.; Armbruster, J.; Reiter, G.; Grützner, P.A.; Gühring, T. Individualized techniques of implant coating with an antibiotic-Loaded, hydroxyapatite/calcium sulphate bone graft substitute. Ther. Clin. Risk Manag. 2020, 16, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.L.; Moriarty, T.F.; Boot, W.; Richards, R.G.; Jaiprakash, A. Single tage revision of MRSA orthopedic device-related infection in sheep with an antibiotic-loaded hydrogel. J. Orthop. Res. 2019, 15, 65–70. [Google Scholar] [CrossRef]
- Lian, X.J.; Mao, K.Z.; Liu, X.; Wang, X.M.; Cui, F.Z. In vivo osteogenesis of vancomycin loaded nano- hydroxyapatite/collagen/calcium sulfate composite for treating infectious bone defect induced by chronic osteomyelitis. J. Nanomater. 2015, 5, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Liu, H.; Wang, X.; Xu, S.; Cui, F.; Bai, X. Antibacterial and biocompatible properties of vancomycin- loaded nano-hydroxyapatite/collagen/poly (lactic acid) bone substitute. Prog. Nat. Sci. 2013, 23, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Suchý, T.; Vištejnová, L.; Šupová, M.; Klein, P.; Bartoš, M.; Kolinko, Y.; Blassová, T.; Tonar, Z.; Pokorný, M.; Sucharda, Z. Vancomycin-loaded collagen/hydroxyapatite layers electrospun on 3D Printed titanium implants prevent bone destruction associated with S. epidermidis infection and enhance osseointegration. Biomedicines 2021, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.F.; Liao, X.; Chen, H.W. Antibiotic-loaded MMT/PLL-based coating on the surface of endosseous implants to suppress bacterial infections. Int. J. Nanomed. 2021, 16, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.K.; Mistry, B.D. Soft-tissue infection caused by streptococcus anginosus after intramucosal hyaluronidase injection: A rare complication related to dermal filler injection. Dermatol. Surg. 2018, 44, 51–53. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Prevention the formation of biofilm on orthopedic implants by melittin thin layer on chitosan/bioactive glass/vancomycin coatings. J. Mater. Sci. Mater. Med. 2021, 32, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Mandell, G.L.; Vest, T.K. Killing of intraleukocytie Staphylococcus aureus by rifampin: In-vitro and in-vivo studies. J. Infect. Dis. 1972, 125, 486–490. [Google Scholar] [CrossRef]
- Renz, N.; Trampuz, A.; Zimmerli, W. Controversy about the role of rifampin in biofilm infections: Is it justified? Antibiotics 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Widmer, A.F.; Blatter, M.; Frei, R. Role of rifampinfor treatment of orthopedic implant-related staphylococcal infections: A randomized controlled trial. JAMA 1998, 279, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; Bentley, K.L.D.M.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Calcium phosphate spacers for the local delivery of sitafloxacin and rifampin to treat orthopedic infections: Efficacy and proof of concept in a mouse model of single-stage revision of device-associated osteomyelitis. Pharmaceutics 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsaei, S.; Ganeshraj, N.; Gu, A.; Liu, Q.; Lane, M.A. 691 Rifampin in the treatment of staphylococcal prosthetic joint infections. Open Forum Infect. Dis. 2014, 1, 195–196. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, Y.E.; Borgen, P.; Bragnes, B.; Figved, W.; Westberg, M. Rifampin combination therapy in staphylococcal prosthetic joint infections: A randomized controlled trial. J. Orthop. Surg. Res. 2020, 15, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- López, E.S.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Machado, A.L.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.J.; Ceri, H.; Stremick, C.A.; Turner, R.J. Biofilm susceptibility to metaltoxicity. Environ. Microbiol. 2004, 6, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Fageria, L.; Pareek, V.; Dilip, R.V.; Bhargava, A.; Pasha, S.S.; Laskar, I.R.; Saini, H.; Dash, S.; Chowdhury, R.; Panwar, J. Biosynthesized protein-capped silver nanoparticles induce ROS-dependent pro-apoptotic signals and pro-survival autophagyin cancer cells. ACS Omega 2017, 2, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Marquis, C.P.; Amal, R.; Sotiriou, G.A.; Rice, S.A.; Harry, E.J. Widespread and indiscriminate nanosilver use: Genuine potential for microbial resistance. ACS Nano 2017, 11, 3438–3445. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C. Antibacterial surface treatment for orthopaedic implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klasen, H.J.A. A historical review of the use of silver in the treatment of burns. II. renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ouay, B.L.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Braekling, T.; Streitbuerger, A.; Gosheger, G.; Boettner, F.; Nottrott, M.; Ahrens, H.; Dieckmann, R.; Guder, W.; Andreou, D.; Hauschild, G.; et al. Silver-coated megaprostheses: Review of the literature. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Nasirpour, M.; Carrola, J.; Oliveira, H.; Freire, C.S.R.; Duarte, I.F. Antimicrobial properties and therapeutic applications of silver nanoparticles and nanocomposites. In Antimicrobial Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 223–259. [Google Scholar] [CrossRef]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Menno, L.W.K.; Leo, H.K. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Yun, A.Q.; Lin, C.; Li, R.Y.; Liu, G.C.; Zhang, Y.B.; Tang, X.F.; Wang, J.C.; Liu, H.; Qin, Y.G. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Paladini, F.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Sannino, A.; Pollini, M. Surface chemical and biological characterization of flax fabrics modified with silver nanoparticles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, H.; Liu, X.F.; Sun, H.J.; Wang, S.X.; Zhang, R. pH-responsive release behavior and anti-bacterial activity of bacterial cellulose-silver nanocomposites. Int. J. Biol. Macromol. 2015, 76, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dasari, T.P.S.; Deng, H.; Yu, H.T. Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health Part. C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Payne, J.N.; Waghwani, H.K.; Connor, M.G.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel synthesis of kanamycin conjugated gold nanoparticles withpotent antibacterial activity. Front. Microbio. 2016, 7, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.R.; Scott, M.E. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of pseudomonas aeruginosa and enterobacter aerogenes and methicillin-resistant staphylococcus aureus. Appl. Environ. Microb. 2012, 78, 2768–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottagisio, M.; Lovati, A.; Galbusera, F.; Lorenzo, D.; Giuseppe, B. A precautionary approach to guide the use of transition metal-based nanotechnology to prevent orthopedic infections. Materials 2019, 12, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Lorente, Á.I.; Cardenas, S.; Gonzalez-Sanchez, Z.I. Effect of synthesis, purification and growth determination methods on the antibacterial and antifungal activity of gold nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109805. [Google Scholar] [CrossRef]
- Su, C.; Huang, K.; Li, H.H.; Lu, Y.G.; Zheng, D.L. Antibacterial properties of functionalized gold nanoparticles and their application in oral biology. J. Nanomater. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Yougbare, S.; Chang, T.K.; Tan, S.H.; Kuo, J.C.; Kuo, T. Antimicrobial gold nanoclusters: Recent developments and future perspectives. Int. J. Mol. Sci. 2019, 20, 2924. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Moghaddam, R.R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.M.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against streptococcus mutans and porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Zheng, X.; Chen, Y.; Li, M.; Liu, K. Alteration of intracellular protein expressions as a key mechanism of the deterioration of bacterial denitrification caused by copper oxide nanoparticles. Sci. Rep. 2015, 5, 15824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Marta, P.; Anna, G.; Ukasz, R.; Ewelina, K.; Agnieszka, S.; Adriana, Z.M. The antibacterial and antifungal textile properties functionalized by bimetallic nanoparticles of Ag/Cu with different structures. J. Nanomater. 2016, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.Z.; Yahia, L.H.; Sacher, E. Antimicrobial properties of the Ag, Cu nanoparticle system. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, J.F.; Wang, X.; Wang, Y.Y.; Hang, Y.Q.; Huang, X.B.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO2 coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C 2018, 82, 110–120. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current statusand future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Sign. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Mulla, N.A.; Otari, S.V.; Bohara, R.A.; Yadav, G.M.; Pawar, S.H. Rapid and size-controlled biosynthesis of cytocompatible selenium nanoparticles by azadirachta indica leaves extract for antibacterial activity. Mater. Lett. 2020, 264, 127353. [Google Scholar] [CrossRef]
- Mojtaba, M.; Hamid, F.; Yaser, G.; Tayebe, M.K.; Reza, S.M. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of staphylococcus aureus, pseudomonas aeruginosa, and proteus mirabilis. J. Trace. Elem. Med. Biol. 2015, 29, 235–241. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Chen, Q.; Yu, Q.; Jie, L. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibitionof candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 2017, 13, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Yip, J.; Liu, L.; Wong, K.H.; Leung, P.H.M.; Yuen, C.W.M.; Cheung, M.C. Investigation of antifungal and antibacterial effects of fabric padded with highly stable selenium nanoparticles. J. Appl. Polym. Sci. 2014, 131, 40728. [Google Scholar] [CrossRef]
- Dorazilová, J.; Muchová, J.; Merková, K.; Koiová, S.; Vojtova, L. Synergistic effect of chitosan and selenium nanoparticles on biodegradation and antibacterial properties of collagenous scaffolds designed for infected burn wounds. Nanomaterials 2020, 10, 1971. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant S.aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Li, D. Zinc and zinc transporters: Novel regulators of ventricular myocardial development. Pediatr. Cardiol. 2018, 39, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, J.; Fellner, M.; Zhang, C.; Sui, D.; Hu, J. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 2017, 3, e1700344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Zhang, Y.; Ma, P.; Sutrisno, L.; Cai, K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials 2019, 212, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Yang, Y.; Qing, Y.; Li, R.; Tang, X.; Guo, D.; Qin, Y.G. Enhancing ZnO-NP antibacterial and osteogenesis properties in orthopedic applications: A review. Int. J. Nanomed. 2020, 15, 6247–6262. [Google Scholar] [CrossRef]
- Krol, A.; Pomastowski, P.; Rafinska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef]
- Mamat, B.; Zhang, N.Y.; Yan, L.; Luo, J.H.; Xie, C.M.; Wang, Y.B.; Ma, C.; Ye, T.J. Stable ZnO-doped hydroxyapatite nanocoating for anti-infection and osteogenic on titanium. Colloids Surf. B Biointerfaces 2020, 186, 110731. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.F.; Allaker, R.P. Nanoparticulate zinc oxide as acoating material for orthopedic and dental implants. J. Biomed. Mater. Res. Part A 2015, 103A, 981–989. [Google Scholar] [CrossRef]
- Tengvall, P.; Bertilsson, L.; Bo, L.; Elwing, H.; Lundström, I. Degradation of dried Ti-peroxy gels made from metallic titanium and hydrogen peroxide. J. Colloid Interface Sci. 1990, 139, 575–580. [Google Scholar] [CrossRef]
- Tengvall, P.; Hornsten, E.G.; Elwing, H.; Lundström, I. Bactericidal properties of a titanium-peroxy gel obtained from metallic titanium and hydrogen peroxide. J. Biomed. Mater. Res. 1990, 24, 319–330. [Google Scholar] [CrossRef]
- Tengvall, P.; Wälivaara, B.; Westerling, J.; Lundström, I. Stable titanium superoxide radicals in aqueous Ti-peroxy gels and Ti-peroxide solutions. J. Colloid Interface Sci. 1991, 143, 589–592. [Google Scholar] [CrossRef]
- Ohlin, A.; Mattsson, E.; Rgelin, M.M.; Davies, J.R.; Svensater, G.; Corvec, S.; Tengvall, P.; Riesbeck, K. Titanium granules pre-treated with hydrogen peroxide inhibit growth of bacteria associated with post-operative infections in spine surgery. Eur. Spine J. 2018, 27, 2463–2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itabashi, T.; Narita, K.; Ono, A.; Wada, K.; Tanaka, T.; Kumagai, G.; Yamauchi, R.; Nakane, A.; Ishibashi, Y. Bactericidal and antimicrobial effects of pure titanium and titanium alloy treated with short-term, low-energy UV irradiation. Bone Jt. Res. 2017, 6, 108–112. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical applications of TiO2 nanostructures: Recent advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biot. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Koseki, H.; Asahara, T.; Shida, T.; Yoda, I.; Osaki, M. Clinical and histomorphometrical study on titanium dioxide-coated external fixation pins. Int. J. Nanomed. 2013, 8, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Villatte, G.; Massard, C.; Descamps, S.; Sibaud, Y.; Forestier, C.; Awito, K.O. Photoactive TiO2 antibacterial coating on surgical external fixation pins for clinical application. Int. J. Nanomed. 2015, 10, 3367–3375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hérault, N.; Wagner, J.; Abram, S.L.; Widmer, J.; Horvath, L.; Vanhecke, D.; Bourquin, C.; Fromm, K.M. Silver-containing titanium dioxide nanocapsules for combating multidrug-resistant bacteria. Int. J. Nanomed. 2020, 15, 1267–1281. [Google Scholar] [CrossRef] [Green Version]
- Rijnaarts, H.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Alexander, J.B. Bacterial adhesion under static and dynamic conditions. Appl. Environ. Microb. 1993, 59, 3255–3265. [Google Scholar] [CrossRef] [Green Version]
- Scialla, S.; Martuscelli, G.; Nappi, F.; Singh, S.S.A.; Raucci, M.G. Trends in managing cardiac and orthopaedic device-associated infections by using therapeutic biomaterials. Polymers 2021, 13, 1556. [Google Scholar] [CrossRef]
- Kurtz, I.S.; Schiffman, J.D. Current and emerging approaches to engineer antibacterial and antifouling electrospun nanofibers. Materials 2018, 11, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilgus, T.A. Immune cells in the healing skin wound: Influential players at each stage of repair. Pharmacol. Res. 2008, 58, 112–116. [Google Scholar] [CrossRef]
- Kuang, J.; Messersmith, P.B. Universal surface-initiated polymerization of antifouling zwitterionic brushesusing a mussel-mimetic peptide initiator. Langmuir 2012, 28, 7258–7266. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.H.; Hunley, M.T.; Allen, M.H., Jr.; Long, T.E. Electrospinning zwitterion-containing nanoscale acrylicfibers. Polymer 2009, 50, 4781–4787. [Google Scholar] [CrossRef]
- Govinna, N.; Kaner, P.; Ceasar, D.; Dhungana, A.; Moers, C.; Son, K.; Asatekin, A.; Cebe, P. Electrospun fiber membranes from blends of poly(vinylidene fluoride) with fouling-resistant zwitterionic copolymers. Polym. Int. 2019, 68, 231–239. [Google Scholar] [CrossRef]
- Ozcan, S.; Kaner, P.; Thomas, D.; Cebe, P.; Asatekin, A. Hydrophobic anti-fouling electrospun mats from zwitterionic amphiphilic copolymers. ACS Appl. Mater. Interfaces 2018, 10, 18300–18309. [Google Scholar] [CrossRef] [PubMed]
- Venault, A.; Lai, M.W.; Jhong, J.F.; Yeh, C.C.; Yeh, L.C.; Chang, Y. Superior bio-inert capability of zwitterionic poly(4-vinylpyridine propylsulfobetaine) with standing clinical sterilization for extended medical applications. ACS Appl. Mater. Interfaces 2018, 10, 17771–17783. [Google Scholar] [CrossRef] [PubMed]
- Kolewe, K.W.; Dobosz, K.M.; Rieger, K.A.; Chang, C.C.; Emrick, T.; Schiffman, J.D. Antifouling electrospun nanofiber mats functionalized with polymer zwitterions. ACS Appl. Mater. Interfaces 2016, 8, 27585–27593. [Google Scholar] [CrossRef] [PubMed]
- De Brucker, K.; Delattin, N.; Robijns, S.; Steenackers, H.; Verstraeten, V.; Landuyt, B.; Luyten, W.; Schoofs, L.; Dovgan, B.; Frohlich, M.; et al. Derivatives of the mouse cathelicidin-related antimicrobial peptide (CRAMP) inhibit fungal and bacterial biofilm formation. Antimicrob. Agents Chemother. 2014, 58, 5395–5404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pletzer, D.; Coleman, S.R.; Hancock, R.E. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 2016, 33, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabbene, O.; Azaiez, S.; Di Grazia, A.; Karkouch, I.; Slimene, I.B.; Elkahoui, S.; Alfeddy, M.N.; Casciaro, B.; Luca, V.; Limam, F. Bacillomycin D and its combination with amphotericin B: Promising antifungal compounds with powerful antibiofilm activity and wound-healing potency. J. Appl. Microbiol. 2016, 120, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. BBA Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Zhou, C.; Kallenbach, N.R.; Ren, D. Control of bacterial persister cells by Trp/Arg -containing antimicrobial peptides. Appl. Environ. Microbiol. 2011, 77, 4878–4885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E.W. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyonsaba, F.; Madera, L.; Afacan, N.; Okumura, K.; Ogawa, H.; Hancock, R.E.W. The innate defense regulator peptides IDR-HH2, IDR-1002, and IDR-1018 modulate human neutrophil functions. J. Leukoc. Biol. 2013, 94, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, S.C.; César, F.N.; Hancock, R. Peptide IDR-1018: Modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 2015, 21, 323–329. [Google Scholar] [CrossRef]
- Pletzer, D.; Mansour, S.C.; Hancock, R.E.W.; Yeaman, M.R. Synergy between conventional antibiotics and anti-biofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathog. 2018, 14, e100708. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Leong, S.S. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef]
- Gao, G.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.; Cheng, J.T.J.; Kazemzadeh-Narbat, M.; Kai, Y.; Wang, R.; Straus, S.K.; et al. The biocompatibility and biofilmresistance of implant coatings based on hydrophilic polymerbrushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909. [Google Scholar] [CrossRef]
- Afacan, N.J.; Yeung, A.T.; Pena, O.M.; Robert, E.W.H. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazici, H.; O’Neill, M.B.; Kacar, T.; Wilson, B.R.; Tamerler, C. Engineered chimeric peptides as antimicrobial surface coating agents toward infection-free implants. ACS Appl. Mater. Interfaces 2016, 8, 5070–5081. [Google Scholar] [CrossRef] [Green Version]
- Pajarinen, J.; Cenni, E.; Savarino, L.; Gomez-Barrena, E.; Tamaki, Y.; Takagi, M.; Salo, J.; Konttinen, Y.T. Profile of toll-like receptor-positive cells in septic and aseptic loosening of total hip arthoplasty implants. J. Biomed. Mater. Res. A 2010, 94, 84–92. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, T.H.; Chong, A.; Bai, L.; Yu, H.; Gong, W.; Wooley, P.H.; Yang, S.Y. Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene Ther. 2010, 17, 1262–1269. [Google Scholar] [CrossRef] [Green Version]
- Lasek, A.W.; Janak, P.H.; He, L.; Whistler, J.L.; Heberlein, U. Down regulation of mu opioid receptor by RNA interference in the ventral tegmental area reduces ethanol consumption in mice. Genes Brain Behav. 2007, 6, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Lenz, L.L.; Ley, K. Macrophages at the fork in the road to health or disease. Front. Immunol. 2015, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Lucca, L.E.; Hafler, D.A. Sodium-activated macrophages: The salt mine expands. Cell Res. 2015, 25, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbe, P.; Draijer, C.; Borg, T.R.; Luinge, M.; Timens, W.; Wouters, I.M.; Melgert, B.N.; Hylkema, M.N. Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Zarif, J.C.; Hernandez, J.R.; Sachsenmeier, K.F.; Hui, Z.; Pienta, K.J. Targeting M2-tumor associated macrophages (M2-TAMs) in prostate cancer. Cancer Res. 2015, 75, 2365. [Google Scholar] [CrossRef]
- Nicol, M.Q.; Dutia, B.M. The role of macrophages in influenza A virus infection. Future Virol. 2014, 9, 847–862. [Google Scholar] [CrossRef]
- Meng, X.M.; Tang, P.M.K.; Li, J.; Lan, H.Y. Macrophage phenotype in kidney injury and repair. Kidney Dis. 2015, 1, 138–146. [Google Scholar] [CrossRef]
- Taylor, P.C.; Feldmann, M. Anti-TNF biologic agents: Still the therapy of choice for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.Q.; Huang, D.S.; Zhang, C.; Song, B.; Huang, J.B.; Yue, D. Lentivirus-mediated short hairpin RNA interference targeting TNF-alpha in macrophages inhibits particle-induced inflammation and osteolysis in vitro and in vivo. BMC Musculoskel. Dis. 2016, 17, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Talekar, M.; Raikar, A.; Mansoor, A. Macrophage-targeted delivery systems for nucleic acid therapy of inflammatory diseases. J. Control. Release 2014, 190, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Castanheira, P.; Faria, T.Q.; Goncalves, C.; Madureira, P.; Faro, C.; Domingues, L.; Rui, M.M.B.; Vilanova, M.; Gama, M. Biological activity of heterologous murine interleukin-10 and preliminary studies on the use of a dextrin nanogel as a delivery system. Int. J. Pharm. 2010, 400, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, Y.; Yu, W.; Yin, X.Z.; Weng, J.; Feng, B. Modulation of macrophage phenotype through controlled release of interleukin-4 from gelatine coatings on titanium surfaces. Eur. Cells Mater. 2018, 36, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Miron, R.J.; Zhang, Y. Modulating macrophage polarization on titanium implant surface by poly(dopamine)-assisted immobilization of IL4. Clin. Implant Dent. Relat. Res. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Zhao, D.W.; Zuo, K.Q.; Wang, K.; Sun, Z.Y.; Liu, C. Interleukin-4 assisted calcium-strontium-zinc-phosphate coating induces controllable macrophage polarization and promotes osseointegration on titanium implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111512. [Google Scholar] [CrossRef]
- Li, Q.; Liang, B.; Wang, F.; Wang, Z. Delivery of interleukin 4 from a titanium substrate coated with graphene oxide for enhanced osseointegration by regulating macrophage polarization. ACS Biomater. Sci. Eng. 2020, 6, 5215–5229. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Cao, M.; Xu, S.H.; Shi, J.F.; Mao, X.D.; Yao, X.M.; Liu, C. Luteolin alters macrophage polarization to inhibit inflammation. Inflammation 2020, 43, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Cao, M.; Xu, S.H.; Zhang, J.M.; Wang, Z.G.; Mao, X.D.; Yao, X.M.; Liu, C. Effect of luteolin on inflammatory responses inRAW264.7 macrophages activated with LPS and IFN-γ. J. Funct. Foods 2017, 32, 123–130. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Luong, H.D.; Jin, L.S.; Min, T.; Taeho, K.; Kee, J.D. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Li, X.; Lin, H.; Zhou, Y. Curcumin as a promising antibacterial agent: Effects on metabolism and biofilm formation in S. mutans. Biomed. Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.T.; Chiang, Y.C.; Huang, T.Y.; Chen, P.C.; Chang, P.J.; Lee, C.W. Curcumin nanoparticles are a promising anti-bacterial and anti-inflammatory agent for treating periprosthetic joint infections. Int. J. Nanomed. 2019, 14, 469–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.J.; Liang, Y.P.; Zhang, J.; Bai, L.; Xu, M.G.; Han, Q.; Han, X.Z.; Xiu, J.T.; Li, M.; Zhou, X.L.; et al. Synergistic enhancement of tendon-to-bone healing via anti-inflammatory and pro-differentiation effects caused by sustained release of Mg2+/curcumin from injectable self-healing hydrogels. Theranostics 2021, 11, 5911–5925. [Google Scholar] [CrossRef] [PubMed]
- Murgia, D.; Mauceri, R.; Campisi, G.; Viviana, D.C. Advance on resveratrol application in bone regeneration: Progress and perspectives for use in oral and maxillofacial surgery. Biomolecules 2019, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, D.D.S.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-inflammatory effects of resveratrol: Mechanistic insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [Green Version]
- Ll, L.M.; Yu, M.L.; Li, Y.; Li, Q.; Yang, H.C.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2021, 6, 1255–1266. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; You, Y.; Ma, A.; Song, Y.J.; Jiao, J.; Song, L.T.; Shi, E.Y.; Zhong, X.; Li, Y.; Li, C.Y. Zn-incorporated TiO2 nanotube surface improves osteogenesis ability through influencing immunomodulatory function of macrophages. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.R.; Yang, J.; Shang, Y.L.; Deng, S.; Peng, C. Magnesium-doped nanostructured titanium surface modulates macrophage-mediated inflammatory response for ameliorative osseointegration. Int. J. Nanomed. 2020, 15, 7185–7198. [Google Scholar] [CrossRef] [PubMed]
- Negrescu, A.M.; Necula, M.G.; Gebaur, A.; Golgovici, F.; Nica, C.; Curti, F.; Iovu, H.; Costache, M.; Cimpean, A. In vitro macrophage immunomodulation by poly(ε- caprolactone) based-coated AZ31 Mg alloy. Int. J. Mol. Sci. 2021, 22, 909. [Google Scholar] [CrossRef]
- Qiao, W.; Wong, K.H.M.; Shen, J.; Wang, W.H.; Wu, J.; Li, J.H.; Lin, Z.J.; Chen, T.; Matinlinna, J.P.; Zheng, Y.F.; et al. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat. Commun. 2021, 12, 2885–2900. [Google Scholar] [CrossRef]
- Jin, L.; Chen, C.X.; Li, Y.T.; Yuan, F.; Gong, R.L.; Wu, J.; Zhang, H.; Kang, B.; Yuan, G.Y.; Zeng, H.; et al. A biodegradable Mg-based alloy inhibited the inflammatory response of THP-1 cell-derived macrophages through the TRPM7–PI3K–AKT1 signaling axis. Front. Immunol. 2019, 10, 2798–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Lin, Z.N.; Ding, J.M.; Huang, W.X.; Chen, J.; Wu, D. Inflammatory and biocompatibility evaluation of antimicrobial peptide GL13K immobilized onto titanium by silanization. Colloids Surf. B Biointerfaces 2017, 160, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Zhou, L.; Wu, D.; Huang, W.X.; Lin, Y.J.; Zhou, B.W.; Chen, J. The effects of titanium surfaces modified with an antimicrobial peptide GL13K by silanization on polarization, anti-inflammatory, and proinflammatory properties of macrophages. Biomed. Res. Int. 2020, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, M.L.; Sun, X.L.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.M.; Wang, L.; Xu, H.K.H.K. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Jo, H.S.; Choi, S.; Song, H.G.; Park, K. Lactoferrin-anchored tannylated mesoporous silica nanomaterials for enhanced osteo-differentiation ability. Pharmaceutics 2020, 13, 30. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Meng, F.F.; Kim, B.K.; Hyun, H.; Yeo, Y. Tannic acid-mediated surface functionalization of polymeric nanoparticles. ACS Biomater. Sci. Eng. 2016, 2, 2294–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Lim, H.; Ahn, J.W.; Jang, D.; Lee, S.H. Design of a 3D BMP-2-delivering tannylated PCL scaffold and its anti-oxidant, anti-inflammatory, and osteogenic effects in vitro. Int. J. Mol. Sci. 2018, 19, 3602. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, N.; Sagbas, S.; Aktas, N.; Silan, C. Inherently antioxidant and antimicrobial tannic acid release from poly(tannic acid)nanoparticles with controllable degradability. Colloids Surf. B Biointerfaces 2016, 142, 334–343. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Forget, A.; Shastri, V.P.; Nicolas, H.V.; Blencowe, A. Anti-bacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Choi, S.; Jo, H.S.; Song, H.; Kim, H.J.; Oh, J.K.; Cho, J.W.; Park, K.; Kim, S.E. Multifunctional tannic acid-alendronate nanocomplexes with antioxidant, anti-inflammatory, and osteogenic potency. Nanomaterials 2021, 11, 1812. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yoon, J.S.; Lee, J.Y.; Kim, H.J.; Park, K.; Kim, S.E. Long-term local PDGF delivery using porous microspheres modifiedwith heparin for tendon healing of rotator cuff tendinitis in a rabbit model. Carbohyd. Polym. 2019, 209, 372–381. [Google Scholar] [CrossRef]
- Jung, S.Y.; Hwang, H.; Jo, H.S.; Choi, S.; Park, K. Tannylated calcium carbonate materials with antacid, anti-inflammatory, and antioxidant effects. Int. J. Mol. Sci. 2021, 22, 4614. [Google Scholar] [CrossRef] [PubMed]
- Sawdy, R.; Knock, G.A.; Bennett, P.R.; Poston, L.; Aaronson, P.I. Effect of nimesulide and indomethacin on contractility and the Ca2+ channel current in myometrial smooth muscle from pregnant women. Brit. J. Pharmacol. 2010, 125, 1212–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Bodón, J.; Ruiz-Rubio, L.; Hernáez-Laviña, E.; Vilas-Vilela, J.L.; Moreno-Benítez, M.I. Poly(l-lactide)- based anti-inflammatory responsive surfaces for surgical implants. Polymers 2020, 13, 34. [Google Scholar] [CrossRef]
- Rivera, M.C.; Perni, S.; Sloan, A.; Prokopovich, P. Anti-inflammatory drug-eluting implant model system to prevent wear particle-induced periprosthetic osteolysis. Int. J. Nanomed. 2019, 14, 1069–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.L.; Zhou, Y.X.; Sun, Q.A.; Zhou, C.H.; Hu, S.Y.; Lenahan, C.; Xu, W.L.; Deng, Y.C.; Li, G.H.; Tao, S.F. Update on nanoparticle-based drug delivery system for anti-inflammatory treatment. Front. Bioeng. Biotechnol. 2021, 9, 630352. [Google Scholar] [CrossRef]
- Trujillo-Nolasco, R.M.; Morales-Avila, E.; Ocampo-García, B.E.; Ferro-Flores, G.; Gibbens-Bandala, B.V.; Escudero-Castellanos, A.; Isaac-Olive, K. Preparation and in vitro evaluation of radio labeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109766. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.L.; Zhong, Z.R.; Wang, Y.; Feng, Y.; Li, C.H. Exosome-based biomimetic nanoparticles targeted to inflamed joints for enhanced treatment of rheumatoid arthritis. J. Nanobiotechnol. 2020, 18, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Toita, R.; Kawano, T.; Murata, M.; Kang, J.H. Anti-obesity and anti-inflammatory effects of macrophage-targeted interleukin-10-conjugated liposomes in obese mice. Biomaterials 2016, 110, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, Y.L.; Wang, L.X.; Li, Y.; Pan, J.J.; Fu, X.M.; Luo, Z.Y.; Sui, Y.; Zhang, S.Q.; Wang, L.; et al. Triple- functional polyetheretherketone surface with enhanced bacteriostasis and anti-inflammatory and osseoin- tegrative properties for implant application. Biomaterials 2019, 212, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Soundarapandian, K. 141PErbitux conjugated zinc oxide nanoparticles to enhance antitumor efficiency via targeted drug delivery system for breast cancer therapy. Ann. Oncol. 2017, 28, 658–699. [Google Scholar] [CrossRef]
- Kalangi, S.K.; Swarnakar, N.K.; Sathyavathi, R.; Narayana, R.D.; Sanyog, J.; Pallu, R. Synthesis, characterization, and biodistribution of quantum dot-celecoxib conjugate in mouse paw edema model. Oxidative Med. Cell. Longev. 2018, 2018, 3090517. [Google Scholar] [CrossRef]
- Kumar, R.S.; Shakambari, G.; Ashokkumar, B.; Nelson, D.J.; SJohn, S.A.; Varalakshmi, P. Nitrogen-doped graphene quantum dot-combined sodium 10-amino-2-methoxyundecanoate: Studies of proinflammatory gene expression and live cell imaging. ACS Omega 2018, 3, 11982–11992. [Google Scholar] [CrossRef] [Green Version]
- Bridges, A.W.; Garcia, A.J. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. J. Diabetes Sci. Technol. 2008, 2, 984–994. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, G.; Xu, J.; Chirume, W.M.; Zhang, J.; Zhang, H.; Hu, X. Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review. Coatings 2021, 11, 1401. https://doi.org/10.3390/coatings11111401
Tan G, Xu J, Chirume WM, Zhang J, Zhang H, Hu X. Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review. Coatings. 2021; 11(11):1401. https://doi.org/10.3390/coatings11111401
Chicago/Turabian StyleTan, Gang, Jing Xu, Walter Munesu Chirume, Jieyu Zhang, Hui Zhang, and Xuefeng Hu. 2021. "Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review" Coatings 11, no. 11: 1401. https://doi.org/10.3390/coatings11111401





