Regulating Monolayer Aligned Silver Nanowire Coatings for Energy-Saving Windows
Abstract
:1. Introduction
2. Materials and Methods
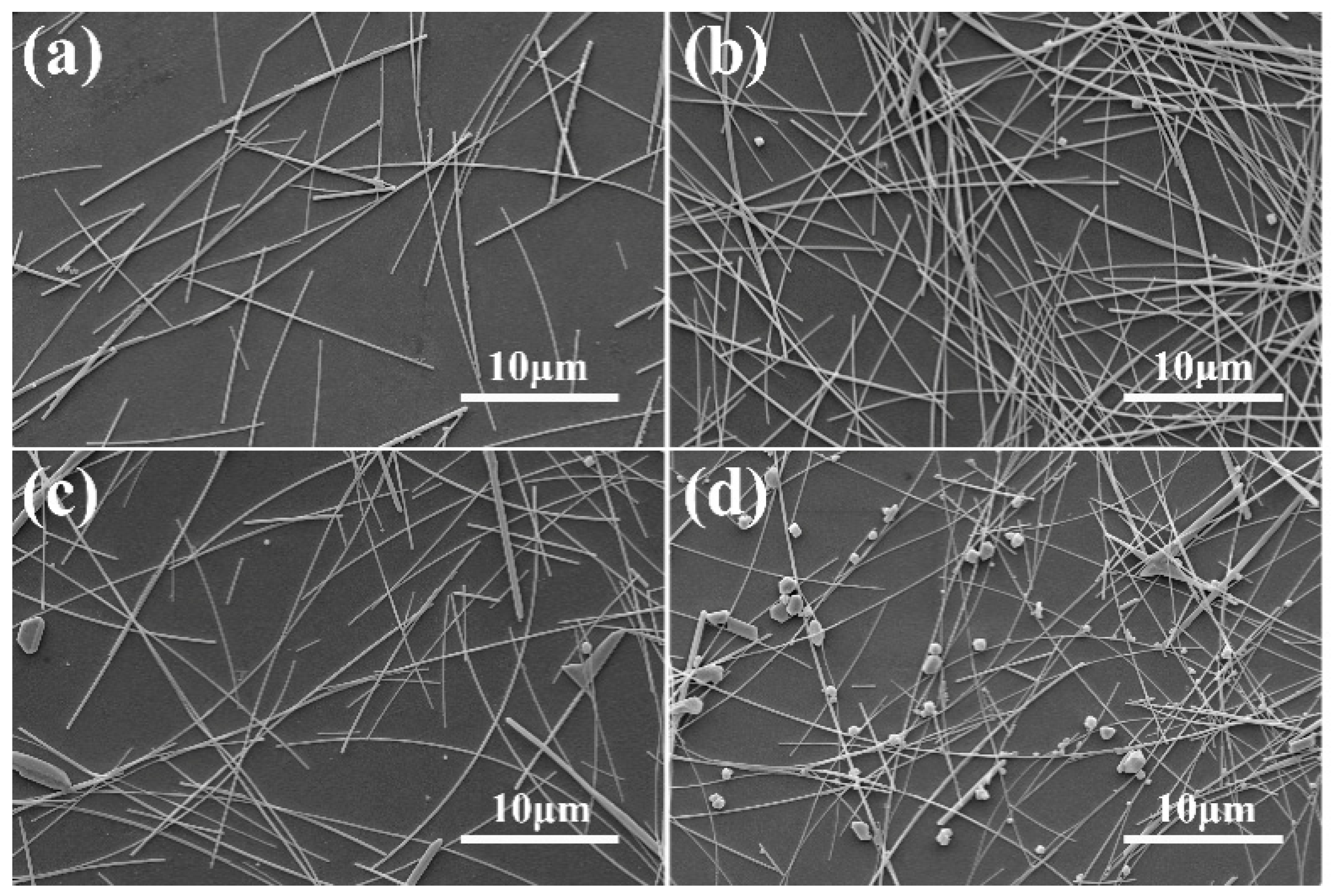
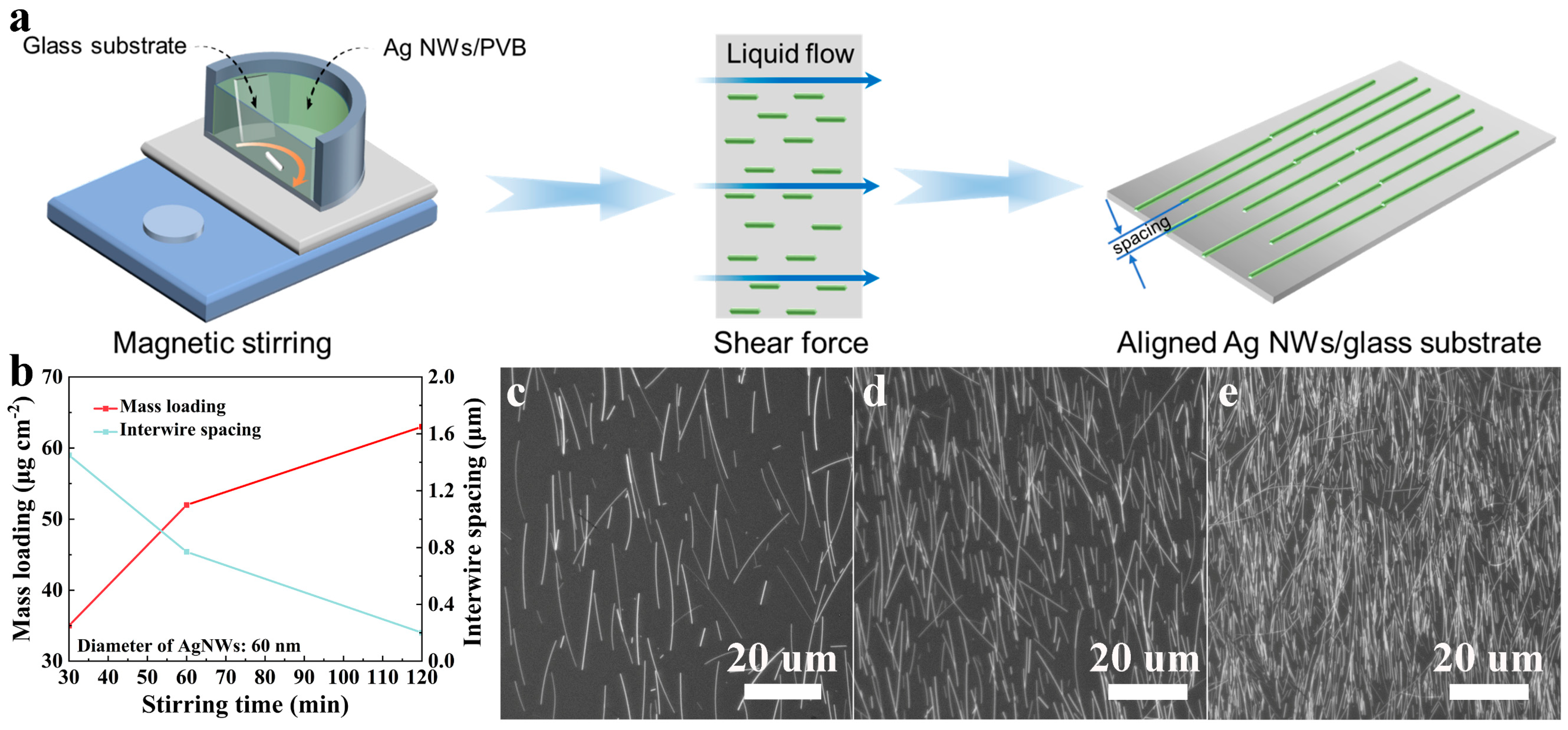
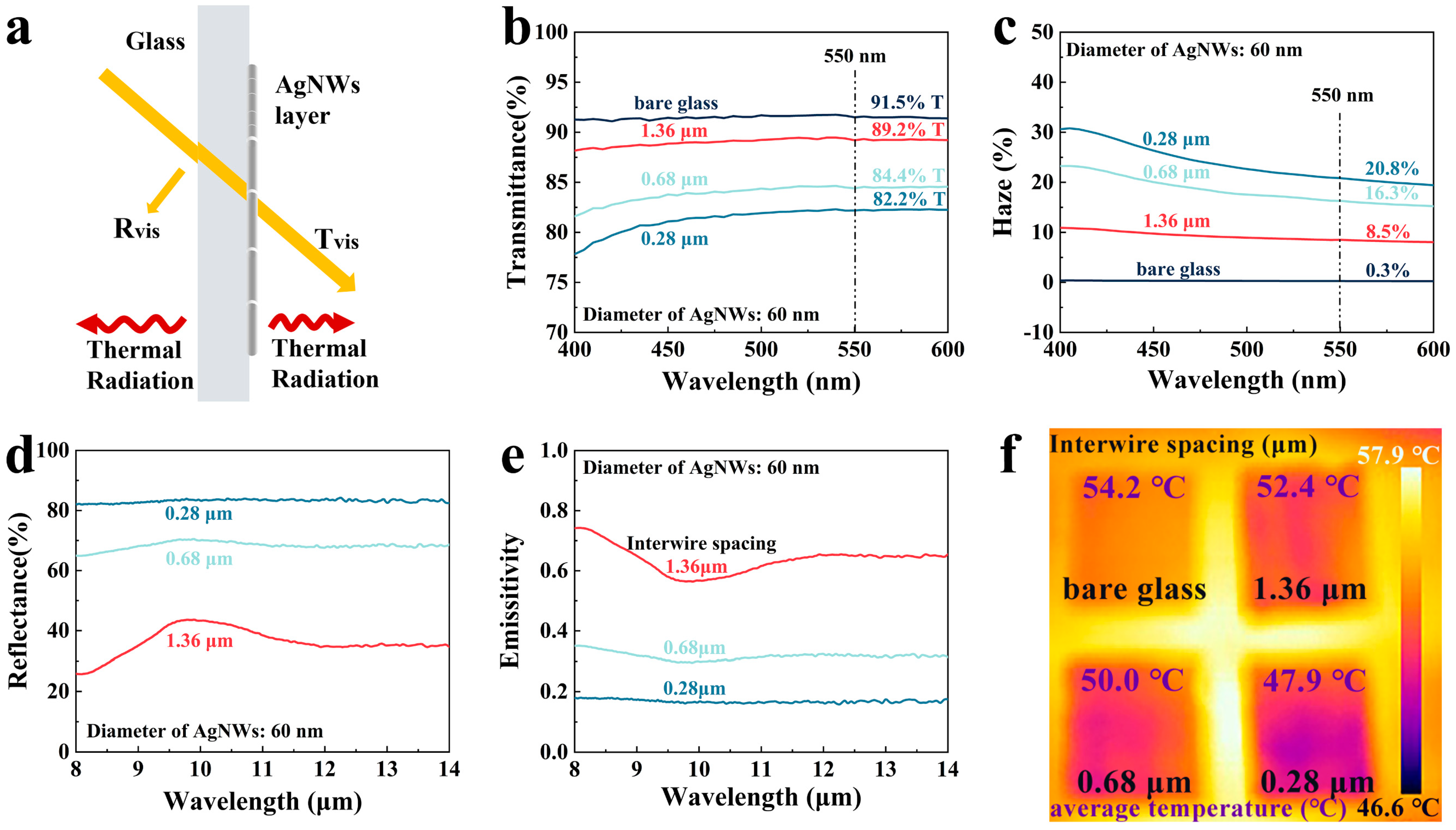
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Zhai, Y.; He, S.M.; Gan, W.T.; Wei, Z.Y.; Heidarinejad, M.; Dalgo, D.; Mi, R.Y.; Zhao, X.P.; Song, J.W.; et al. A radiative cooling structural material. Science 2019, 364, 760–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.X.; Magoules, F. A review on the prediction of building energy consumption. Renew Sustain. Energy Rev. 2012, 16, 3586–3592. [Google Scholar] [CrossRef]
- Perez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Yang, H.J.; Geng, L.; Zhang, Y.T.; Chang, G.; Zhang, Z.L.; Liu, X.; Lei, M.; He, Y.B. Graphene-templated synthesis of palladium nanoplates as novel electrocatalyst for direct methanol fuel cell. Appl. Surf. Sci. 2019, 466, 385–392. [Google Scholar] [CrossRef]
- Wang, X.T.; Cui, Y.; Li, T.; Lei, M.; Li, J.B.; Wei, Z.M. Recent Advances in the Functional 2D Photonic and Optoelectronic Devices. Adv. Opt. Mater. 2019, 7, 1801274. [Google Scholar] [CrossRef]
- Hoffmann, S.; Lee, E.S.; Clavero, C. Examination of the technical potential of near-infrared switching thermochromic windows for commercial building applications. Sol. Energy Mat. Sol. Cells 2014, 123, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.D.; Cortie, M.B.; Stevens, M. Effect of glass pre-treatment on the nucleation of semi-transparent gold coatings. Mater. Chem. Phys. 2005, 94, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Martin-Palma, R.J.; Vazquez, L.; Martinez-Duart, J.M.; Malats, R. Silver-based low-emissivity coatings for architectural windows: Optical and structural properties. Sol. Energy Mat. Sol. Cells 1998, 53, 55–66. [Google Scholar] [CrossRef]
- Wu, J.Y.; Qian, X.H.; Liu, C.Y.; Ji, Y.L.; Yu, S.W. Design and realization of neutral-tinted low-E film. Vacuum 2022, 203, 111228. [Google Scholar] [CrossRef]
- Deng, Z.S.; Wang, J.; Wu, A.M.; Shen, J.; Zhou, B. High strength SiO2 aerogel insulation. J. Non-Cryst. Solids 1998, 225, 101–104. [Google Scholar] [CrossRef]
- Schaefer, C.; Brauer, G.; Szczyrbowski, J. Low emissivity coatings on architectural glass. Surf. Coat. Technol. 1997, 93, 37–45. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Yin, Z.W.; Li, W.J.; Yang, C.P.; Sun, W.H.; Zhang, H.L.; Li, Y. Enhanced Electrochromic Performance of All-Solid-State Electrochromic Device Based on W-Doped NiO Films. Coatings 2022, 12, 118. [Google Scholar] [CrossRef]
- Mouratis, K.; Tudose, I.V.; Romanitan, C.; Pachiu, C.; Popescu, M.; Simistiras, G.; Couris, S.; Suchea, M.P.; Koudoumas, E. WO3 Films Grown by Spray Pyrolysis for Smart Windows Applications. Coatings 2022, 12, 545. [Google Scholar] [CrossRef]
- Kaklauskas, A.; Zavadskas, E.K.; Raslanas, S.; Ginevicius, R.; Komka, A.; Malinauskas, P. Selection of low-e windows in retrofit of public buildings by applying multiple criteria method COPRAS: A Lithuanian case. Energy Build. 2006, 38, 454–462. [Google Scholar] [CrossRef]
- Yu, Q.Q.; Guo, M.; Xu, W.X.; Shi, X.D.; Ma, Y.; Yu, J.Y.; Ding, B. Spray-assembly of thermos plasmonic nanoparticles: A speed-up fabrication strategy for energy-saving smart windows. Sol. Energy 2022, 238, 9–16. [Google Scholar] [CrossRef]
- Hsu, P.C.; Liu, X.G.; Liu, C.; Xie, X.; Lee, H.R.; Welch, A.J.; Zhao, T.; Cui, Y. Personal Thermal Management by Metallic Nanowire-Coated Textile. Nano Lett. 2015, 15, 365–371. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Orendorff, C.J. One-dimensional colloidal gold and silver nanostructures. Inorg. Chem. 2006, 45, 7544–7554. [Google Scholar] [CrossRef]
- Lin, C.J.; Hur, J.; Chao, C.Y.H.; Liu, G.Z.; Yao, S.H.; Li, W.H.; Huang, B.L. All-weather thermochromic windows for synchronous solar and thermal radiation regulation. Sci. Adv. 2022, 8, 7359. [Google Scholar] [CrossRef]
- Hanauer, S.; Celle, C.; Crivello, C.; Szambolics, H.; Munoz-Rojas, D.; Bellet, D.; Simonato, J.P. Transparent and Mechanically Resistant Silver-Nanowire-Based Low-Emissivity Coatings. ACS Appl. Mater. Interfaces 2021, 13, 21971–21978. [Google Scholar] [CrossRef]
- Lin, S.; Wang, H.Y.; Zhang, X.N.; Wang, D.; Zu, D.; Song, J.A.; Liu, Z.L.; Huang, Y.; Huang, K.; Tao, N.A.; et al. Direct spray-coating of highly robust and transparent Ag nanowires for energy saving windows. Nano Energy 2019, 62, 111–116. [Google Scholar] [CrossRef]
- Hu, H.B.; Wang, S.C.; Wang, S.C.; Liu, G.W.; Cao, T.; Long, Y. Aligned Silver Nanowires Enabled Highly Stretchable and Transparent Electrodes with Unusual Conductive Property. Adv. Funct. Mater. 2019, 29, 1902922. [Google Scholar] [CrossRef]
- Johan, M.R.; Aznan, N.A.K.; Yee, S.T.; Ho, I.H.; Ooi, S.W.; Singho, N.D.; Aplop, F. Synthesis and Growth Mechanism of Silver Nanowires through Different Mediated Agents (CuCl2 and NaCl) Polyol Process. J. Nanomater. 2014, 2014, 105454. [Google Scholar] [CrossRef] [Green Version]
- Trung, T.N.; Arepalli, V.K.; Gudala, R.; Kim, E.T. Polyol synthesis of ultrathin and high-aspect-ratio Ag nanowires for transparent conductive films. Mater. Lett. 2017, 194, 66–69. [Google Scholar] [CrossRef]
- Rui, Y.J.; Zhao, W.L.; Zhu, D.W.; Wang, H.Y.; Song, G.L.; Swihart, M.T.; Wan, N.; Gu, D.W.; Tang, X.B.; Yang, Y.; et al. Understanding the Effects of NaCl, NaBr and Their Mixtures on Silver Nanowire Nucleation and Growth in Terms of the Distribution of Electron Traps in Silver Halide Crystals. Nanomaterials 2018, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.G.; Mayers, B.; Herricks, T.; Xia, Y.N. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Kottmann, J.P.; Martin, O.J.F.; Smith, D.R.; Schultz, S. Plasmon resonances of silver nanowires with a nonregular cross section. Phys. Rev. B 2001, 64, 235402. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.B.; Feng, J.Y.; Ma, X.; Wu, C.; Zhao, X.J. One-dimensional silver nanowires synthesized by self-seeding polyol process. J. Nanopart. Res. 2012, 14, 1–15. [Google Scholar] [CrossRef]
- Jo, H.A.; Jang, H.W.; Hwang, B.Y.; Kim, Y.H.; Kim, J.Y. Synthesis of small diameter silver nanowires via a magnetic-ionic-liquid-assisted polyol process. RSC Adv. 2016, 6, 104273–104279. [Google Scholar] [CrossRef]



| Material | Supplier | Purity |
|---|---|---|
| AgNO3 | Tianjin YingDa Chemical Reagents Co., Ltd. (Tianjin, China) | AR |
| CuCl2.2H2O | Shanghai Macklin Biochemical Co. Ltd. (Shanghai, China) | AR |
| PVP, Mw = 58,000 | Shanghai Macklin Biochemical Co. Ltd. (Shanghai, China) | AR |
| PVB, Mw = 170,000–250,000 | Shanghai Macklin Biochemical Co. Ltd. (Shanghai, China) | AR |
| EG | Tianjin FuChen Chemical Reagents Co., Ltd. (Tianjin, China) | 99.5% |
| Ethanol | Tianjin FuChen Chemical Reagents Co., Ltd. (Tianjin, China) | 99.7% |
| KBr | Tianjin FuChen Chemical Reagents Co., Ltd. (Tianjin, China) | AR |
| Deionized water | UPT-II-20T, Chengdu Chaochun Technology Co., Ltd. (Chengdu, China) | Resistivity = 18.2 MΩ cm |
| Abbreviation | Meaning |
|---|---|
| T | Transmittance |
| R | Reflectance |
| εMIR | Emissivity of mid-infrared region |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| FTIR spectrometer | Fourier transform infrared spectrometer |
| UV–Vis | UV-visible spectrophotometer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Ma, H.; Shao, Y.; Yu, X.; Chen, J.; Dong, C.; Mao, Z.; Wang, D. Regulating Monolayer Aligned Silver Nanowire Coatings for Energy-Saving Windows. Coatings 2022, 12, 1552. https://doi.org/10.3390/coatings12101552
Yu Z, Ma H, Shao Y, Yu X, Chen J, Dong C, Mao Z, Wang D. Regulating Monolayer Aligned Silver Nanowire Coatings for Energy-Saving Windows. Coatings. 2022; 12(10):1552. https://doi.org/10.3390/coatings12101552
Chicago/Turabian StyleYu, Zhongrui, Huirong Ma, Yuqiu Shao, Xiaole Yu, Jingjing Chen, Chenlong Dong, Zhiyong Mao, and Dajian Wang. 2022. "Regulating Monolayer Aligned Silver Nanowire Coatings for Energy-Saving Windows" Coatings 12, no. 10: 1552. https://doi.org/10.3390/coatings12101552





