Eco-Friendly Electrowinning for Metals Recovery from Waste Electrical and Electronic Equipment (WEEE)
Abstract
:1. Introduction
2. Brief Review on Experimental Set-Up for Electrowinning
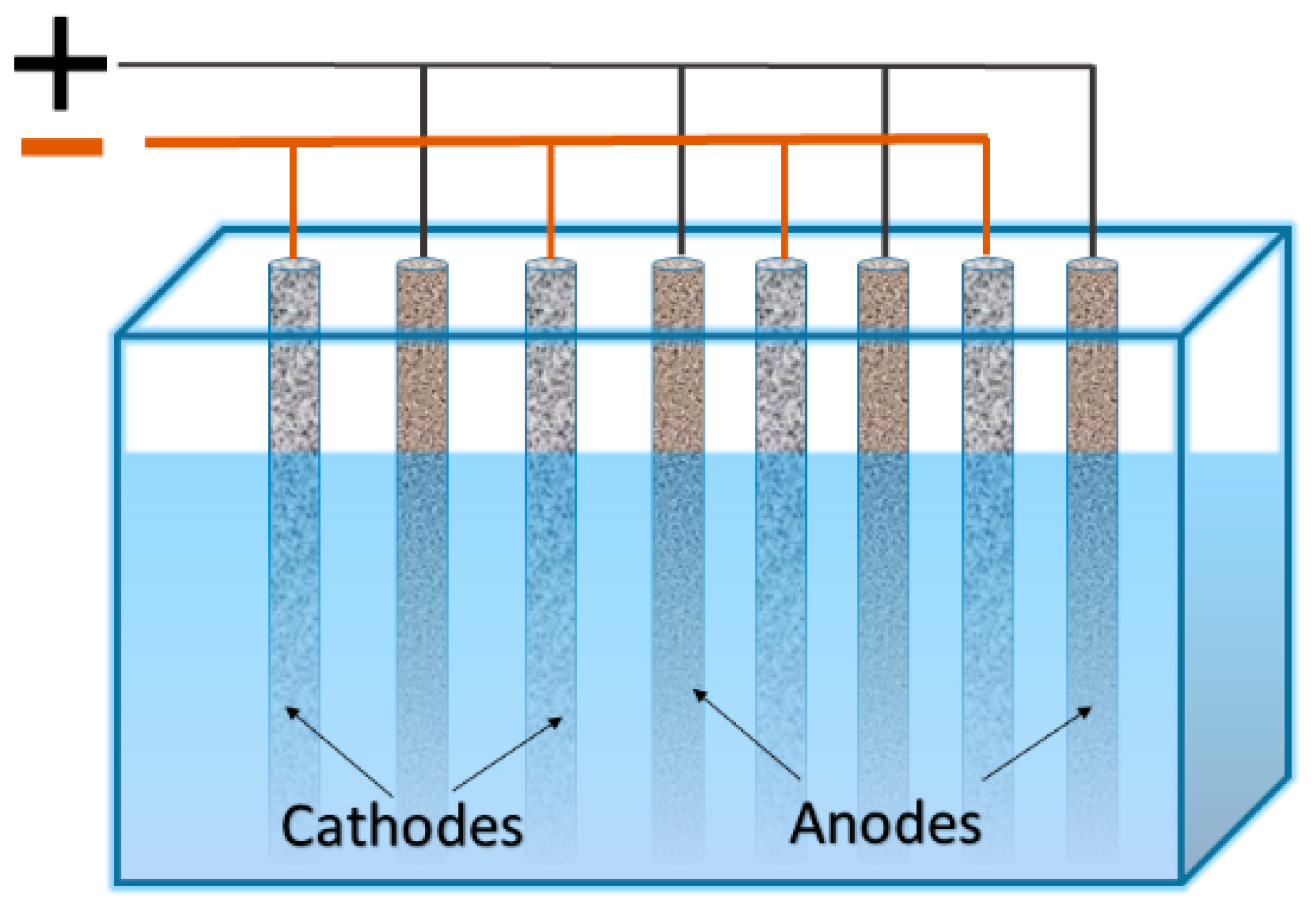
2.1. Cathode Configurations
2.2. Solutions for Electrowinning
3. Results
3.1. Methodology of Experiments
3.2. Perspectives of Tin Electrowinning from Citrate-Based Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waste from Electrical and Electronic Equipment (WEEE). Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-electrical-and-electronic-equipment-weee_en (accessed on 27 December 2022).
- Hagelüken, C.; Goldmann, D. Recycling and circular economy—Towards a closed loop for metals in emerging clean technologies. Miner Econ. 2022, 35, 539–562. [Google Scholar] [CrossRef]
- Talens Peiró, L.; Nuss, P.; Mathieux, F.; Blengini, G.A. Towards Recycling Indicators Based on EU Flows and Raw Materials System Analysis Data; JRC Technical Report; EUR 29435 EN; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Žiūkaitė, S.; Ivanauskas, R.; Tatariants, M.; Denafas, G. Feasibilities for hydrometallurgical recovery of precious metals from waste printed circuit boards in Lithuania. Chemija 2017, 28, 109–116. [Google Scholar]
- Tunsu, C.; Retegan, T. Hydrometallurgical processes for the recovery of metals from WEEE. In WEEE Recycling; Chagnes, A., Cote, G., Ekberg, C., Nilsson, M., Retegan, T., Eds.; Elsevier Science BV: Amsterdam, The Netherlands, 2016; pp. 139–175. [Google Scholar]
- Kamarska, K. Citric acid as an eco-friendly inhibitor for the EN AW-2024 aluminum alloy corrosion in acidic medium. J. Ecol. Eng. 2023, 24, 307–311. [Google Scholar] [CrossRef]
- Beukes, N.T.; Badenhorst, J. Copper electrowinning: Theoretical and practical design. In Proceedings of the hydrometallurgy conference SAIMM, Gauteng, South Africa, 24–26 February 2009; pp. 213–240. [Google Scholar]
- Haeger, A.; Forrestal, C.; Xu, P.; Ren, Z.J. High performance spiral wound microbial fuel cell with hydraulic characterization. Bioresour. Technol. 2014, 174, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Rao, S. Hydrometallurgical processes. In Waste Management Series; Ramachandra Rao, S., Ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2006; Volume 7, pp. 71–108. [Google Scholar]
- Vainoris, M.; Cesiulis, H.; Tsyntsaru, N. Metal foam electrode as a cathode for copper electrowinning. Coatings 2020, 10, 822. [Google Scholar] [CrossRef]
- Liu, C.K.; Li, C.W. Simultaneous recovery of copper and surfactant by an electrolytic process from synthetic solution prepared to simulate a concentrate waste stream of a micellar-enhanced ultrafiltration process. Desalination 2004, 169, 185–192. [Google Scholar] [CrossRef]
- Almeida, L.C.; Gasparotto, L.H.S.; Bocchi, N.; Rocha-Filho, R.C.; Biaggio, S.R. Galvanostatic Pb(II) removal from a simulated wastewater by using a stainless-steel wool cathode in a flow-through cell: A factorial-design study. J. Appl. Electrochem. 2008, 38, 167–173. [Google Scholar] [CrossRef]
- Chang, S.H.; Wang, K.S.; Hu, P.I.; Lui, I.C. Rapid recovery of dilute copper from a simulated Cu–SDS solution with low-cost steel wool cathode reactor. J. Hazard. Mater. 2009, 163, 544–549. [Google Scholar] [CrossRef]
- Naumov, K.D.; Lobanov, V.G.; Zelyakh, Y.D. Gold Electrowinning from cyanide solutions using three-dimensional cathodes. Metallurgist 2017, 61, 249–253. [Google Scholar] [CrossRef]
- Lister, T.E.; Wang, P.; Anderko, A. Recovery of critical and value metals from mobile electronics enabled by electrochemical processing. Hydrometallurgy 2014, 149, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Pilone, D.; Kelsall, G.H. Metal recovery from electronic scrap by leaching and electrowinning IV. In Electrometallurgy and environmental hydrometallurgy; Young, C., Alfantazi, A., Anderson, C., James, A., Dreisinger, D., Harris, B., Eds.; Wiley: Hoboken, NJ, USA, 2013; Volume 2, pp. 1565–1575. [Google Scholar]
- Vegliò, F.; Quaresima, R.; Fornari, P.; Ubaldini, S. Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning. Waste Manag. 2003, 23, 245–252. [Google Scholar] [CrossRef]
- Preetam, A.; Modak, A.; Jadhao, P.R.; Naik, S.N.; Pant, K.K.; Kumara, V. A comprehensive study on the extraction of transition metals from waste random access memory using acetic acid as a chelating solvent. J. Environ. Chem. Eng. 2022, 10, 108761. [Google Scholar] [CrossRef]
- Belfqueh, S.; Seron, A.; Chapron, S.; Arrachart, G.; Menad, N. Evaluating organic acids as alternative leaching reagents for rare earth elements recovery from NdFeB magnets. J. Rare Earths 2022, in press. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar]
- Mohammadi, M.; Nakhaie, D.; Asselin, E.; Alfantazi, A. Failure investigation of stainless steel anodes used in gold electrowinning. Eng. Fail. Anal. 2019, 106, 104183. [Google Scholar] [CrossRef]
- Soleymani, M.; Sadri, F.; Zhang, S.; Ghahreman, A. The role of thiosulfate and sulfite in gold thiosulfate electrowinning process: An electrochemical view. Process. Saf. Environ. Prot. 2022, 166, 232–240. [Google Scholar] [CrossRef]
- Kasper, A.C.; Carrillo, A.J.; García Gabaldón, M.; Veit, H.M.; Pérez, H.V. Determination of the potential gold electrowinning from an ammoniacal thiosulphate solution applied to recycling of printed circuit board scraps. Waste Manag. Res. 2016, 34, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Urbanski, T.S.; Fornari, P.; Abbruzzese, C. Gold electrowinning from aqueous–alcoholic thiourea solutions. Hydrometallurgy 2000, 55, 137–152. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Birloaga, I.; Ferella, F.; Centofanti, M.; Vegliò, F. Preliminary study on gold recovery from high grade e-waste by thiourea leaching and electrowinning. Minerals 2021, 11, 235. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, C.; Yliniemi, K.; Lundström, M. Recovery of high-purity silver from spent silver oxide batteries by sulfuric acid leaching and electrowinning. ACS Sustain. Chem. Eng. 2020, 8, 15573–15583. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Bafghi, M.S.; Moradkhani, D.; Ilkhchi, M.O. A review on hydrometallurgical extraction and recovery of cadmium from various resources. Miner. Eng. 2007, 20, 211–220. [Google Scholar] [CrossRef]
- Takemoto, K.; Imori, T.; Ouchi, T.; Takahashi, H. Method For Manufacturing High Purity Tin, Electrowinning Apparatus for High Purity Tin and High Purity Tin. U.S. Patent 20160097139, 2016. Available online: https://www.freepatentsonline.com/y2016/0097139.html (accessed on 27 December 2022).
- Kanazawa, T.; Morimoto, K.; Yamaguchi, I. Tin Electrowinning Method. Japan Patent JP2020117798A, 6 August 2020. [Google Scholar]
- Ishiwatari, M. Method and apparatus for electrolytic purification of high purity tin. Japan Patent JP3882608B2, 21 February 2020. [Google Scholar]
- Guo, X.; Qin, H.; Tian, Q.; Li, D. Recovery of metals from waste printed circuit boards by selective leaching combined with cy-clone electrowinning process. J. Hazard. Mater. 2020, 384, 121355. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, W.Q.; Wang, Z.Y.; Yang, Y.J.-Q.; Zheng, A.-N.; Yang, F.-Z.; Zhan, D.; Wu, D.-Y.; Tian, Z.-Q. Novel, simple, and green citrate-based copper electronic electroplating bath in microvia void-free filling for printed circuit board application. ACS Sustain. Chem. Eng. 2022, 10, 14204–14211. [Google Scholar] [CrossRef]
- Bigos, A.; Wolowicz, M.; Janusz-Skuza, M.; Starowicz, Z.; Szczerba, M.J.; Bogucki, R.; Beltowska-Lehman, E. Citrate-based baths for electrodeposition of nanocrystalline nickel coatings with enhanced hardness. J. Alloys Compd. 2021, 850, 156857. [Google Scholar] [CrossRef]
- Sharma, A.; Min Park, Y.; Lee, S.; Ahn, B. Morphology, resistivity and corrosion behavior of tin coatings plated from citric acid bath. Mater. Res. Express 2019, 6, 116589. [Google Scholar] [CrossRef]
- Halli, P.; Agarwal, V.; Partinen, J.; Lundström, M. Recovery of Pb and Zn from a citrate leach liquor of a roasted EAF dust using precipitation and solvent extraction. Sep. Purif. Technol. 2020, 236, 116264. [Google Scholar] [CrossRef]
- Tsurusaki, T.; Ohgai, T. Mechanical properties of solder-jointed copper rods with electrodeposited Sn-Zn alloy films. Materials 2020, 13, 1330. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Chen, C.; Li, Q.; Yuan, J.; Li, H.; Xue, Y.; Dong, H.; Li, B.; Niu, Y. Process and theoretical research on electroplating Cu–Sn alloys of low Sn. J. Appl. Electrochem. 2021, 51, 1287–1299. [Google Scholar] [CrossRef]
- Guo, F.Y.; Yu, J.K.; Xiao, J.J.; Qiao, Q.; Yang, H.B.; Guo, Y.Q. Preparation of FeCoNiCr high entropy alloy coatings and optimization of process parameters. Rare Metal. Mat. Eng. 2021, 50, 2337–2342. [Google Scholar]
- Deng, D.; Deng, C.; Liu, T.; Xue, D.; Gong, J.; Tan, R.; Mi, X.; Gong, B.; Wang, Z.; Liu, C.; et al. Selective recovery of copper from electroplating sludge by integrated EDTA mixed with citric acid leaching and electrodeposition. Sep. Purif. Technol. 2022, 301, 121917. [Google Scholar] [CrossRef]
- Jadhav, U.; Su, C.; Hocheng, H. Leaching of metals from large pieces of printed circuit boards using citric acid and hydrogen peroxide. Environ. Sci. Pollut. Res. 2016, 23, 24384–24392. [Google Scholar] [CrossRef] [PubMed]








| Composition of g/L | |||||||
|---|---|---|---|---|---|---|---|
| Solution | Tag | Pb | Sn | Cu | Al | Zn | Ni |
| Sn–citric acid | “Ref” | - | 20 | - | - | - | - |
| leachate | “L1” | 1.63 | 20.38 | 0.02 | 0.06 | 0.10 | 0.09 |
| leachate | “L2” | 1.36 | 4.80 | 2.29 | 0.02 | 0.031 | 0.10 |
| Metal | Content of Elements in the Deposit at Various pHs, wt.% | ||
|---|---|---|---|
| 6.5 | 4.3 | 3.2 | |
| Al | 0.26 | 0.18 | 0.31 |
| Cu | 0.18 | 0.46 | 0.52 |
| Pb | 88.3 | 92.0 | 68.4 |
| Sn | 11.23 | 7.40 | 30.8 |
| Metal | Content in the Electrodeposit, wt.% |
|---|---|
| Cu | 88.6 |
| Al | 0.9 |
| Sn | 1.3 |
| Pb | 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesiulis, H.; Tsyntsaru, N. Eco-Friendly Electrowinning for Metals Recovery from Waste Electrical and Electronic Equipment (WEEE). Coatings 2023, 13, 574. https://doi.org/10.3390/coatings13030574
Cesiulis H, Tsyntsaru N. Eco-Friendly Electrowinning for Metals Recovery from Waste Electrical and Electronic Equipment (WEEE). Coatings. 2023; 13(3):574. https://doi.org/10.3390/coatings13030574
Chicago/Turabian StyleCesiulis, Henrikas, and Natalia Tsyntsaru. 2023. "Eco-Friendly Electrowinning for Metals Recovery from Waste Electrical and Electronic Equipment (WEEE)" Coatings 13, no. 3: 574. https://doi.org/10.3390/coatings13030574
APA StyleCesiulis, H., & Tsyntsaru, N. (2023). Eco-Friendly Electrowinning for Metals Recovery from Waste Electrical and Electronic Equipment (WEEE). Coatings, 13(3), 574. https://doi.org/10.3390/coatings13030574








