Performance Assessment of Differently Dried Coating Systems for Potential Application in the Power Transformer Industry
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. IR Drying
3.2. Coating Thickness Measurement
3.3. Salt Spray Test Result
3.4. OCP and EIS Study
3.5. Metallurgical Analysis
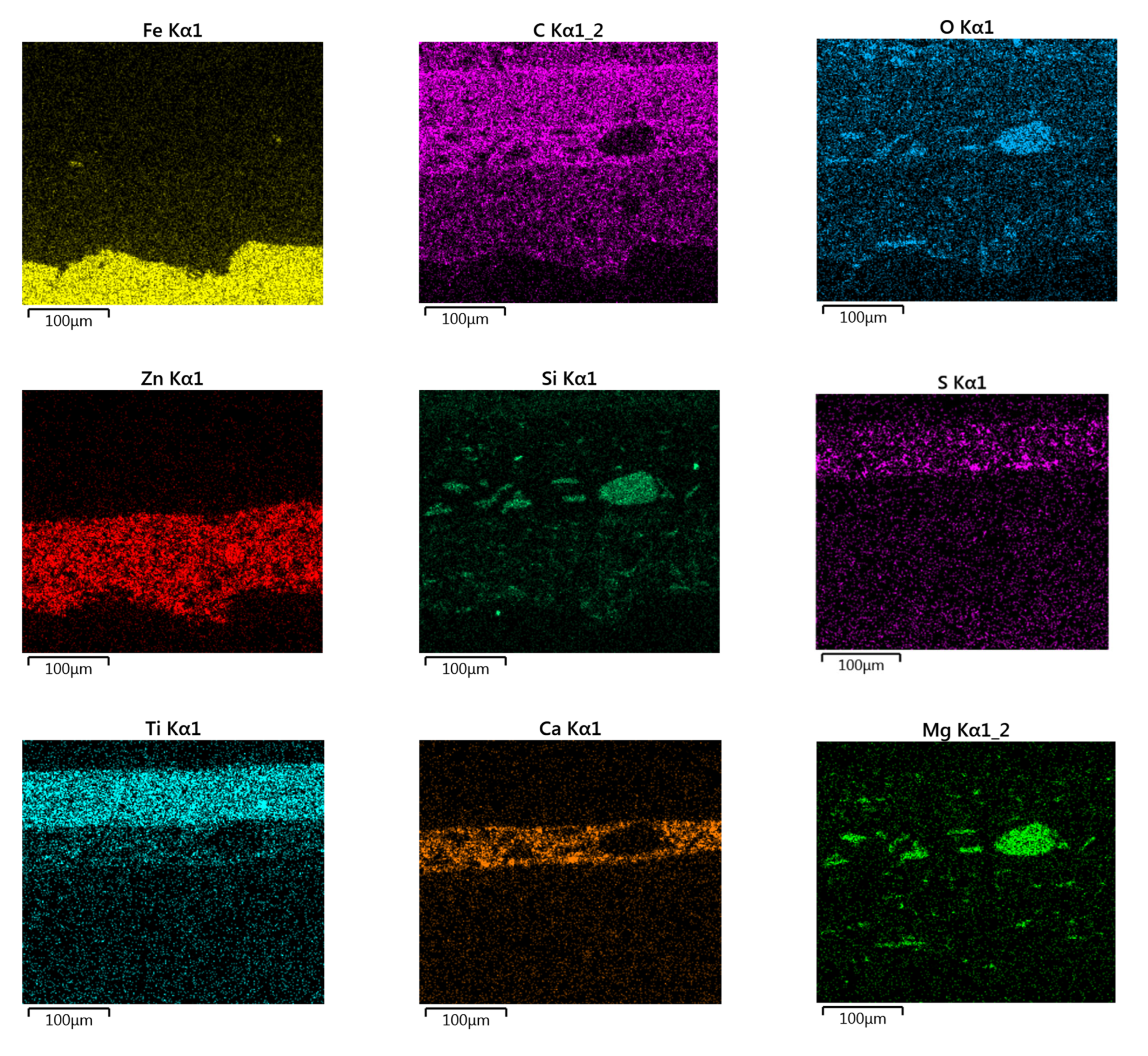
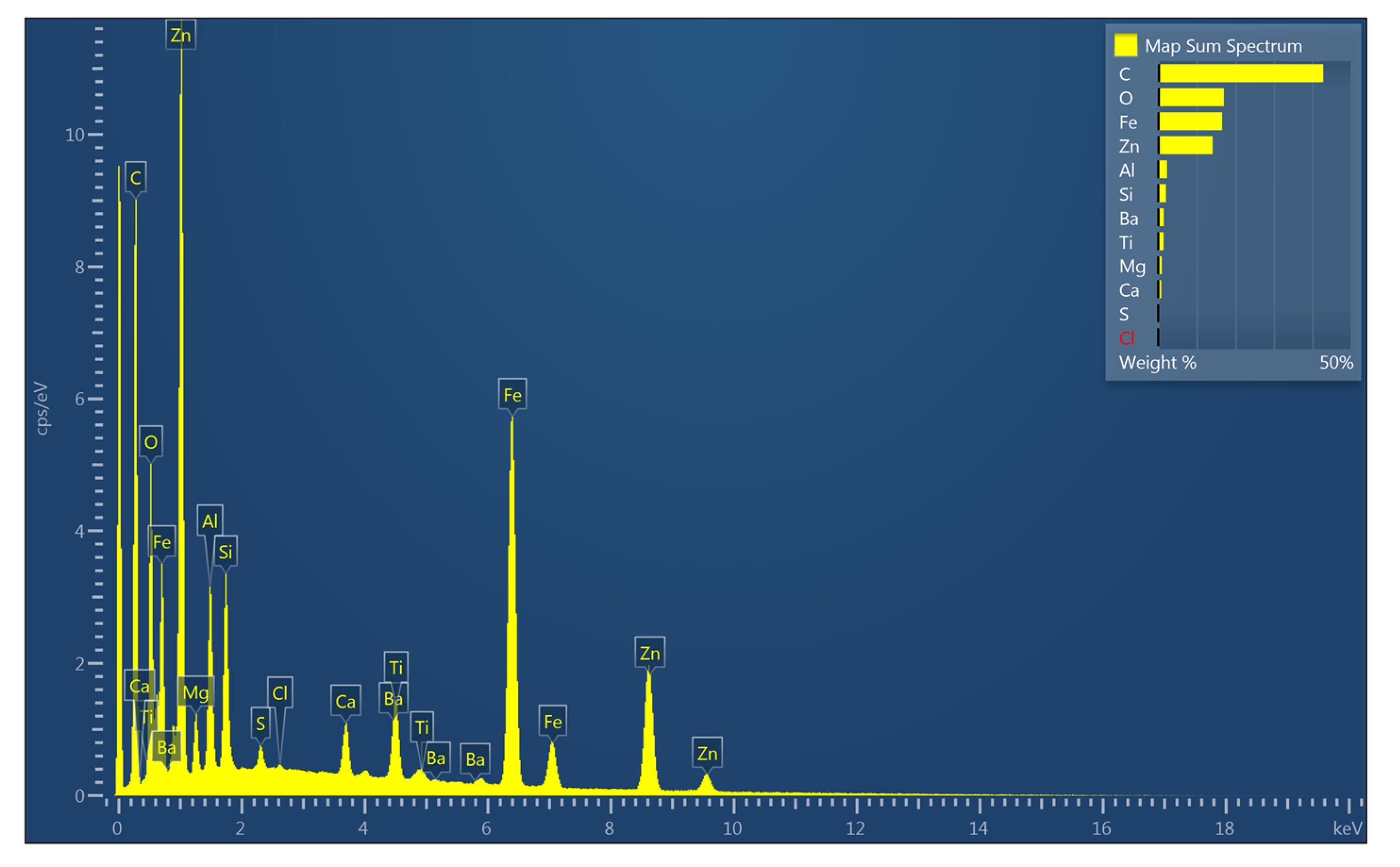
3.6. Morphology and EDX Spectra
4. Conclusions
- Catalytic IR curing technology drastically reduces the intercoating interval between layers and speeds up the anti-corrosive protection process.
- Coatings displayed good adhesion to the steel substrate even after accelerated corrosion test, regardless of the drying method.
- Morphology analysis showed both drying methods provide the coatings uniform thicknesses and evenly distributed elements within layers with no defects.
- The EIS results show that coatings acted as a pure capacitor at the early stages of immersion with very high resistances. Coating capacitance and pore resistance dropped after 10 days of immersion with the formation of a new liquid–metal interface under the coating. Double-layer capacitance was still significantly low for all samples in the observed time, indicating no significant delamination. Bode diagrams showed all coatings had very good resistance even after 500 h of immersion in 3% NaCl, with values exceeding 108 Ωcm2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behzadnasab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Odio, B.O.; Chinwuko, E.C.; Chukwuneke, J.L.; Sinebe, J.E. Investigation of the Effect Of Corrosion On Mild Steel In Five Different Environments. Int. J. Sci. Technol. Res. 2014, 3, 306–310. [Google Scholar]
- López Miguel, A.; Pérez Quiroz, J.T.; Ortega-Borges, R.; Martínez Madrid, M.; Rendón Belmonte, M.; Salgado López, J.M.; Trejo, G.; Meas-Vong, Y. Comparative Study between NiCoB and IrO2-Ta2O5/Ti Anodes for Application in Impressed Current Cathodic Protection (ICCP). Coatings 2020, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Reis, S.T.; Chen, G.; Koenigstein, M.L. Corrosion Resistance of Pipeline Steel with Damaged Enamel Coating and Cathodic Protection. Coatings 2018, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Alibakhshi, E.; Akbarian, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. Evaluation of the corrosion protection performance of mild steel coated with hybrid sol-gel silane coating in 3.5 wt.% NaCl solution. Prog. Org. Coat. 2018, 123, 190–200. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Sun, W.; Li, S.; Zhu, T.; Liu, W.; Liu, G. Superhydrophobic epoxy coating modified by fluorographene used for anti-corrosion and self-cleaning. Appl. Surf. Sci. 2017, 401, 146–155. [Google Scholar] [CrossRef]
- Taghavikish, M.; Dutta, N.K.; Roy Choudhury, N. Emerging Corrosion Inhibitors for Interfacial Coating. Coatings 2017, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Lyon, S.B.; Bingham, R.; Mills, D.J. Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Stojanović, I.; Šimunović, V.; Alar, V.; Kapor, F. Experimental Evaluation of Polyester and Epoxy–Polyester Powder Coatings in Aggressive Media. Coatings 2018, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Cao, J.; Gao, L.; Yan, C. A Mechanistic Study of Corrosion of Graphene and Low zinc- rich Epoxy Coatings on Carbon Steel in Salt Environment. Int. J. Electrochem. Sci. 2019, 14, 9671–9681. [Google Scholar] [CrossRef]
- Xing, C.; Wang, W.; Qu, S.; Tang, Y.; Zhao, X.; Zuo, Y. Degradation of zinc-rich epoxy coating in 3.5% NaCl solution and evolution of its EIS parameters. J. Coat. Technol. Res. 2021, 18, 843–860. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Park, K.C. Study on corrosive electrochemical behaviors of zinc-rich and graphite-filled epoxy coatings in 3.5 wt% NaCl solution. Mater. Corros. 2011, 62, 1008–1014. [Google Scholar] [CrossRef]
- Schmitz, C.; Strehmel, B. NIR LEDs and NIR lasers as feasible alternatives to replace oven processes for treatment of thermal-responsive coatings. J. Coat. Technol. Res. 2019, 16, 1527–1541. [Google Scholar] [CrossRef]
- Putranto, A.; Chen, X.D.; Webley, P.A. Infrared and convective drying of thin layer of polyvinyl alcohol (PVA)/glycerol/water mixture—The reaction engineering approach (REA). Chem. Eng. Process. 2010, 49, 348–357. [Google Scholar] [CrossRef]
- ISO 8501-1; Preparation of Steel Substrates before Application of Paints and Related Products—Visual Assessment of Surface Cleanliness—Part 1: Rust Grades and Preparation Grades of Uncoated Steel Substrates and of Steel Substrates after Overall Removal of Previous Coatings. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 8503-1; Preparation of Steel Substrates before Application of Paints and Related Products—Surface Roughness Characteristics of Blast-Cleaned Steel Substrates—Part 1: Specifications and Definitions for ISO Surface Profile Comparators for the Assessment of Abrasive Blast-Cleaned Surfaces. International Organization for Standardization: Geneva, Switzerland, 2012.
- Inone, P.C.; Garcia, C.M.; Rúvolo-Filho, A. Evaluating barrier properties of organic coatings by water permeation and electrochemical methods. J. Coat. Technol. 2003, 75, 29–36. [Google Scholar] [CrossRef]
- ISO 2808; Paints and Varnishes—Determination of Film Thickness. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 4624; Paints and Varnishes—Pull-Off Test for Adhesion. International Organization for Standardization: Geneva, Switzerland, 2016.
- Šoić, I.; Martinez, S.; Dubravić, M. Gel-Electrolyte EIS setup used for probing of IR Dried/Cured industrial coatings. Prog. Org. Coat. 2019, 137, 105331. [Google Scholar] [CrossRef]
- ISO 9227; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 4628; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and Intensity of Uniform Changes in Appearance. International Organization for Standardization: Geneva, Switzerland, 2016.
- Cottis, R.A. 2.30—Electrochemical methods. Shreir’s Corros. 2010, 2, 1341–1373. [Google Scholar]
- ISO 12944-5; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 5: Protective Paint Systems. International Organization for Standardization: Geneva, Switzerland, 2019.
- Chaudhari, S.; Sainkar, S.R.; Patil, P.P. Poly(o-ethylaniline) coatings for stainless steel protection. Prog. Org. Coat. 2007, 58, 54–63. [Google Scholar] [CrossRef]
- Vida, T.A.; Freitas, E.S.; Cheung, N.; Garcia, A.; Osorió, W.R. Electrochemical Corrosion Behavior of as-cast Zn-rich Zn-Mg Alloys in a 0.06M NaCl Solution. Int. J. Electrochem. Sci. 2017, 12, 5264–5283. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Bonatti, R.S.; Bortolozo, A.D.; Osorió, W.R. Electrochemical behavior and compressive strength of Al-Cu/xCu composites in NaCl solution. J. Solid State Electrochem. 2021, 25, 1303–1317. [Google Scholar] [CrossRef]
- Snoke, J. Qualification of Protective Coatings using EIS. In Proceedings of the Proceedings Eurocorr 2008, Edinburgh, UK, 7–11 September 2008. [Google Scholar]
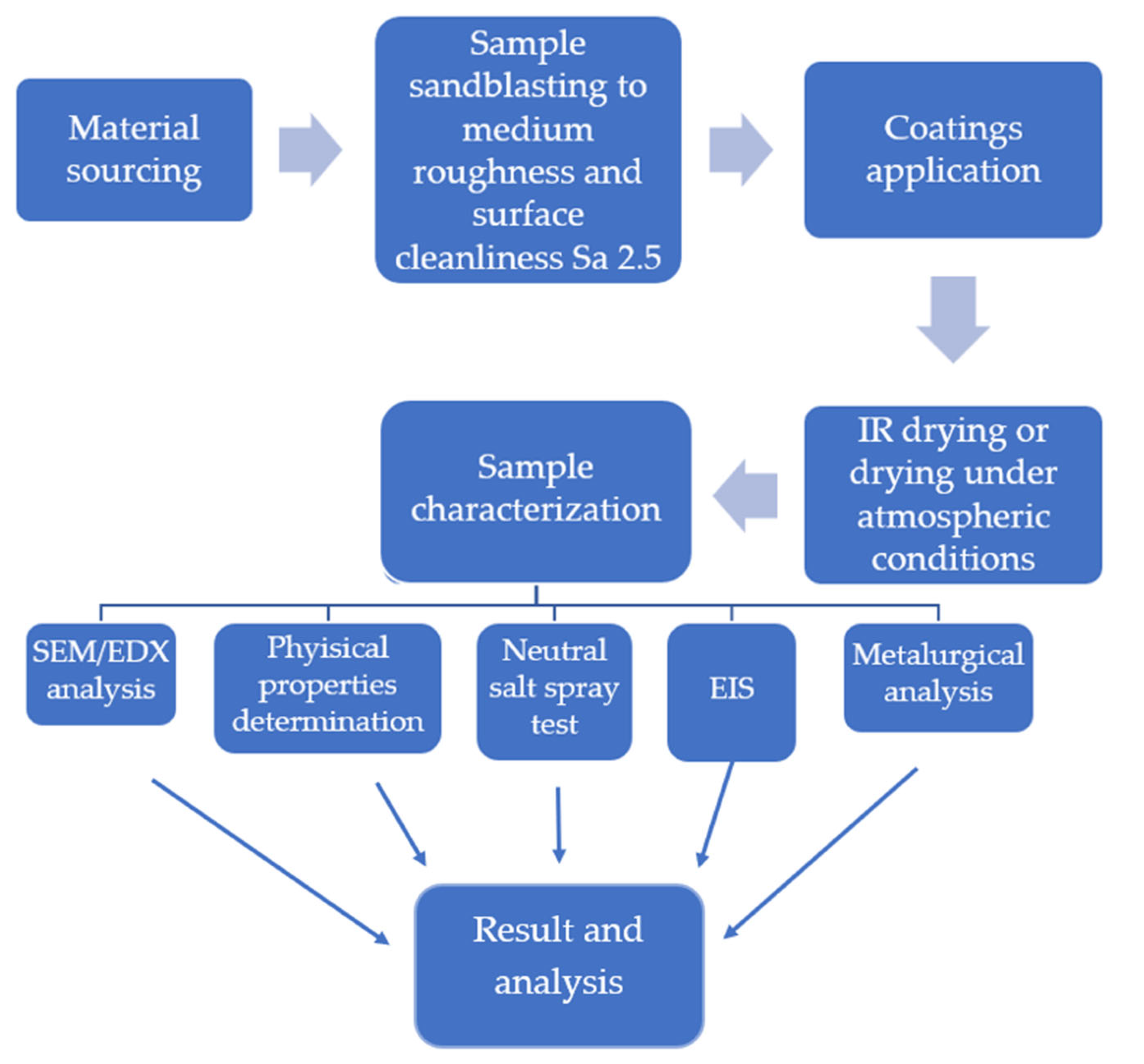





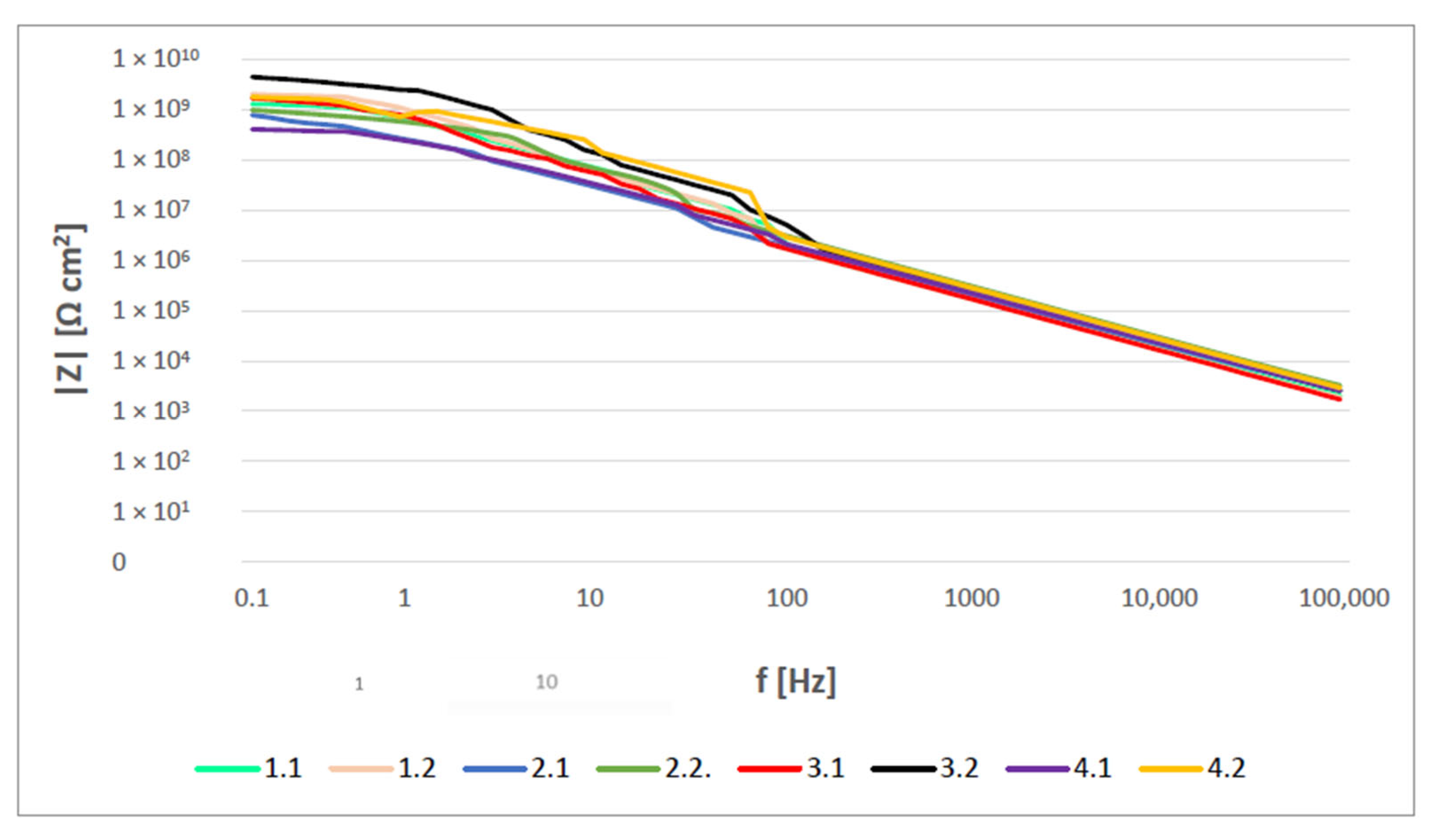
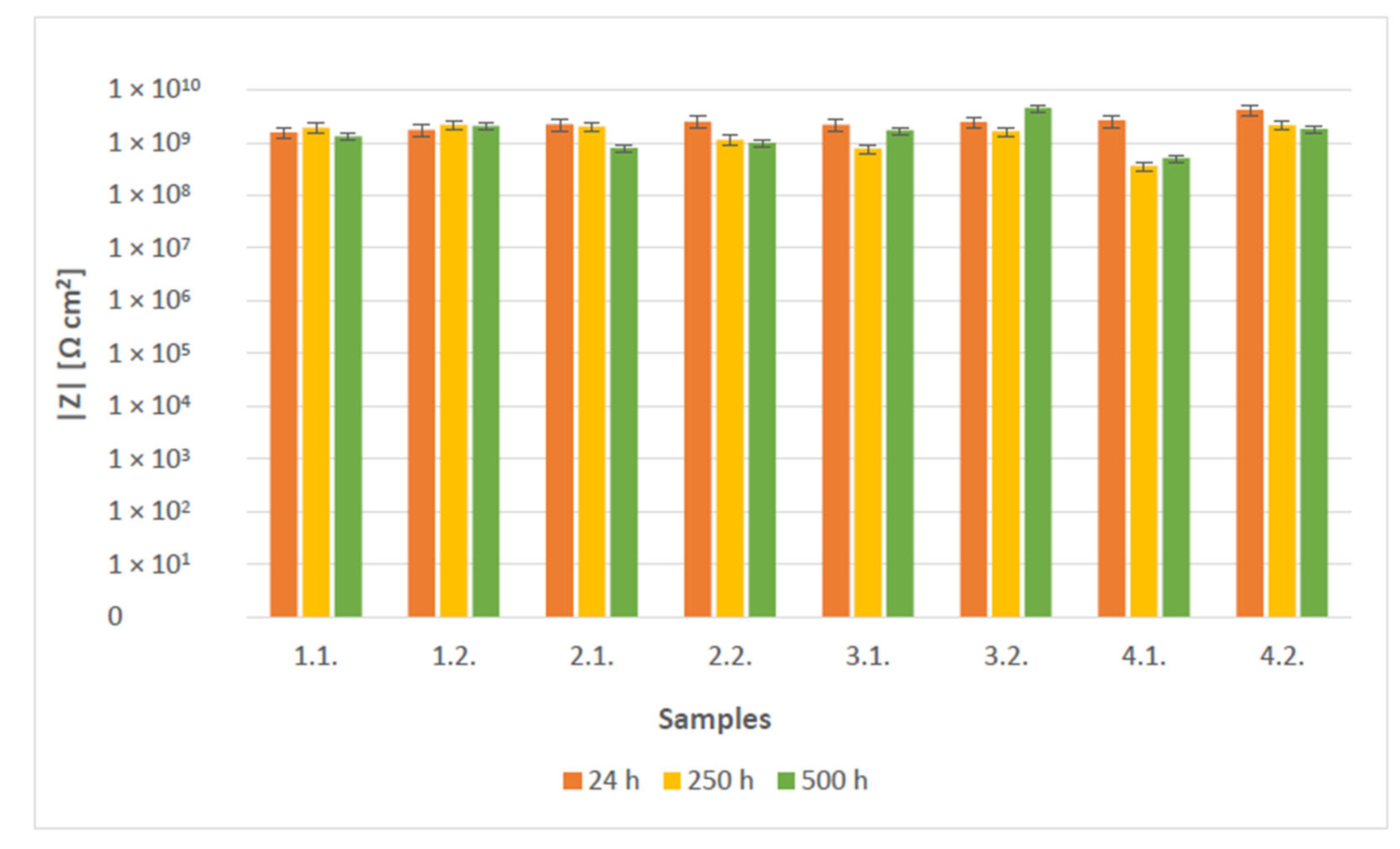



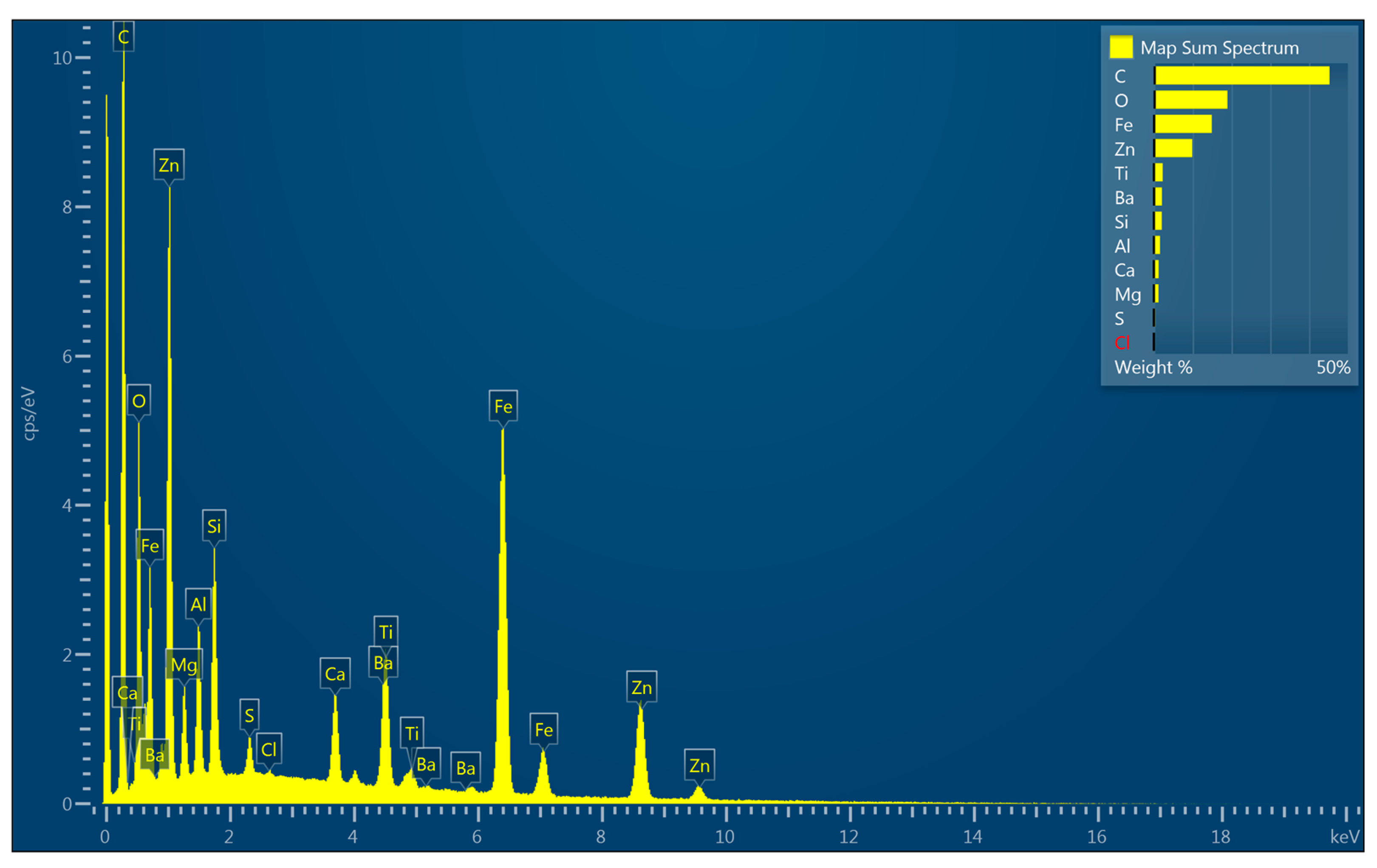

| Coating | Gloss | Solids by Volume [Vol. %] | Recommended Thickness [µm] | Minimum Overcoating Interval at 20 °C |
|---|---|---|---|---|
| Primer: Zn (R) | Mat | 51 ± 3 | 70–80 | 6–8 h |
| Primer: Epoxy | Mat | 59 | 60–100 | 6–8 h |
| Intermediate: Epoxy | Mat | 73 ± 3 | 80–140 | 2 h |
| Topcoat: Polyurethane | Mat | 64 ± 3 | 80–100 | 5 h |
| Coating | Gloss | Solids by Volume [Vol. %] | Recommended Thickness [µm] | Minimum Overcoating Interval at 20 °C |
|---|---|---|---|---|
| Primer: Zn (R) | Mat | 65 ± 2 | 40–100 | 45 min |
| Primer/Intermediate: Epoxy | Mat | 85 ± 2 | 100–250 | 3 h |
| Topcoat: Polyurethane | Glossy | 67 ± 2 | 50–125 | 6 h |
| Manufacturer | Sample | Applicated Coating Systems (Primer–Intermediate–Topcoat) | Drying Method |
|---|---|---|---|
| A | 1.1 | Zn(R) EP-EP-PUR | Atm. |
| 1.2 | Zn(R) EP-EP-PUR | IR | |
| 2.1 | EP-EP-PUR | Atm. | |
| 2.2 | EP-EP-PUR | IR | |
| B | 3.1 | Zn(R) EP-EP-PUR | Atm. |
| 3.2 | Zn(R) EP-EP-PUR | IR | |
| 4.1 | EP-EP–PUR | Atm. | |
| 4.2 | EP-EP-PUR | IR |
| Samples | DFTmean | σDFT | Rusting | Cracking | Flaking | Blistering | Pull off [MPa] |
|---|---|---|---|---|---|---|---|
| [µm] | ISO 4628-3 | ISO 4628-4 | ISO 4628-5 | ISO 4628-2 | |||
| 1.1 | 256 (±2.5) | 24.8 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 8.7 (±0.4) |
| 1.2 | 249 (±2.5) | 21.3 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 10.4 (±0.4) |
| 2.1 | 305 (±2.5) | 11.3 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 11.4 (±0.4) |
| 2.2 | 286 (±2.5) | 22.2 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 9.6 (±0.4) |
| 3.1 | 207 (±2.5) | 11.8 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 11.8 (±0.4) |
| 3.2 | 210 (±2.5) | 11.4 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 10.7 (±0.4) |
| 4.1 | 248 (±2.5) | 5.64 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 9.7 (±0.4) |
| 4.2 | 251 (±2.5) | 9.11 | Ri 0 | 0(S0) | 0(S0) | 0(S0) | 11.3 (±0.4) |
| Sample | DFTmean [µm] | σDFT | Ecorr vs. SCE (V) | ||
|---|---|---|---|---|---|
| 24 h | 250 h | 500 h | |||
| 1.1 | 248 (±2.5) | 21.2 | −0.405 | −0.408 | −0.411 |
| 1.2 | 244 (±2.5) | 12.9 | −0.252 | −0.250 | −0.243 |
| 2.1 | 272 (±2.5) | 15.3 | −0.217 | −0.149 | −0.110 |
| 2.2 | 281 (±2.5) | 24.1 | −0.115 | −0.160 | −0.139 |
| 3.1 | 189 (±2.5) | 2.67 | −0.230 | −0.296 | −0.548 |
| 3.2 | 188 (±2.5) | 5.38 | +0.889 | +0.584 | −0.432 |
| 4.1 | 226 (±2.5) | 4.52 | −0.235 | −0.164 | −0.181 |
| 4.2 | 228 (±2.5) | 7.6 | −0.352 | −0.151 | −0.053 |
| Sample | RS, (102 Ωcm2) | nc | CC, (10−10 Fcm2) | RC, (109 Ωcm2) | χ2 |
|---|---|---|---|---|---|
| 1.1. | 7.631 | - | 9.309 | 1.622 | 5.67 × 10−2 |
| 1.2. | 6.527 | - | 9.107 | 1.947 | 5.01 × 10−2 |
| 2.1. | 7.377 | - | 6.113 | 1.905 | 4.64 × 10−2 |
| 2.2. | 9.532 | - | 5.778 | 2.706 | 6.74 × 10−2 |
| 3.1. | 5.762 | - | 7.689 | 2.259 | 7.99 × 10−2 |
| 3.2. | 5.468 | - | 6.597 | 2.513 | 4.52 × 10−2 |
| 4.1. | 7.044 | - | 7.214 | 2.607 | 9.97 × 10−2 |
| 4.2. | 1.254 | - | 8.096 | 3.862 | 7.17 × 10−2 |
| Sample | RS, (102 Ωcm2) | nc | CC/CPEC, (10−10 Fcm2) | RC, (105 Ωcm2) | ndl | Cdl, (10−10 Fcm2) | Rct, (109 Ωcm2) | χ2 |
|---|---|---|---|---|---|---|---|---|
| 1.1. | 4.494 | - | 7.390 | 0.504 | - | 3.012 | 1.678 | 6.764 × 10−2 |
| 1.2. | 4.186 | - | 8.590 | 0.499 | - | 3.131 | 1.377 | 4.693 × 10−2 |
| 2.1. | 7.989 | 0.96 | 0.108 | 4452 | - | 0.109 | 0.889 | 5.169 × 10−2 |
| 2.2. | 8.754 | - | 5.436 | 9598 | - | 5.497 | 0.906 | 1.374 × 10−2 |
| 3.1. | 0.475 | - | 6.750 | 2446 | - | 0.114 | 1.130 | 7.98 × 10−2 |
| 3.2. | 3.324 | - | 5.912 | 1.535 | - | 1.120 | 2.723 | 5.38 × 10−2 |
| 4.1. | 4.984 | 0.9655 | 6.144 | 3.105 | - | 2.469 | 0.413 | 5.26 × 10−2 |
| 4.2. | 4.807 | - | 5.545 | 6423 | - | 9.448 | 1.244 | 6.04 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, I.; Cindrić, I.; Janković, L.; Šimunović, V.; Franjić, H. Performance Assessment of Differently Dried Coating Systems for Potential Application in the Power Transformer Industry. Coatings 2022, 12, 331. https://doi.org/10.3390/coatings12030331
Stojanović I, Cindrić I, Janković L, Šimunović V, Franjić H. Performance Assessment of Differently Dried Coating Systems for Potential Application in the Power Transformer Industry. Coatings. 2022; 12(3):331. https://doi.org/10.3390/coatings12030331
Chicago/Turabian StyleStojanović, Ivan, Ivan Cindrić, Lara Janković, Vinko Šimunović, and Hrvoje Franjić. 2022. "Performance Assessment of Differently Dried Coating Systems for Potential Application in the Power Transformer Industry" Coatings 12, no. 3: 331. https://doi.org/10.3390/coatings12030331
APA StyleStojanović, I., Cindrić, I., Janković, L., Šimunović, V., & Franjić, H. (2022). Performance Assessment of Differently Dried Coating Systems for Potential Application in the Power Transformer Industry. Coatings, 12(3), 331. https://doi.org/10.3390/coatings12030331






