Synthesis of the Porous ZnO Nanosheets and TiO2/ZnO/FTO Composite Films by a Low-Temperature Hydrothermal Method and Their Applications in Photocatalysis and Electrochromism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 2D Porous ZnO Nanosheets
2.2. Preparation of ZnO/FTO Composite Film
2.3. The Experimental Procedure of Preparing TiO2/ZnO Composite Film
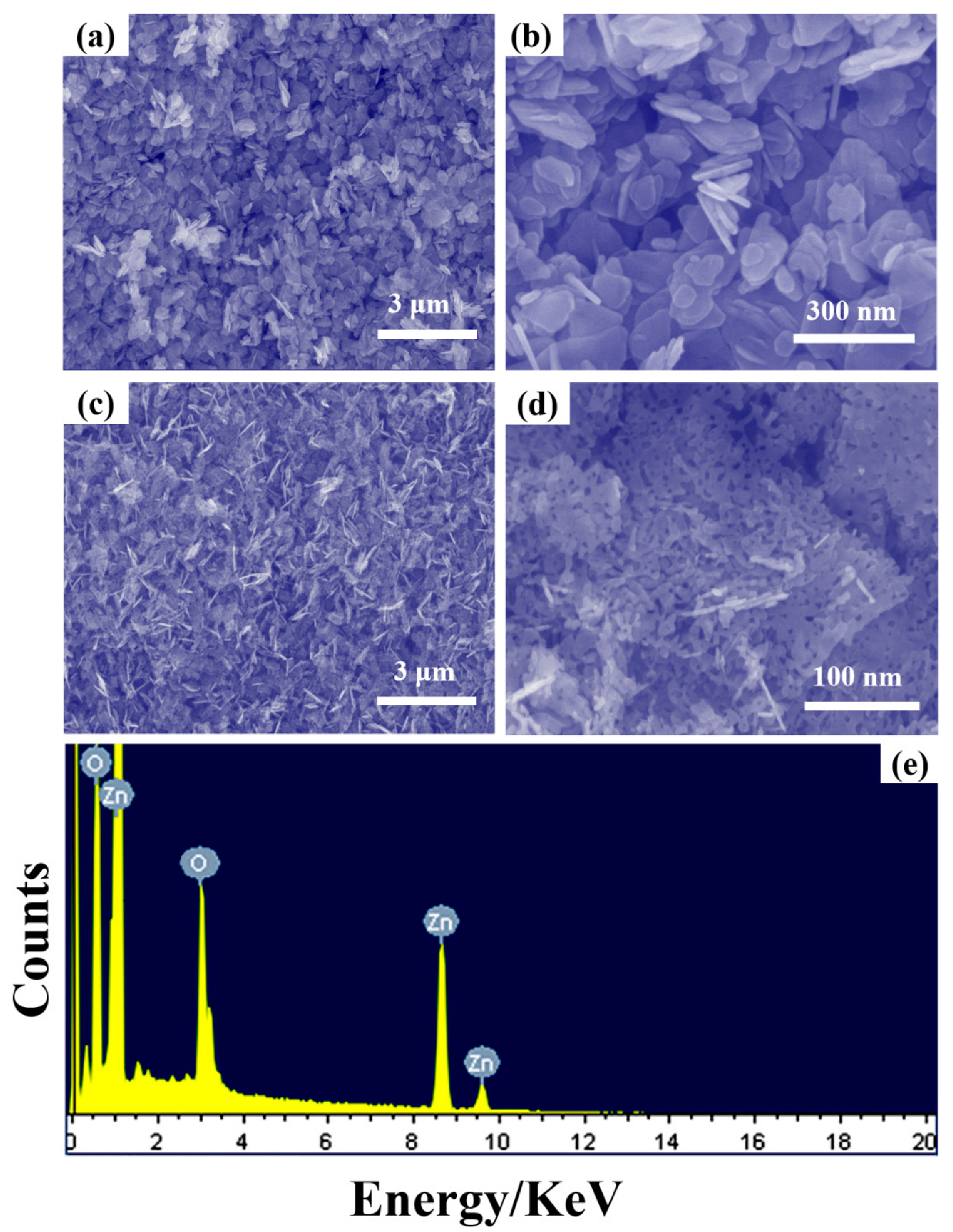
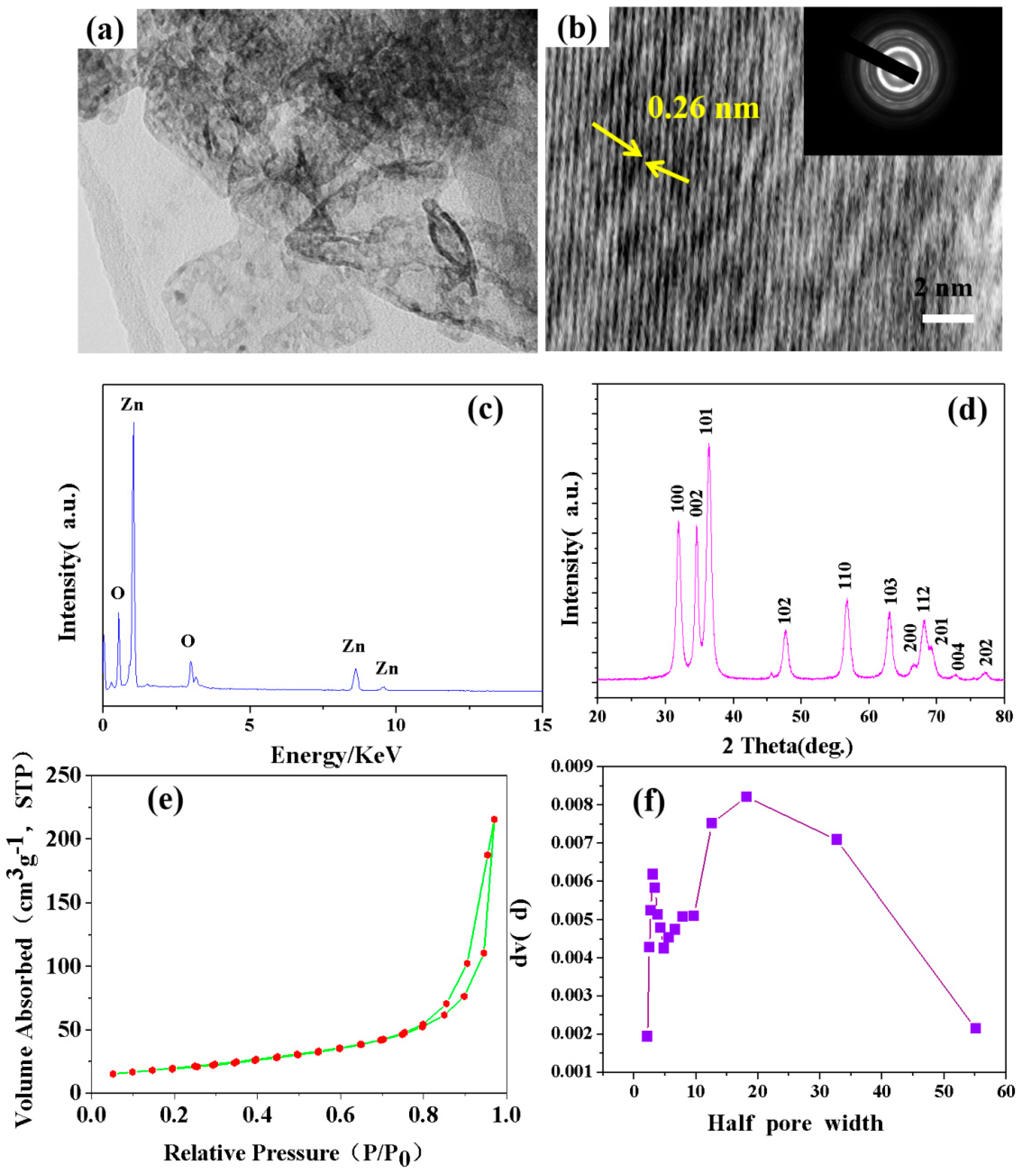
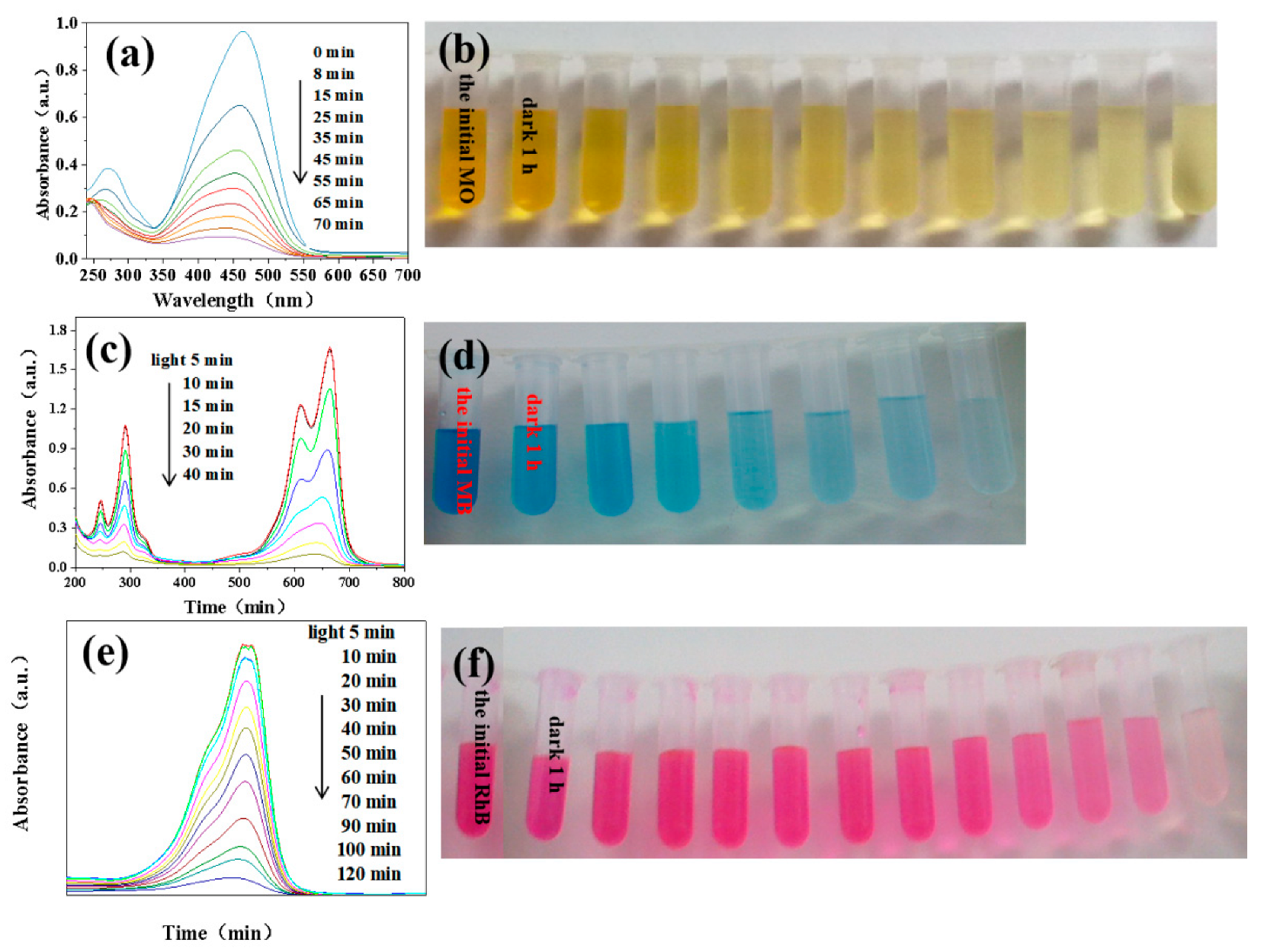
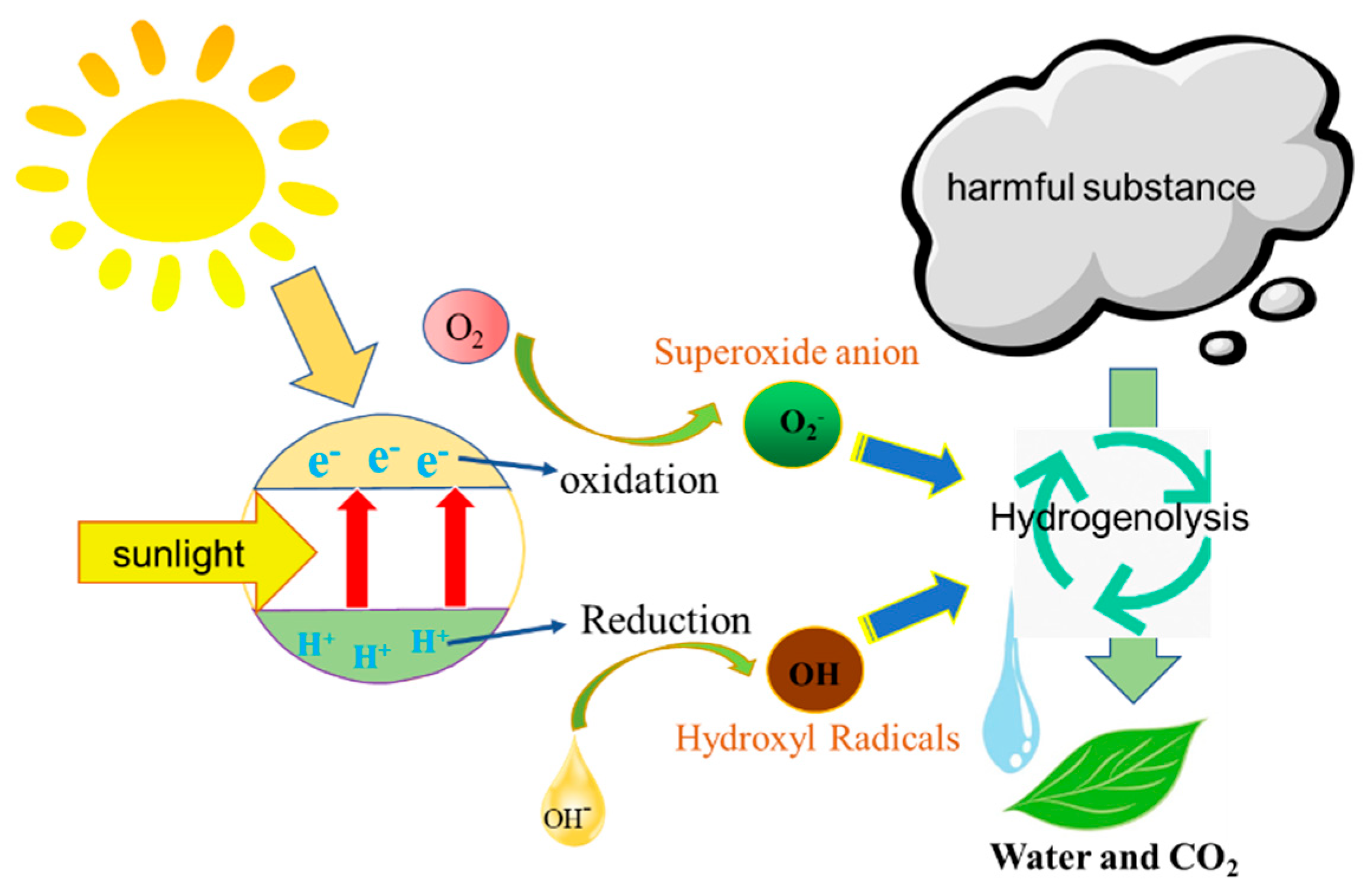
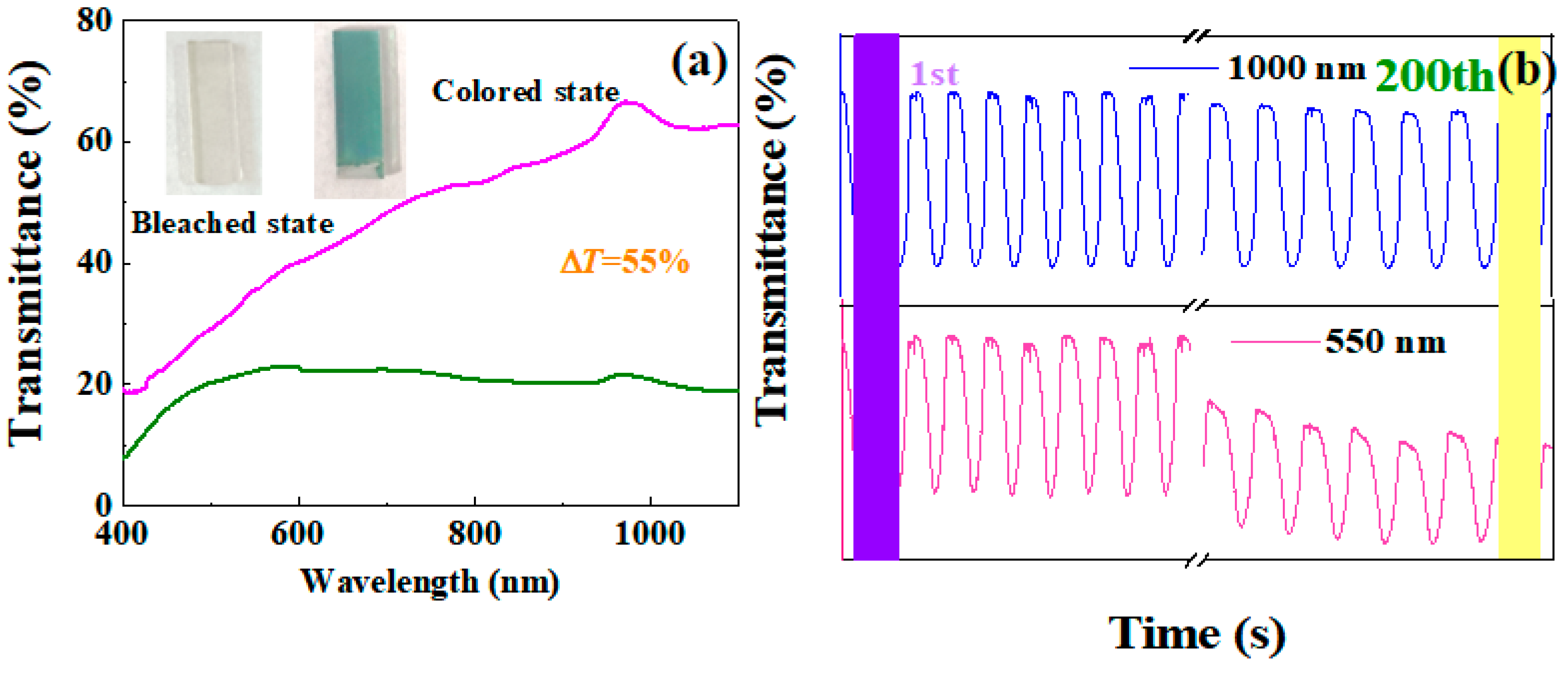
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sher, F.; Kawai, A.; Güleç, F.; Sadiq, H. Sustainable energy saving alternatives in small buildings. Sustain. Energy Technol. Assess. 2019, 32, 92–99. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Griffiths, S. The cultural barriers to a low-carbon future: A review of six mobility and energy transitions across 28 countries. Renew. Sustain. Energy Rev. 2020, 119, 109569. [Google Scholar] [CrossRef]
- Fiory, A. Recent developments in rapid thermal processing. J. Electron. Mater. 2002, 31, 981–987. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, A.; Krishnan, V. Nanoscale zinc oxide based heterojunctions as visible light active photocatalysts for hydrogen energy and environmental remediation. Catal. Rev. 2020, 62, 346–405. [Google Scholar] [CrossRef]
- Saravanan, R.; Agarwal, S.; Gupta, V.K.; Khan, M.M.; Gracia, F.; Mosquera, E.; Narayanan, V.; Stephen, A. Line defect Ce3+ induced Ag/CeO2/ZnO nanostructure for visible-light photocatalytic activity. J. Photochem. Photobiol. A 2018, 353, 499–506. [Google Scholar] [CrossRef]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Jońca, J.; Kahn, M.; Fajerwerg, K.; Chaudret, B.; Chapelle, A.; Ménini, P.; Shim, C.H.; Gaudon, A.; Fau, P. Organometallic synthesis of ZnO nanoparticles for gas sensing: Towards selectivity through nanoparticles morphology. J. Nanopart. Res. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and characterization of efficient ZnO/g-C3N4 nanocomposites photocatalyst for photocatalytic degradation of methylene blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Lu, J.; Ali, H.; Hurh, J.; Han, Y.; Batjikh, I.; Rupa, E.J.; Anandapadmanaban, G.; Park, J.K.; Yang, D.-C. The assessment of photocatalytic activity of zinc oxide nanoparticles from the roots of Codonopsis lanceolata synthesized by one-pot green synthesis method. Optik 2019, 184, 82–89. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Akbar, M.B. Highly efficient g-C3N4/Cr-ZnO nanocomposites with superior photocatalytic and antibacterial activity. J. Photochem. Photobiol. A 2020, 401, 112776. [Google Scholar] [CrossRef]
- Ali, R.S.; Sharba, K.S.; Jabbar, A.M.; Chiad, S.S.; Abass, K.H.; Habubi, N.F. Characterization of ZnO thin film/p-Si fabricated by vacuum evaporation method for solar cell applications. NeuroQuantology 2020, 18, 26. [Google Scholar] [CrossRef]
- Yang, S.H.; Yang, J.-H. Enhancement on electrochromic properties of WO3-based electrode prepared with hierarchical ZnO nanobricks. Vacuum 2020, 179, 109460. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Hao, T.; Xu, M.; Xue, J.; Zhao, J.; Li, Y. 3D conifer-like WO3 branched nanowire arrays electrode for boosting electrochromic-supercapacitor performance. Appl. Surf. Sci. 2022, 577, 151889. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Hao, T.; Wang, P.; Zhang, X.; Zhang, H.; Xue, J.; Zhao, J.; Li, Y. In situ XRD and operando spectra-electrochemical investigation of tetragonal WO3-x nanowire networks for electrochromic supercapacitors. Npg Asia Mater. 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Zhao, J.; Li, Y. Two-dimensional WO3 nanosheets for high-performance electrochromic supercapacitors. Inorg. Chem. Front. 2022, 9, 514–523. [Google Scholar] [CrossRef]
- Mahmud, R.A.; Shafawi, A.N.; Ali, K.A.; Putri, L.K.; Rosli, N.I.M.; Mohamed, A.R. Graphene nanoplatelets with low defect density as a synergetic adsorbent and electron sink for ZnO in the photocatalytic degradation of Methylene Blue under UV–vis irradiation. Mater. Res. Bull. 2020, 128, 110876. [Google Scholar] [CrossRef]
- Qu, Y.; Huang, R.; Qi, W.; Shi, M.; Su, R.; He, Z. Controllable synthesis of ZnO nanoflowers with structure-dependent photocatalytic activity. Catal. Today. 2020, 355, 397–407. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Cheng, Z.; Luo, S.; Nguyen, T.T.; Guo, M.; Gao, X. Promoting effect of cellulose-based carbon dots at different concentrations on multifunctional photocatalytic degradation of dyes by ZnO. Opt. Mater. 2021, 121, 111591. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Mane, R.S.; Min, B.K.; Hwang, Y.J.; Joo, O.S. D-sorbitol-induced phase control of TiO2 nanoparticles and its application for dye-sensitized solar cells. Sci. Rep. 2016, 6, 20103. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Shaikh, S.F.; Tamboli, A.M.; Marium, A.; Ijaz, M.F.; Ubaidullah, M.; Moydeen Abdulhameed, M.; Ekar, S.U. Hybrid ZnO Flowers-Rods Nanostructure for Improved Photodetection Compared to Standalone Flowers and Rods. Coatings 2021, 11, 1464. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Raizada, P.; Thakur, V.K.; Saini, A.K.; Mittal, D.; Nguyen, V.-H.; Ahamad, T.; Nguyen, C.C.; Kim, S.Y.; et al. Synergistic photocatalytic dye mitigation and bacterial disinfection using Carbon quantum dots decorated dual Z-scheme Manganese Indium Sulfide/Cuprous Oxide/Silver oxide heterojunction. Mater. Lett. 2022, 313, 131716. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Ruksana; Alhabarah, A.N.; Alshehri, S.M. N/S doped highly porous magnetic carbon aerogel derived from sugarcane bagasse cellulose for the removal of bisphenolA. Int. J. Biol. Macromol. 2019, 132, 1031–1038. [Google Scholar] [CrossRef]
- Reddy, I.N.; Reddy, C.V.; Shim, J.; Akkinepally, B.; Cho, M.; Yoo, K.; Kim, D. Excellent visible-light driven photocatalyst of (Al, Ni) co-doped ZnO structures for organic dye degradation. Catal. Today 2020, 340, 277–285. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, J. Cerium modified rod-like ZnO graphene complex and its photocatalytic properties under visible-light irradiation. Opt. Mater. 2020, 108, 110203. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloy. Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Kegel, J.; Povey, I.M.; Pemble, M.E. Zinc oxide for solar water splitting: A brief review of the material’s challenges and associated opportunities. Nano Energy 2018, 54, 409–428. [Google Scholar] [CrossRef]
- Mandal, S.K.; Paul, S.; Datta, S.; Jana, D. Nitrogenated CQD decorated ZnO nanorods towards rapid photodegradation of rhodamine B: A combined experimental and theoretical approach. Appl. Surf. Sci. 2021, 563, 150315. [Google Scholar] [CrossRef]
- Shah, A.A.; Chandio, A.D.; Sheikh, A.A. Boron doped ZnO nanostructures for photo degradation of methylene blue, methyl orange and rhodamine B. J. Nanosci. Nanotechno. 2021, 21, 2483–2494. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and enhanced photocatalytic activity of zinc oxide nanoparticles toward organosulfur pollutants. Sci. Rep.-UK 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, J.; Rawat, M. Green synthesis of zinc oxide nanoparticles using Punica Granatum leaf extract and its application towards photocatalytic degradation of Coomassie brilliant blue R-250 dye. SN Appl. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Abebe, B.; Nagaswarupa, H.; Murthy, H.; Ravikumar, C.; Sabir, F.K. Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: Its applications in dye decolorization and as supercapacitors. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Hunge, Y.; Kulkarni, S. Synthesis of multifunctional FeCo2O4 electrode using ultrasonic treatment for photocatalysis and energy storage applications. Ultrason. Sonochem. 2019, 58, 104663. [Google Scholar] [CrossRef]
- Bantawal, H.; Sethi, M.; Shenoy, U.S.; Bhat, D.K. Porous graphene wrapped SrTiO3 nanocomposite: Sr–C bond as an effective coadjutant for high performance photocatalytic degradation of methylene blue. ACS Appl. Nano Mater. 2019, 2, 6629–6636. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dhiman, P.; Vo, D.-V.N.; Stadler, F.J. Fe3O4/ZnO/Si3N4 nanocomposite based photocatalyst for the degradation of dyes from aqueous solution. Mater. Lett. 2020, 278, 128359. [Google Scholar] [CrossRef]
- Ma, H.; Hao, B.; Song, W.; Guo, J.; Li, M.; Zhang, L. A High-Efficiency TiO2/ZnO Nano-Film with Surface Oxygen Vacancies for Dye Degradation. Materials 2021, 14, 3299. [Google Scholar] [CrossRef]
- Ju, X.; Yang, F.; Zhu, X.; Jia, X. Zinc ion intercalation/deintercalation of metal organic framework-derived nanostructured NiO@ C for low-transmittance and high-performance electrochromism. ACS Sustain. Chem. Eng. 2020, 8, 12222–12229. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.A.; Zhang, D.; Tong, Z.; Ji, H.; Qu, H.Y. Zn2+ intercalation/de-intercalation-based aqueous electrochromic titanium dioxide electrode with Zn-ion storage. Ionics 2021, 27, 4429–4437. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Han, G.; Zhao, H. Controllable synthesis of hexagonal WO3 nanorod-cluster films with high electrochromic performance in NIR range. J. Alloy. Compd. 2022, 890, 161833. [Google Scholar] [CrossRef]
- Ling, H.; Yeo, L.P.; Wang, Z.; Li, X.; Mandler, D.; Magdassi, S.; Tok, A.I.Y. TiO2–WO3 core–shell inverse opal structure with enhanced electrochromic performance in NIR region. J. Mater. Chem. C 2018, 6, 8488–8494. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, C.; Xu, G.; Tan, S.; Zhang, J. Amorphous titanium dioxide film with improved electrochromism in near-infrared region. Opt. Mater. 2019, 89, 191–196. [Google Scholar] [CrossRef]




| Material | Dye | Degradation Efficiency | Cycle Stability | Ref |
|---|---|---|---|---|
| ZnO NPS | DBT | 95% (190 min) | 93% (5 cycles) | [32] |
| ZnO NPS | R-250 | 93% (180 min) | [33] | |
| TiO2 -Fe2O3 | Titan Yellow | 92.98% (60 min) | [34] | |
| FeCo2O4 | Crystal Violet | 94.19% (160 min) | 92.06% (5 cycles) | [35] |
| SrTiO3 | MB | 92% (120 min) | [36] | |
| Fe3O4/ZnO/Si3N4 | MO | 96% (80 min) | 90% (6 cycles) | [37] |
| Fe3O4/ZnO/Si3N4 | Sunset Yellow | 90% (80 min) | 90% (6 cycles) | [37] |
| ZnO/TiO2 | RhB | 90.8% (120 min) | [38] | |
| ZnO | MB | 99% (40 min) | 99% (20 cycles) | this paper |
| ZnO | MO | 97.7% (90 min) | this paper | |
| TiO2/ZnO | RhB | 96.8% (120 min) | this paper |
| Material | Wavelength (nm) | Transmittance | Retention | References |
|---|---|---|---|---|
| NiO@C | 1200 | 35% | [39] | |
| TiO2 | 980 | 45% | 93% (100 cycles) | [40] |
| NiO | 650 | 41.08% | 86% (100 cycles) | [13] |
| WO3 | 1600 | 46% | 96% (1000 cycles) | [41] |
| TiO2-WO3 | 1200 | 55% | Over 90% (1200 cycles) | [42] |
| TiO2 | 900 | 28% | [43] | |
| TiO2/ZnO/FTO | 1000 | 55% | 96.6% (200 cycles) | this paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, G.; Zhi, H.; Dong, J.; Hao, J.; Zhang, X.; Wang, J.; Li, D.; Liu, B. Synthesis of the Porous ZnO Nanosheets and TiO2/ZnO/FTO Composite Films by a Low-Temperature Hydrothermal Method and Their Applications in Photocatalysis and Electrochromism. Coatings 2022, 12, 695. https://doi.org/10.3390/coatings12050695
Liu X, Wang G, Zhi H, Dong J, Hao J, Zhang X, Wang J, Li D, Liu B. Synthesis of the Porous ZnO Nanosheets and TiO2/ZnO/FTO Composite Films by a Low-Temperature Hydrothermal Method and Their Applications in Photocatalysis and Electrochromism. Coatings. 2022; 12(5):695. https://doi.org/10.3390/coatings12050695
Chicago/Turabian StyleLiu, Xusong, Gang Wang, Hui Zhi, Jing Dong, Jian Hao, Xiang Zhang, Jing Wang, Danting Li, and Baosheng Liu. 2022. "Synthesis of the Porous ZnO Nanosheets and TiO2/ZnO/FTO Composite Films by a Low-Temperature Hydrothermal Method and Their Applications in Photocatalysis and Electrochromism" Coatings 12, no. 5: 695. https://doi.org/10.3390/coatings12050695
APA StyleLiu, X., Wang, G., Zhi, H., Dong, J., Hao, J., Zhang, X., Wang, J., Li, D., & Liu, B. (2022). Synthesis of the Porous ZnO Nanosheets and TiO2/ZnO/FTO Composite Films by a Low-Temperature Hydrothermal Method and Their Applications in Photocatalysis and Electrochromism. Coatings, 12(5), 695. https://doi.org/10.3390/coatings12050695







