Analysis of the Causes of Differences between the Upper and Lower Surfaces of Electroless Ni–P Coating on LZ91 Magnesium–Lithium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Coating Preparation
2.3. Performance and Characterization
3. Results
3.1. Microscopic Morphology and Composition Analysis
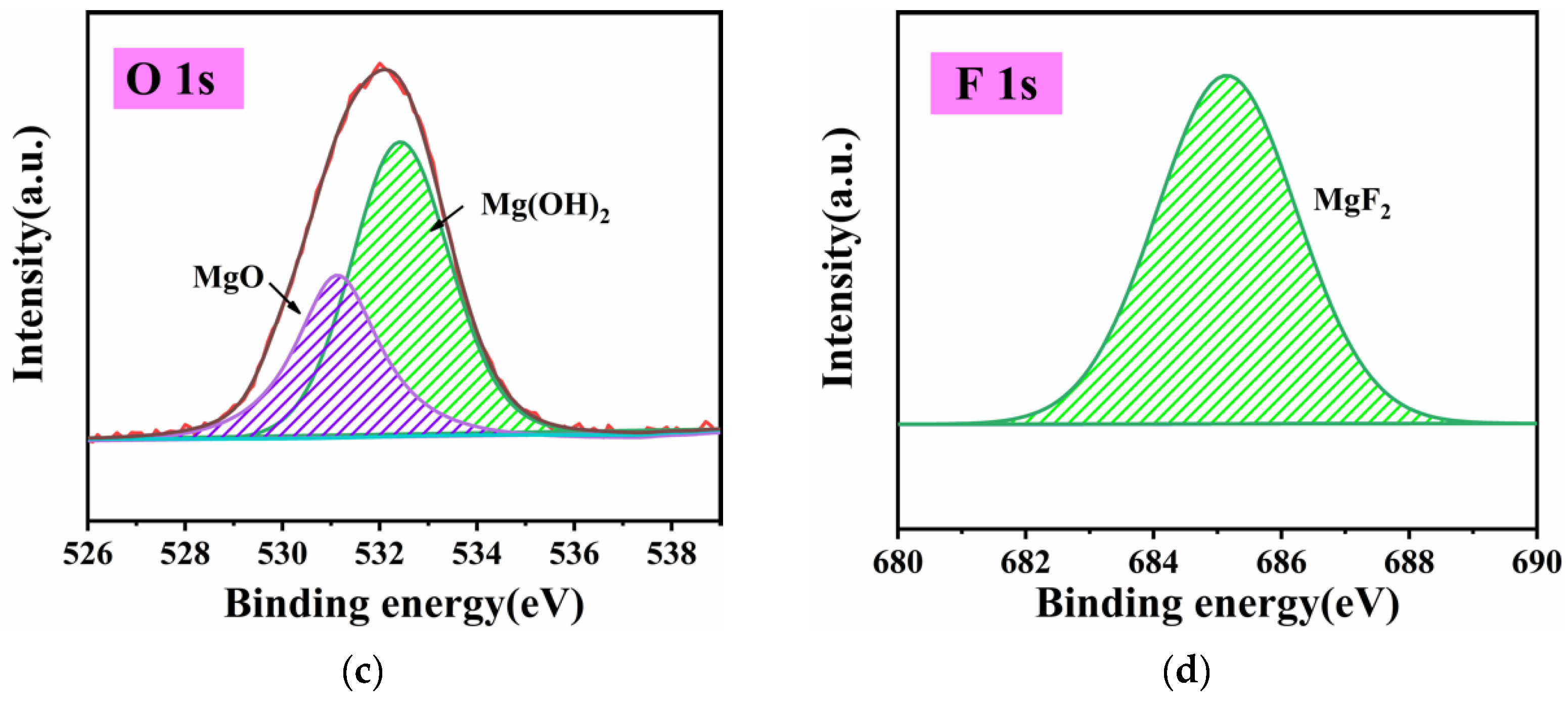
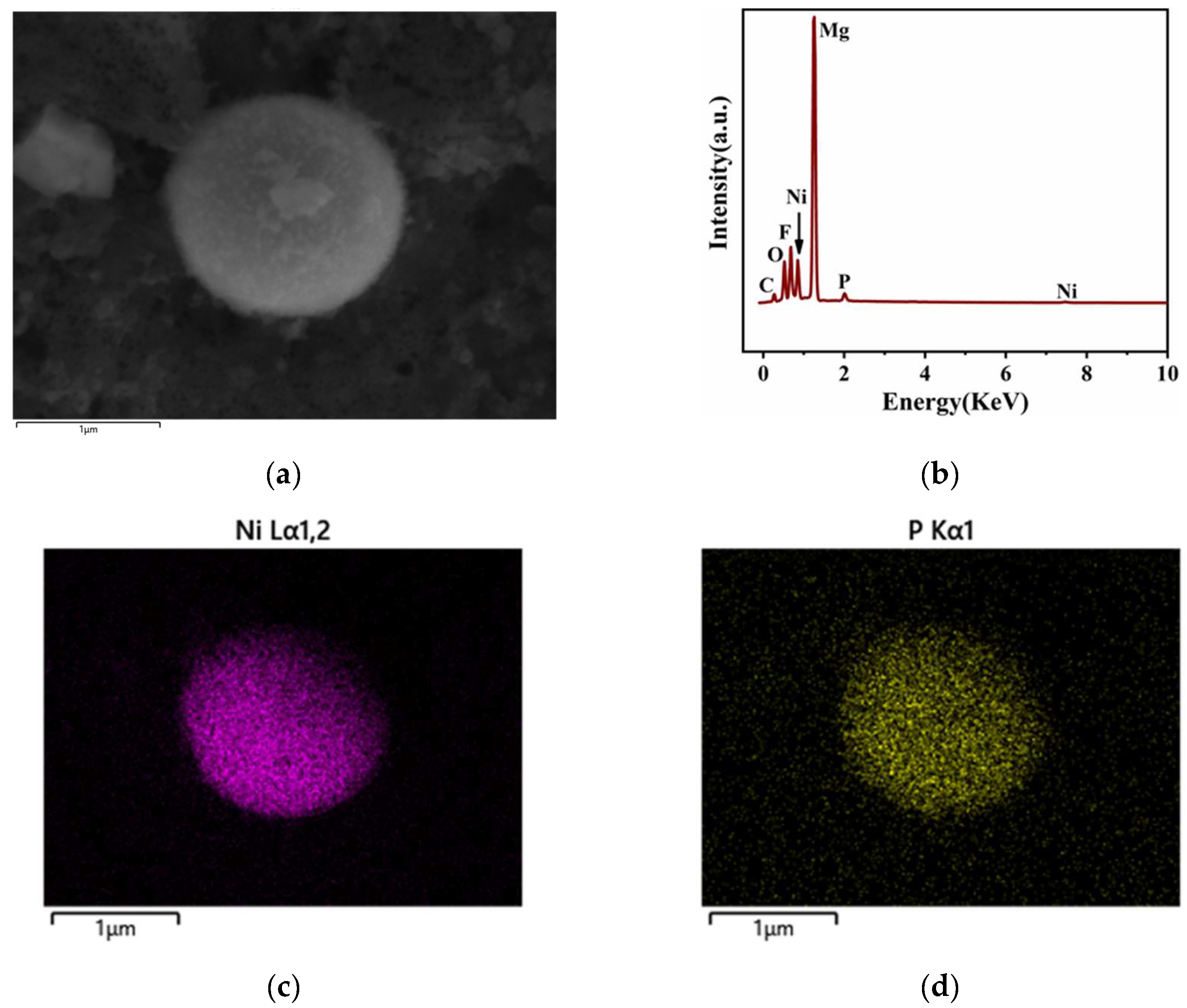
3.1.1. Analysis of Surface Composition after Activation
3.1.2. Analysis of Surface Composition at the Initial Stage of the Reaction
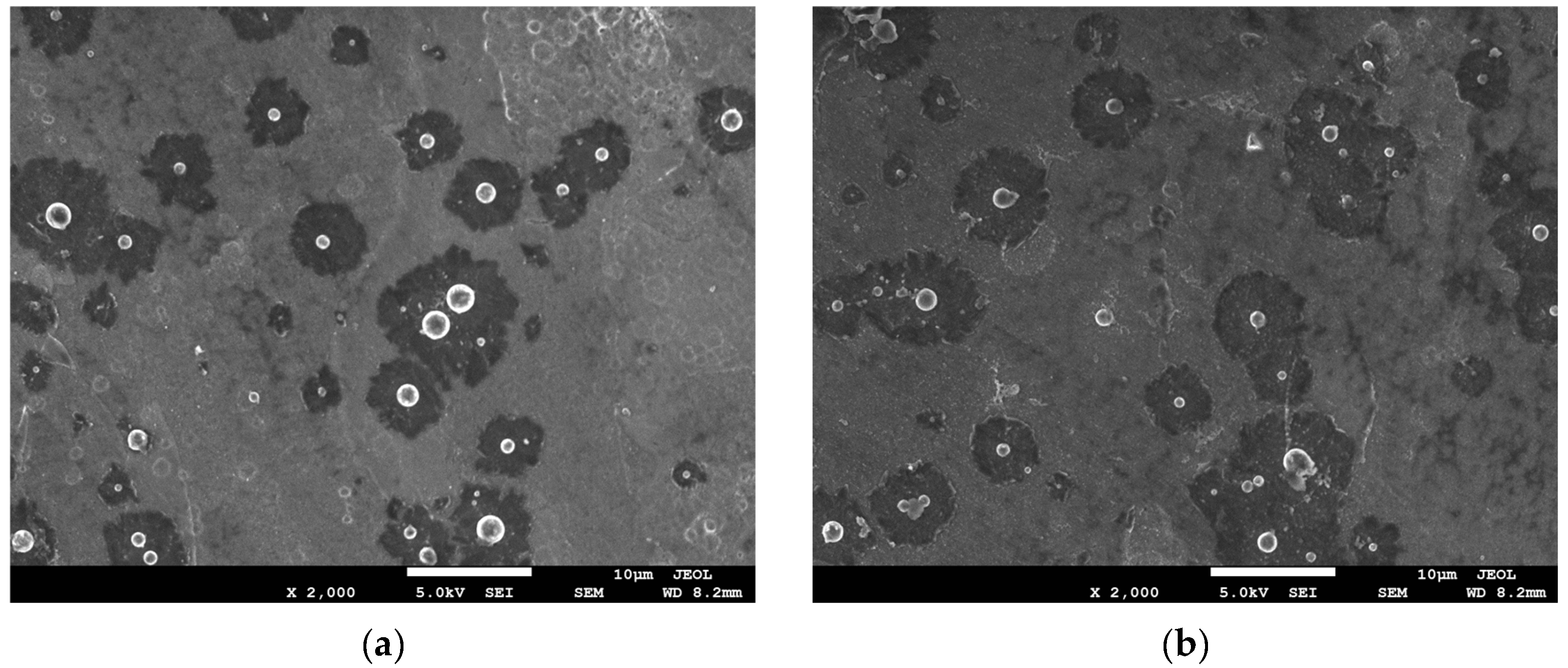
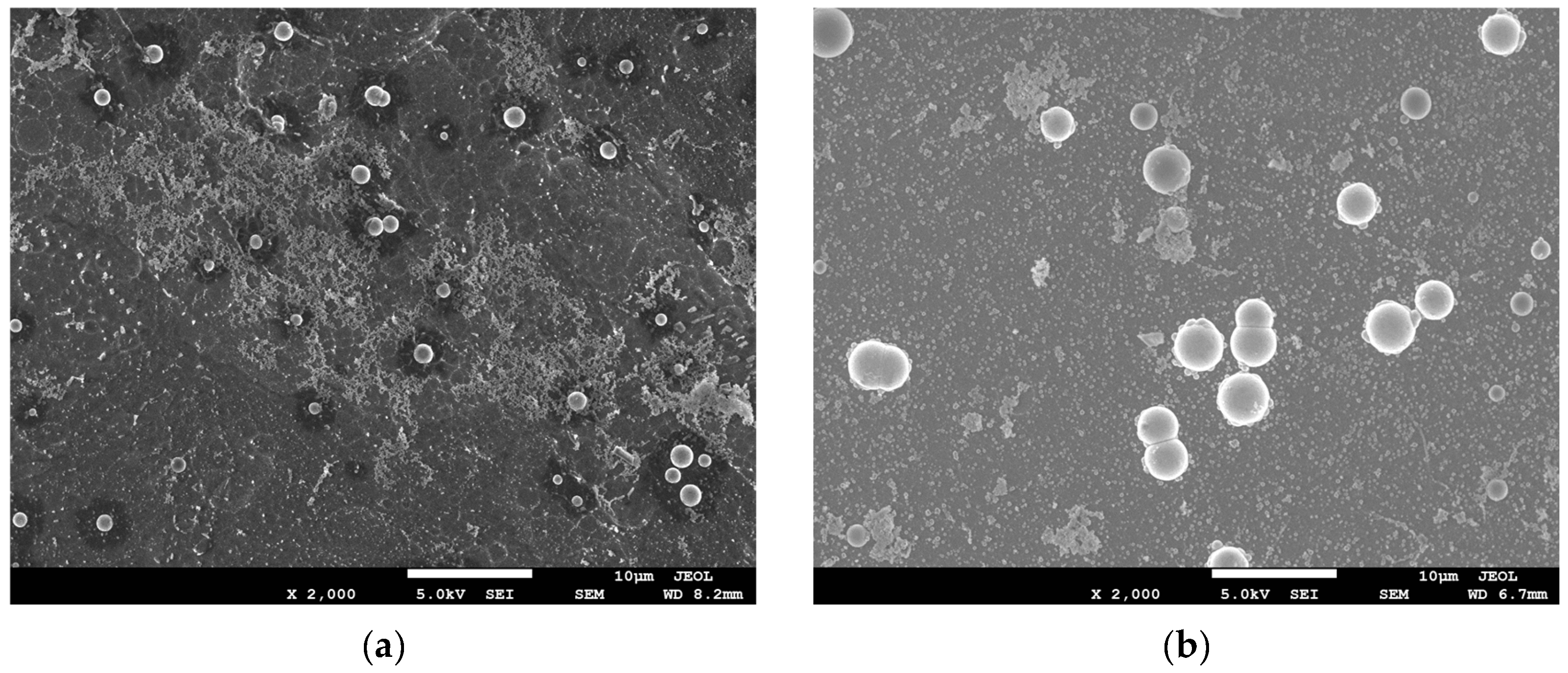
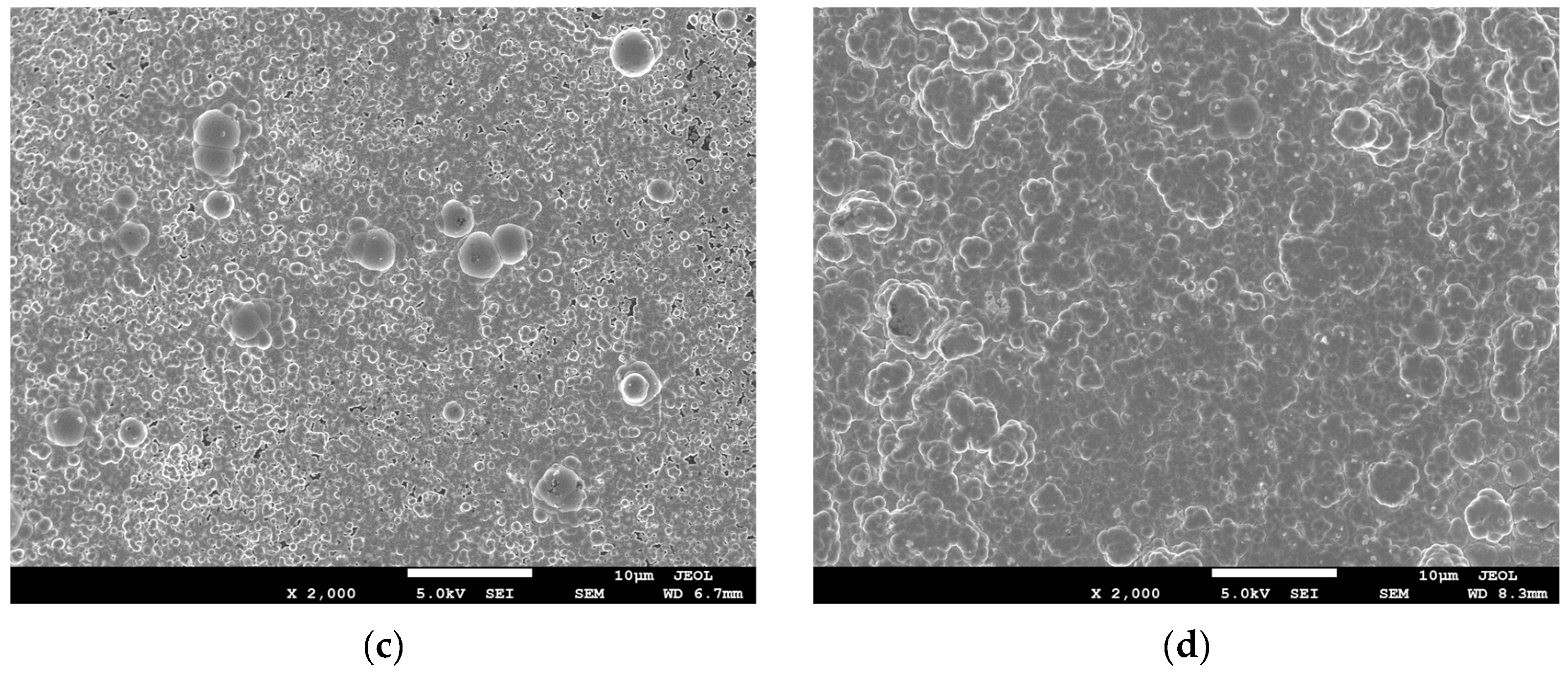
3.1.3. The Evolution of the Upper and Lower Surface Morphology of the Mg–Li Alloy with Reaction Time
3.2. Deposition Process Inference
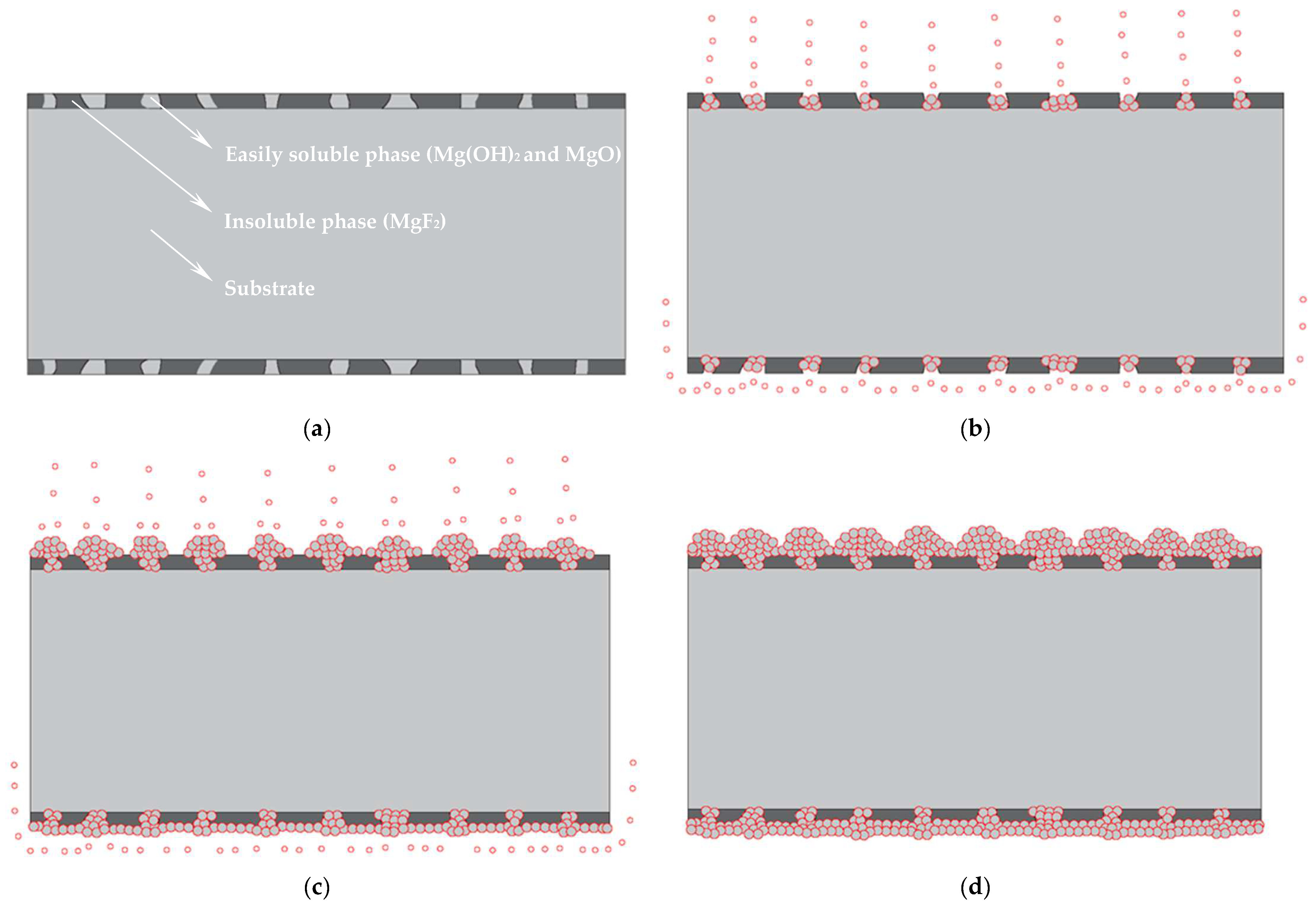
- Due to the special characteristics of Mg–Li alloy material, to be used as a substrate it must be activated with fluoride first. This led to the formation of the surface initial state with Mg(OH)2 and MgO as the easily soluble phase and MgF2 as the insoluble phase on the sample surface. At the first stage, the region where the easily soluble phase was concentrated took the lead in dissolving and forming the catalytic core of Ni and started depositing. Meanwhile, the region where the insoluble phase was concentrated dissolved slowly, leading to a time difference of the deposition reaction in different regions.
- At the third stage, due to the direct upward flow of the bubbles generated at the catalytic core on the upper surface, which drove [H] to flow directly upward. The region where the insoluble phase was concentrated lacked [H] coming from horizontal diffusion, resulting in a lag in the deposition reaction in this region. While the bubbles generated at the catalytic core on the lower surface, under the effect of buoyancy, the bubbles flowed uniformly along the horizontal direction, thus promoting the horizontal flow of [H], which facilitated the occurrence of the reaction Equations (6)–(8) in the insoluble phase concentration region, which caused the Ni–P particles on the lower surface to spread rapidly in the horizontal direction. This eventually led to the formation of obvious differences between the upper and lower surfaces of the plating.
3.3. The Evolution the Optimized Upper Surface Morphology of the Mg–Li Alloy with Reaction Time
3.4. Corrosion Resistance Test of Coating
3.4.1. Potentiodynamic Polarization Test
3.4.2. Electrochemical Impedance Spectroscopy Test
3.5. Coating Adhesion Test
3.6. Analysis of the Surface Composition of Coatings
4. Discussion
5. Conclusions
- 1.
- The reasons for the significant differences formed on the upper and lower surfaces were considered as follows:
- Due to the special characteristics of Mg–Li alloy material, when used as a substrate it must be activated with fluoride first, leading to the formation of the surface initial state. At the stage of dissolution of the easily soluble phase (the first stage), the region where the easily soluble phase was concentrated took the lead in dissolving and forming the catalytic core of Ni and started depositing Ni–P earlier. Meanwhile, the region where the insoluble phase was concentrated dissolved slowly, leading to a time difference of the deposition reaction in different regions;
- At the autocatalytic reaction stage (the third stage), due to the direct upward flow of the bubbles generated at the catalytic core on the upper surface, which drove [H] to flow directly upward, the region where the insoluble phase was concentrated lacked [H] coming from horizontal diffusion, resulting in a lag in the deposition reaction in this region. The bubbles generated at the catalytic core on the lower surface, under the effect of buoyancy, flowed uniformly along the horizontal direction, thus promoting the horizontal flow of [H], which facilitated the occurrence of Ni–P deposition reaction in the insoluble phase concentration region, which caused the Ni–P particles on the lower surface to spread rapidly in the horizontal direction. It eventually led to the formation of obvious differences between the upper and lower surfaces of the plating.
- 2.
- In order to promote the dissolution of the easily soluble phase and accelerate the horizontal flow of [H] on the upper surface, two optimization measures were proposed:
- Preheating the substrate to the same temperature as the coating solution before electroless plating;
- Placing a baffle in parallel 1 mm above the substrate during electroless plating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, B.; Hou, J.; Luan, J.; Xu, D.; Sun, H.; Sun, J. The Corrosion Behaviors of an As-Rolled Mg-8Li (in wt.%) Alloy in Two Differently Concentrated NaCl Solutions. Coatings 2022, 12, 406. [Google Scholar] [CrossRef]
- Mineta, T.; Hasegawa, K.; Sato, H. High strength and plastic deformability of Mg–Li–Al alloy with dual BCC phase produced by a combination of heat treatment and multi-directional forging in channel die. Mater. Sci. Eng. A 2020, 773, 138867. [Google Scholar] [CrossRef]
- Shao, B.; Wu, S.; Shan, D.; Guo, B.; Zong, Y. The effect of thermal cycling process between high and low temperatures on the microstructure and properties of Mg-10Li-3Al-3Zn-0.25Si alloy. Mater. Lett. 2019, 254, 167–170. [Google Scholar] [CrossRef]
- Wen, S.; Wang, X.; Ren, Z. Microstructure and Corrosion Resistance of an HVAF-Sprayed Al-Based Amorphous Coating on Magnesium Alloys. Coatings 2022, 12, 425. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, B.; Tang, A.; Chai, Y.; Liu, B.; Sheng, H.; Yang, T.; Huang, G.; Zhang, D.; Pan, F. Deformation behavior and texture evolution inan extruded MgLi sheet with non-basal texture during tensile deformation. Mater. Charact. 2020, 159, 110041. [Google Scholar] [CrossRef]
- Tang, S.; Xin, T.; Luo, T.; Ji, F.; Li, C.; Feng, T.; Lan, S. Grain boundary decohesion in body-centered cubic Mg-Li-Al alloys. J. Alloys Compd. 2022, 902, 163732. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Y.; Song, B.; Yao, G. Surfacial Modification of Magnesium-Lithium Alloy. Adv. Mater. Res. 2014, 905, 113–118. [Google Scholar] [CrossRef]
- Yu, B.; Dai, J.; Ruan, Q.; Liu, Z.; Chu, P. Corrosion Behavior and Mechanism of Carbon Ion-Implanted Magnesium Alloy. Coatings 2020, 8, 734. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Wu, R. Molybdate and molybdate/permanganate conversion coatings on Mg-8.5Li alloy. Appl. Surf. Sci. 2012, 258, 2648–2654. [Google Scholar] [CrossRef]
- Georgiza, E.; Novakovic, J.; Vassiliou, P. Characterization and corrosion resistance of duplex electroless Ni-P composite coatings on magnesium alloy. Surf. Coat. Technol. 2013, 232, 432–439. [Google Scholar] [CrossRef]
- Brito, M.M.; Artisiani, R.A.; Carlos, I.A. The electrodeposition of Ni-Cu and Ni-Cu-P from aspartate-based baths. J. Alloys Compd. 2022, 890, 161761. [Google Scholar] [CrossRef]
- Leng, Z.; Li, T.; Wang, X.; Zhang, S.; Zhou, J. Effect of Graphite Content on the Conductivity, Wear Behavior, and Corrosion Resistance of the Organic Layer on Magnesium Alloy MAO Coatings. Coatings 2022, 12, 434. [Google Scholar] [CrossRef]
- Chang, L.M.; Wang, P.; Liu, W. Effect of Amino Acids on the Structure and Corrosion Resistance of Mg-Li Alloy Anodic Oxide Film. Adv. Mater. Res. 2011, 146, 785–788. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, G.C.; Wang, S.L.; Liu, Y.H.; Luo, H.J. A chrome-free conversion coating for magnesium–lithium alloy by a phosphate–permanganate solution. Surf. Coat. Technol. 2008, 202, 1825–1830. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.W.; Liu, S.Y.; Chen, D.; Wang, G.X.; Wang, Y.Y.; Zhang, M.L.; Chen, Y.H. Ultrasonic-Assisted Electroless Ni-P Plating on Dual Phase Mg-Li Alloy. J. Electrochem. Soc. 2014, 162, C64–C70. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, X.; Hou, A.; Li, J.; Wang, K.; Cai, H. In Situ Preparation of Mg-Al-Co Layered Double Hydroxides on Microarc Oxidation Ceramic Coating of LA103Z Magnesium-Lithium Alloy for Enhanced Corrosion Resistance. J. Mater. Eng. Perform. 2021, 30, 8490–8499. [Google Scholar] [CrossRef]
- Radwan, A.B.; Ali, K.; Shakoor, R.A.; Mohammed, H.; Alsalama, T.; Kahraman, R.; Yusuf, M.M.; Abdullah, A.M.; Montemor, M.F.; Helal, M. Properties enhancement of Ni-P electrodeposited coatings by the incorporation of nanoscale Y2O3 particles. Appl. Surf. Sci. 2018, 457, 956–967. [Google Scholar] [CrossRef]
- Buchtík, M.; Doskočil, L.; Brescher, R.; Doležal, P.; Másilko, J.; Wasserbauer, J. The Effect of Crystallization and Phase Transformation on the Mechanical and Electrochemical Corrosion Properties of Ni-P Coatings. Coatings 2021, 11, 447. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, H.C.; Tseng, Y.C. Structural morphology and magnetism of electroless-plated NiP films on a surface-modified Si substrate. Thin Solid Films 2011, 520, 1102–1108. [Google Scholar] [CrossRef]
- Jian, S.Y.; Chu, Y.R.; Lin, C.S. Permanganate conversion coating on AZ31 magnesium alloys with enhanced corrosion resistance. Corros. Sci. 2015, 93, 301–309. [Google Scholar] [CrossRef]
- Chang, J.K.; Lin, C.S.; Wang, W.R. Oxidation and corrosion behavior of commercial 5wt% Al-Zn coated steel during austenitization heat treatment. Surf. Coat. Technol. 2018, 350, 880–889. [Google Scholar] [CrossRef]
- Milcius, D.; Grbović-Novaković, J.; Zostautienė, R.; Lelis, M.; Girdzevicius, D.; Urbonavicius, M. Combined XRD and XPS analysis of ex-situ and in-situplasma hydrogenated magnetron sputtered Mg films. J. Alloys Compd. 2015, 647, 790–796. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, Z.; Guo, J.; Han, X.; Mu, Q.; Zhu, X. A novel chemical mechanical polishing slurry for yttrium aluminum garnet crystal. Appl. Surf. Sci. 2019, 496, 43601. [Google Scholar] [CrossRef]
- Jin, Z.; Jia, Y.; Zhang, K.S.; Kong, L.T.; Sun, B.; Shen, W.; Meng, F.L.; Liu, J.H. Effective removal of fluoride by porous MgO nanoplates and its adsorption mechanism. J. Alloys Compd. 2016, 675, 292–300. [Google Scholar] [CrossRef]
- Newberg, J.T.; Starr, D.E.; Yamamoto, S.; Kaya, S.; Kendelewicz, T.; Mysak, E.R.; Porsgaard, S.; Salmeron, M.B.; Brown, G.E.; Nilsson, A.; et al. Formation of hydroxyl and water layers on MgO films studied with ambient pressure XPS. Surf. Sci. 2011, 605, 89–94. [Google Scholar] [CrossRef]
- Khajuria, P.; Mahajan, R.; Kumar, S.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Surface and spectral investigation of Sm3+ doped MgO-ZrO2 phosphor. Optik 2020, 216, 164909. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zou, B.; Gu, L.; Huang, W.; Cao, X. Preparation and corrosion resistance of MAO/Ni–P composite coat on Mg alloy. Appl. Surf. Sci. 2013, 277, 272–280. [Google Scholar] [CrossRef]
- Shang, W.; Zhan, X.; Wen, Y.; Li, Y.; Zhang, Z.; Wu, F.; Wang, C. Deposition mechanism of electroless nickel plating of composite coatings on magnesium alloy. Chem. Eng. Sci. 2019, 207, 1299–1308. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, S.; Xu, G.; Zhao, H.; Li, Y.; Wang, X.; Huang, K.; Ren, M.; Wu, X. Crack self-healing of phytic acid conversion coating on AZ31 magnesium alloy by heat treatment and the corrosion resistance. Appl. Surf. Sci. 2014, 313, 896–904. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, P.; Sun, P.; Niu, X.; Hu, C. Electroless Ni-P Plating on Mullite Powders and Study of the Mechanical Properties of Its Plasma-Sprayed Coating. Coatings 2022, 12, 18. [Google Scholar] [CrossRef]
- Sharma, A.K.; Suresh, M.R.; Bhojraj, H.; Narayanamurthy, H.; Sahu, R.P. Electroless nickel plating on magnesium alloy. Met. Finish. 1998, 96, 10–16. [Google Scholar] [CrossRef]
- Hu, R.; Su, Y.; Liu, H. Deposition behaviour of nickel phosphorus coating on magnesium alloy in a weak corrosive electroless nickel plating bath. J. Alloys Compd. 2016, 658, 555–560. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Wen, C.; Li, S.; Li, J.; Cheng, D.; Bai, J.; Cui, Q.; Zhang, L. Effect of initial deposition behavior on properties of electroless Ni-P coating on ZK60 and ME20 magnesium alloys. Trans. Nonferr. Met. Soc. 2021, 31, 2307–2322. [Google Scholar] [CrossRef]
- Shao, Z.; Cai, Z.; Hu, R.; Wei, S. The study of electroless nickel plating directly on magnesium alloy. Surf. Coat. Technol. 2014, 249, 42–47. [Google Scholar] [CrossRef]
- Song, Z.; Xie, Z.; Yu, G.; Hu, B.; He, X.; Zhang, X. A novel palladium-free surface activation process for electroless nickel deposition on micro-arc oxidation film of AZ91D Mg alloy. J. Alloys Compd. 2015, 623, 274–281. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Han, E. High corrosion resistance of electroless composite plating coatings on AZ91D magnesium alloys. Electrochim. Acta 2008, 53, 2135–2143. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Han, E. Investigation of surface oxide film on magnesium-lithium alloy. J. Alloys Compd. 2009, 484, 585–590. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Wang, Y.; Wang, H.; Gao, K.; Han, Z.; Ren, L. Superhydrophobic and superoleophobic surface by electrodeposition on magnesium alloy substrate: Wettability and corrosion inhibition. J. Colloid Interface Sci. 2016, 478, 164–171. [Google Scholar] [CrossRef]
- Hu, C.; Xu, M.; Zhang, J.; Hu, B.; Yu, G. High corrosion resistance of electroless Ni/Ni-B coating from fluoride-free baths on AZ31 magnesium alloy. J. Alloys Compd. 2019, 770, 48–57. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, C.; Wu, D.; Zou, Y. Synthesis and corrosion resistance of solid solution Ti3(Al1−xSix)C2. J. Alloys Compd. 2021, 867, 159126. [Google Scholar] [CrossRef]
- Kamde, M.A.; Mahton, Y.; Ohodnicki, J.; Roy, M.; Saha, P. Effect of cerim-based conversion coating on corrosion behavior of squeeze cast Mg-4 wt%Y alloy in 0.1 M NaCl solution. Surf. Coat. Technol. 2021, 421, 127451. [Google Scholar] [CrossRef]
- Zhao, G.H.; He, Y.D. Plasma electroplating Ni coating on pure copper sheet—The effects of H2SO4 concentration on the microstructure and mechanical properties. Surf. Coat. Technol. 2012, 206, 4411–4416. [Google Scholar] [CrossRef]
- Aleksandra, F.; Sławomir, Z.; Agnieszka, K.; Alicja, Ł.; Tomasz, M. Electrophoretic Co-deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy. Materials 2012, 13, 15. [Google Scholar]
- Gan, R.; Wang, D.M.; Xie, Z.H.; He, L. Improving surface characteristic and corrosion inhibition of coating on Mg alloy by trace stannous (II) chloride. Corros. Sci. 2017, 123, 147–157. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Kefallinou, Z.; Mordarski, G.; Jagoda, Z.; Spencer, B. Surface functionalisation by the introduction of self-healing properties into electroless Ni-P coatings. Electrochim. Acta 2019, 297, 427–434. [Google Scholar] [CrossRef]
- Zhan, X.Q.; Shang, W.; Wen, Y.Q.; Li, Y.Q.; Ma, M.M. Preparation and corrosion resistance of a three-layer composite coatings on the Mg alloy. J. Alloys Compd. 2019, 774, 522–531. [Google Scholar] [CrossRef]







| Materials | Coating | Surface Treatment | Ecorr/V | icorr/(A/cm−2) | RCT/Ω·cm2 | Reference |
|---|---|---|---|---|---|---|
| Mg–Li | Anodic oxide film | Anodizing | −1.69 | 5.07 × 10−5 | 700 | [13] |
| Mg–Li | MAO ceramic film | Micro-arc oxidation | −1.5 | 2.39 × 10−5 | 3652 | [16] |
| Mg–Li | phosphate-permanganate | chemical conversion | −1.57 | - | - | [14] |
| Mg–Li | Ni–P | Electroless plating | −0.34 | 8 × 10−6 | 25,000 | [15] |
| Composition and Conditions | Concentration |
|---|---|
| NiCO3·2Ni(OH)2·4H2O | 15 g/L |
| NaH2PO2·H2O | 20 g/L |
| C6H8O7·H2O | 7 g/L |
| HF | 14 mL/L |
| NH4HF2 | 10 g/L |
| CH3CHOHCOOH | 15 mL/L |
| Thiourea | 1 mg/L |
| NH3·H2O | a little |
| pH | 5.5–6.0 |
| Temperature | 358.15 K |
| Samples | RS/Ω·cm2 | RCT/Ω·cm2 | RL/Ω·cm2 | L/H·cm−2 | CPE/(Ω−1·Sn·cm−2) | n |
|---|---|---|---|---|---|---|
| A | 168.9 | 228.2 | 142.9 | 643.5 | 0.000206 | 0.87202 |
| B | 109.2 | 30967 | - | - | 1.6384 × 10−5 | 0.93368 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, S.-F.; Li, S.-Q.; Zhong, L.; Cui, K.-F.; Yang, J.; Yang, Z.-G. Analysis of the Causes of Differences between the Upper and Lower Surfaces of Electroless Ni–P Coating on LZ91 Magnesium–Lithium Alloy. Coatings 2022, 12, 1157. https://doi.org/10.3390/coatings12081157
Pei S-F, Li S-Q, Zhong L, Cui K-F, Yang J, Yang Z-G. Analysis of the Causes of Differences between the Upper and Lower Surfaces of Electroless Ni–P Coating on LZ91 Magnesium–Lithium Alloy. Coatings. 2022; 12(8):1157. https://doi.org/10.3390/coatings12081157
Chicago/Turabian StylePei, Shi-Feng, Si-Qi Li, Liang Zhong, Kai-Fang Cui, Jun Yang, and Zhi-Gang Yang. 2022. "Analysis of the Causes of Differences between the Upper and Lower Surfaces of Electroless Ni–P Coating on LZ91 Magnesium–Lithium Alloy" Coatings 12, no. 8: 1157. https://doi.org/10.3390/coatings12081157
APA StylePei, S.-F., Li, S.-Q., Zhong, L., Cui, K.-F., Yang, J., & Yang, Z.-G. (2022). Analysis of the Causes of Differences between the Upper and Lower Surfaces of Electroless Ni–P Coating on LZ91 Magnesium–Lithium Alloy. Coatings, 12(8), 1157. https://doi.org/10.3390/coatings12081157






