Epitaxial Growth of Cobalt Oxide Thin Films on Sapphire Substrates Using Atmospheric Pressure Mist Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
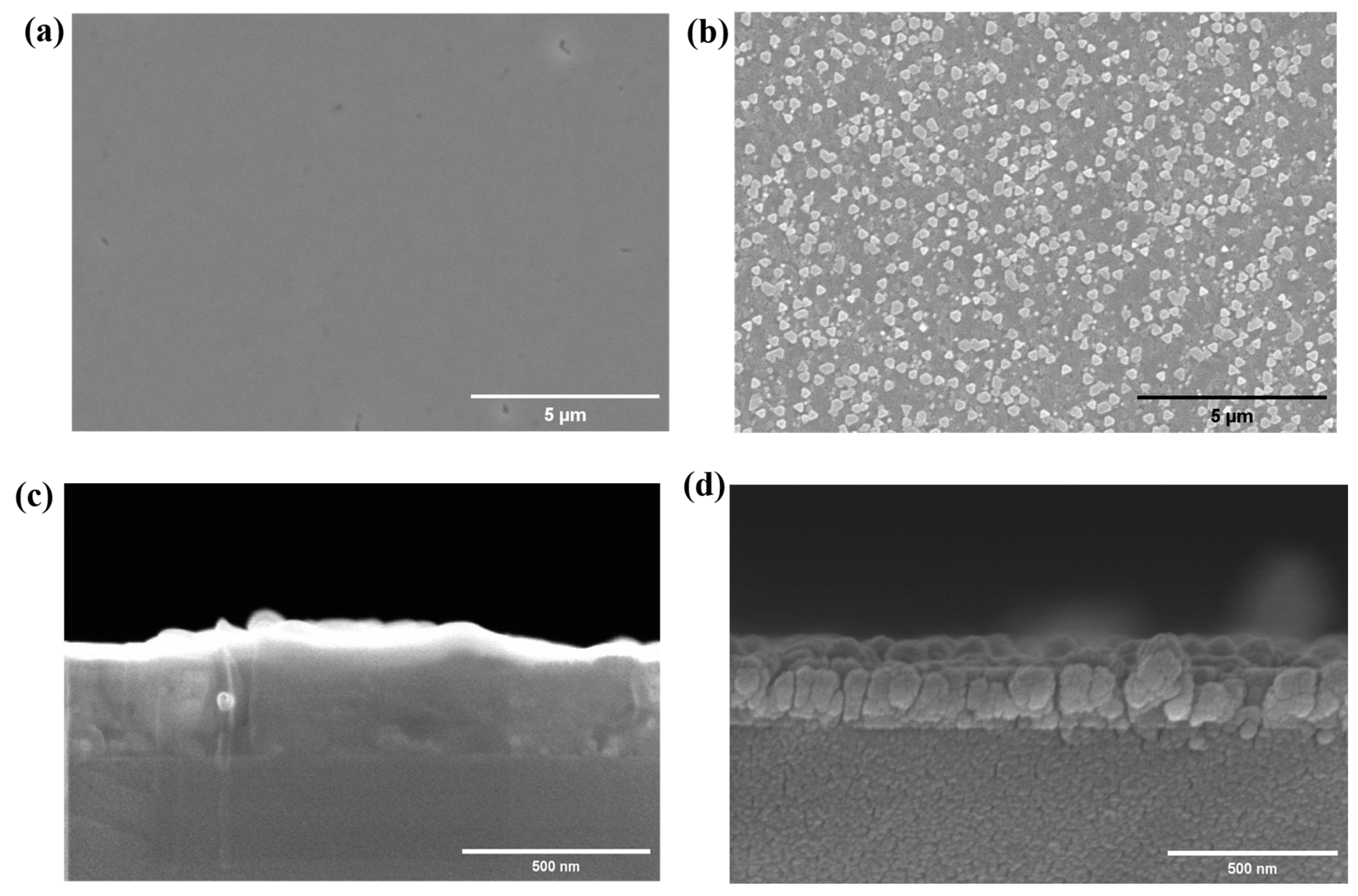
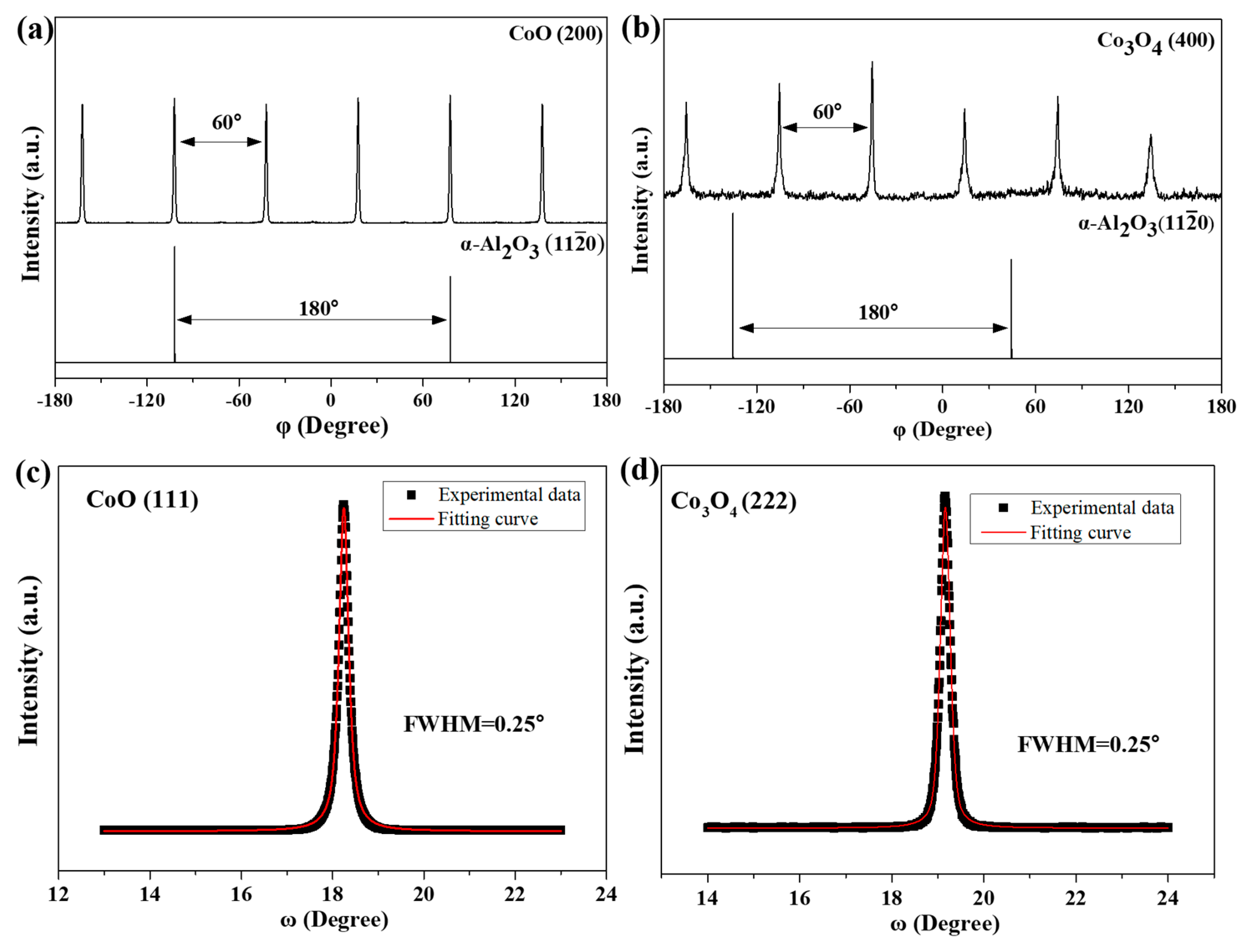
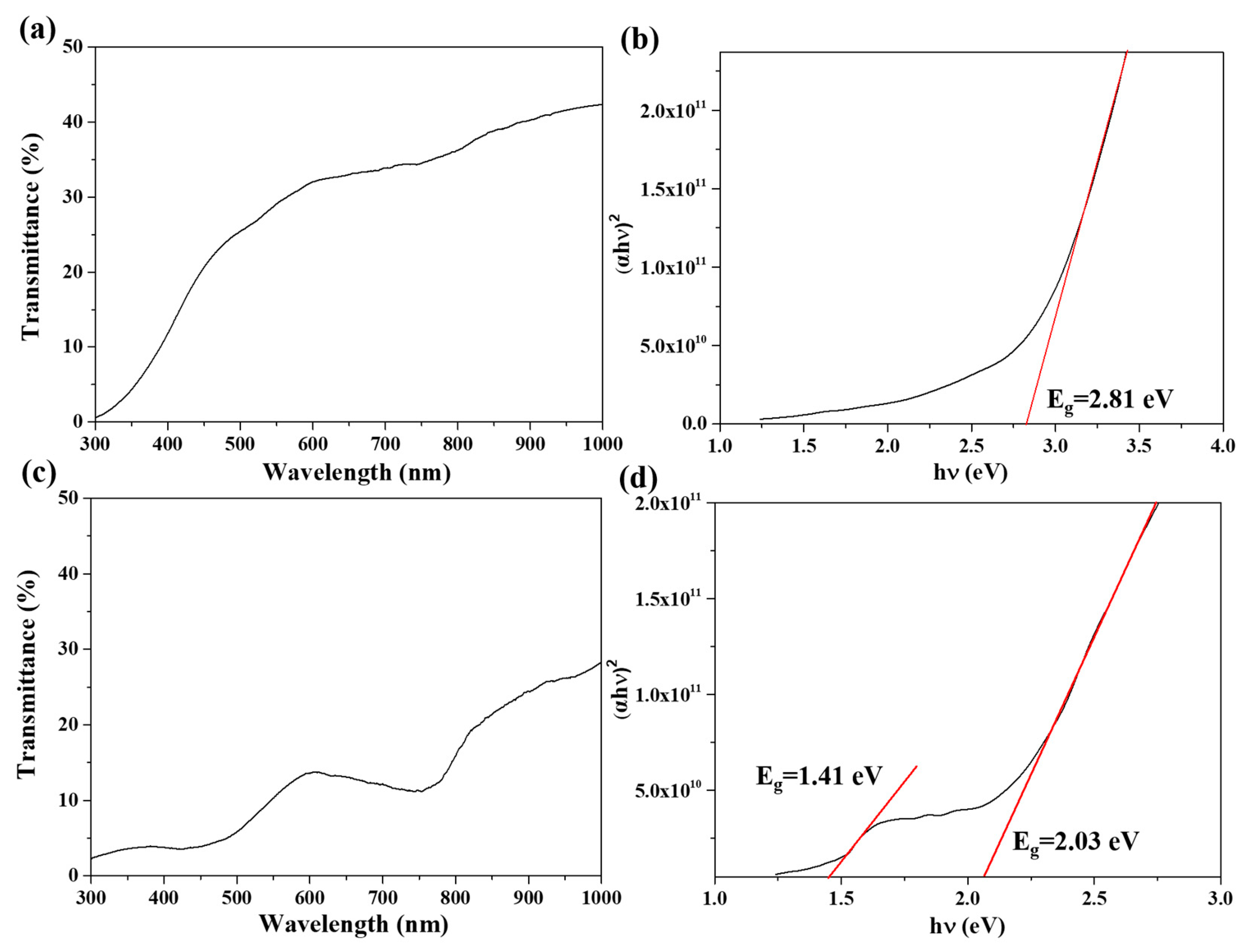
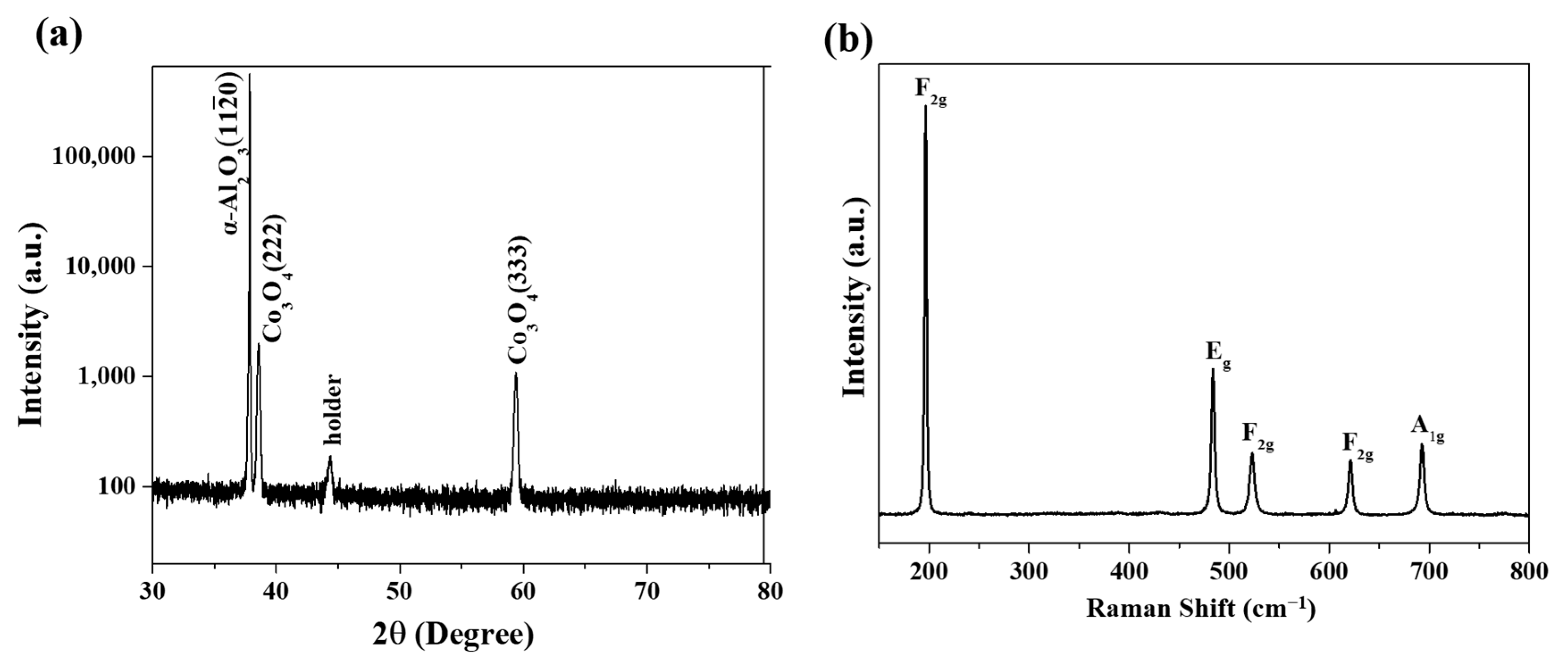
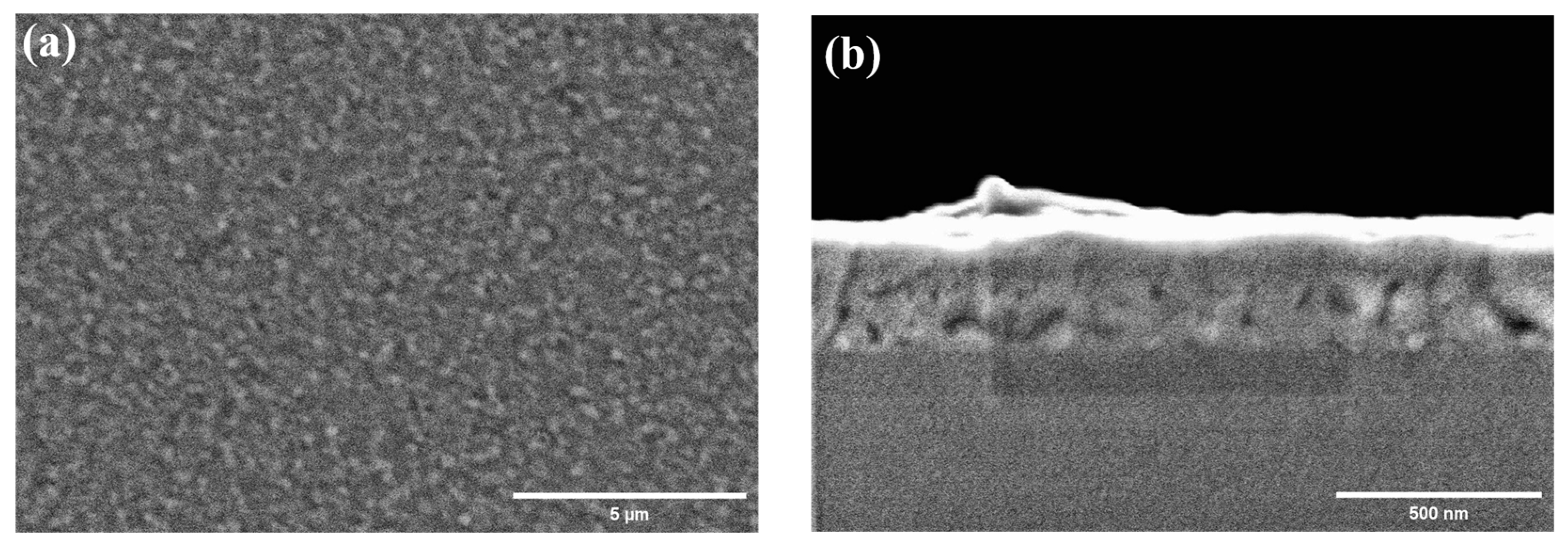
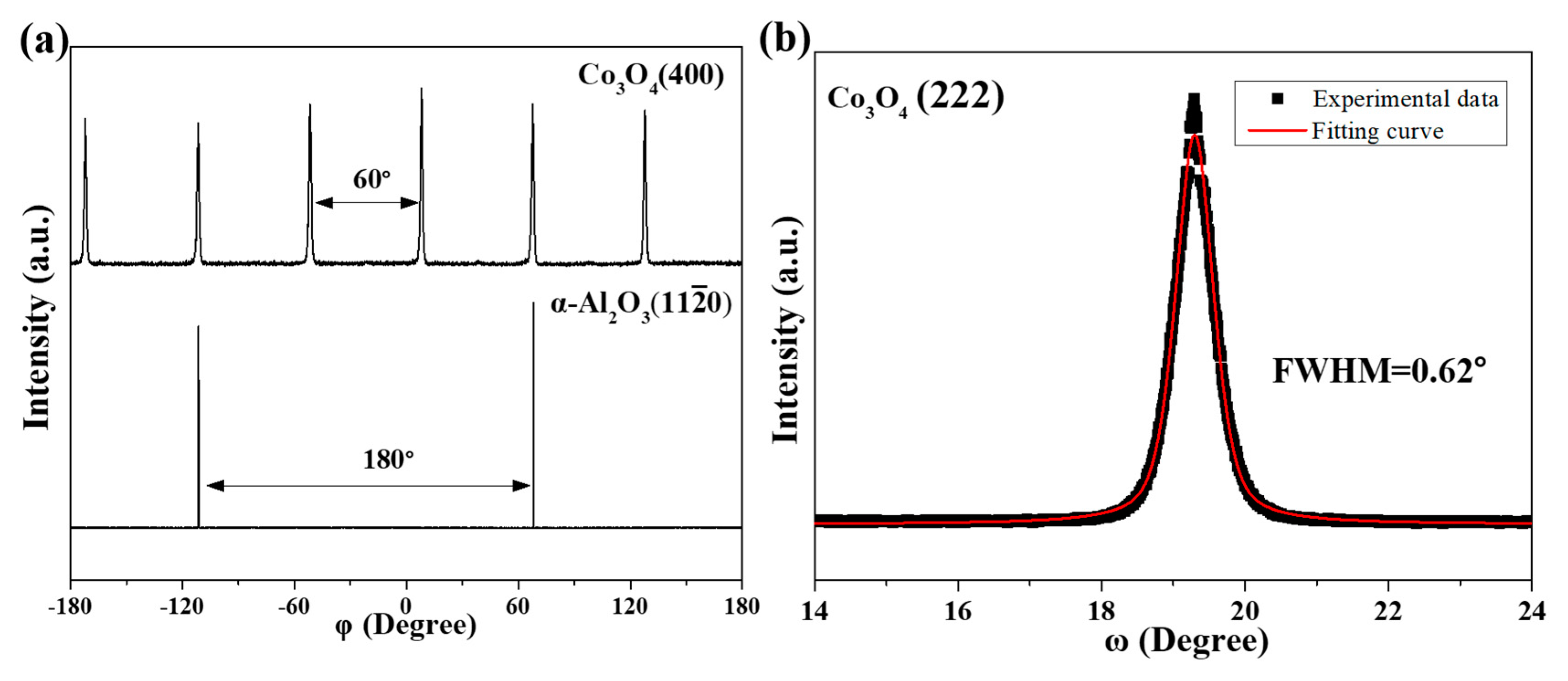
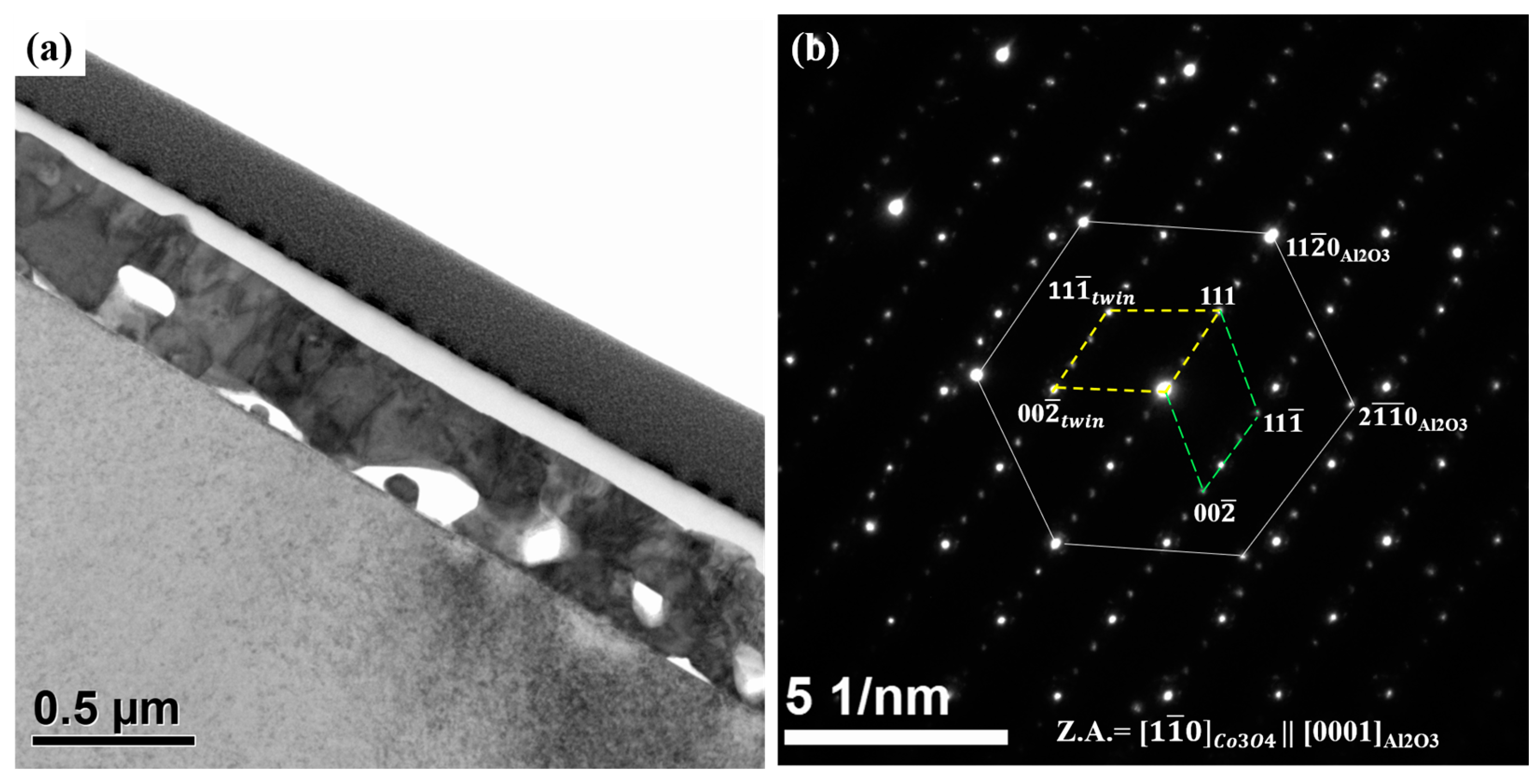
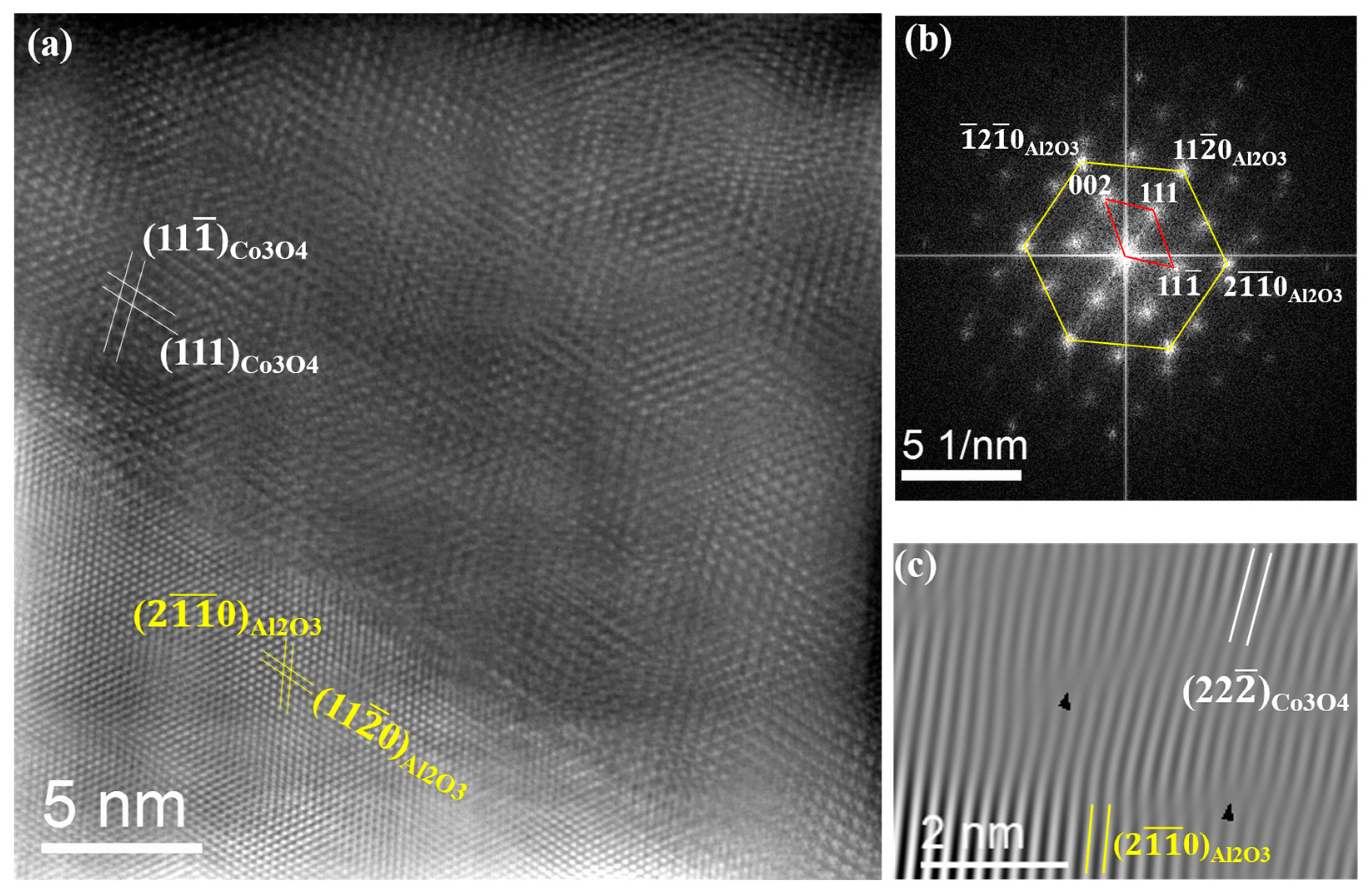
3.1. Epitaxial Growth of CoO and Co3O4 Thin Films on Sapphire Substrates
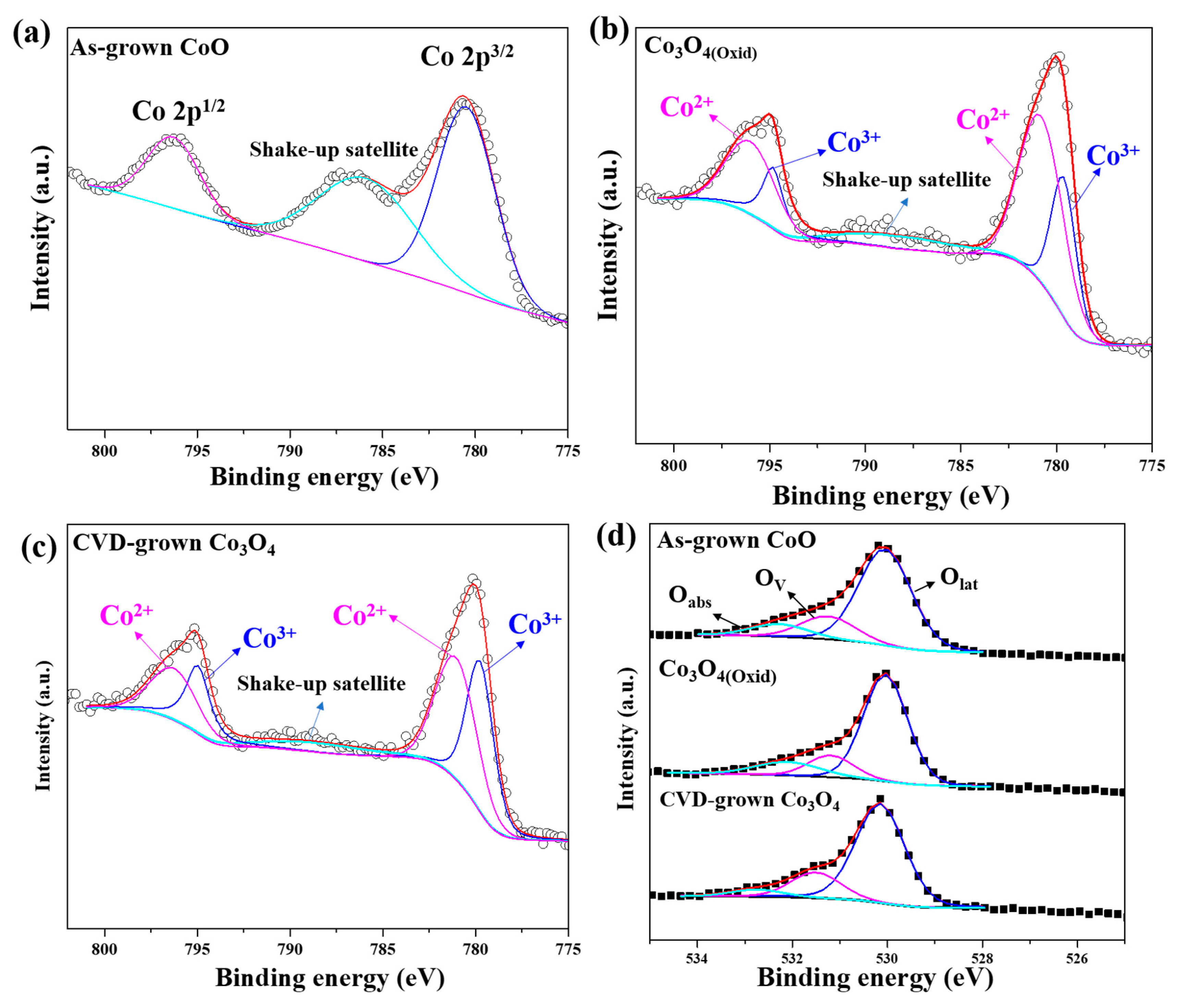
3.2. Thermal Annealing of CoO Epitaxial Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlom, D.G.; Chen, L.-Q.; Pan, X.; Schmehl, A.; Zurbuchen, M.A. A Thin Film Approach to Engineering Functionality into Oxides. J. Am. Ceramic Soc. 2008, 91, 2429–2454. [Google Scholar] [CrossRef]
- Oka, D.; Fukumura, T. Crystal engineering for novel functionalities with oxide thin film epitaxy. CrystEngComm 2017, 19, 2144–2162. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, K.; Wu, S.; Zhou, P.; Fu, Y.; Xia, J.; Li, H.-F. A comprehensive review on the ferroelectric orthochromates: Synthesis, property, and application. Coord. Chem. Rev. 2023, 475, 214873. [Google Scholar] [CrossRef]
- Kumah, D.P.; Ngai, J.H.; Kornblum, L. Epitaxial Oxides on Semiconductors: From Fundamentals to New Devices. Adv. Funct. Mater. 2020, 30, 1901597. [Google Scholar] [CrossRef]
- Zhang, K.H.L.; Xi, K.; Blamire, M.G.; Egdell, R.G. P-type transparent conducting oxides. J. Phys. Condens. Matter. 2016, 28, 383002. [Google Scholar] [CrossRef]
- Kupfer, B.; Majhi, K.; Keller, D.A.; Bouhadana, Y.; Rühle, S.; Barad, H.N.; Anderson, A.Y.; Zaban, A. Thin Film Co3O4/TiO2 Heterojunction Solar Cells. Adv. Energy Mater. 2015, 5, 1401007. [Google Scholar] [CrossRef]
- Patel, M.; Park, S.-H.; Kim, J. Rapid Thermal Treatment of Reactive Sputtering Grown Nanocrystalline Co3O4 for Enhanced All-Oxide Photovoltaics. Phys. Status Solidi A 2018, 215, 1800216. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, L.; Quan, F.; Guo, R. Recent developments of Co3O4-based materials as catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2022, 12, 436–461. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T.; Deng, J.; Zhang, R.; Lou, Z.; Wang, L. P-type Co3O4 nanomaterials-based gas sensor: Preparation and acetone sensing performance. Sens. Actuators B Chem. 2017, 242, 369–377. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Fang, L.; Xu, H.; Zhang, H.; Gu, X.; Wang, Y. Probing the Crystal Plane Effect of Co3O4 for Enhanced Electrocatalytic Performance toward Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 27736–27744. [Google Scholar] [CrossRef]
- Yao, C.; Ismail, M.; Hao, A.; Thatikonda, S.K.; Huang, W.; Qin, N.; Bao, D. Annealing atmosphere effect on the resistive switching and magnetic properties of spinel Co3O4 thin films prepared by a sol–gel technique. RSC Adv. 2019, 9, 12615–12625. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, D.-H.; Kim, S.-W.; Kim, J.; Kang, K. Highly reversible Co3O4/graphene hybrid anode for lithium rechargeable batteries. Carbon 2011, 49, 326–332. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Li, J.; Liao, F.; Yang, G.; Xie, L.; Zhao, C.; Chen, L. Two-dimensional Spinel Structured Co-based Materials for High Performance Supercapacitors: A Critical Review. Chem. Eng. J. 2020, 387, 124081. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, M.; Kim, H.-S.; Park, W.-H.; Choi, E.H.; Kim, J. Reactive sputtering growth of Co3O4 thin films for all metal oxide device: A semitransparent and self-powered ultraviolet photodetector. Mater. Sci. Semicond. Process. 2018, 74, 74–79. [Google Scholar] [CrossRef]
- Negusse, E.; Idzerda, Y.U.; Suci, P.A. Exchange biased magnetoresistance based spin-transport sensor. J. Appl. Phys. 2004, 95, 4506–4508. [Google Scholar] [CrossRef]
- Kadam, L.D.; Patil, P.S. Thickness-dependent properties of sprayed cobalt oxide thin films. Mater. Chem. Phys. 2001, 68, 225–232. [Google Scholar] [CrossRef]
- Tototzintle-Huitle, H.; Prokhorov, E.; Mendoza-Galván, A.; Urbina, J.E.; González-Hernández, J. Study of the formation of Co3O4 thin films using sol–gel method. J. Phys. Chem. Solids 2003, 64, 975–980. [Google Scholar] [CrossRef]
- Matsuda, A.; Yamauchi, R.; Shiojiri, D.; Tan, G.; Kaneko, S.; Yoshimoto, M. Room-temperature selective epitaxial growth of CoO (111) and Co3O4 (111) thin films with atomic steps by pulsed laser deposition. Appl. Surf. Sci. 2015, 349, 78–82. [Google Scholar] [CrossRef]
- Vaz, C.; Wang, H.-Q.; Ahn, C.; Henrich, V.; Baykara, M.; Schwendemann, T.; Pilet, N.; Albers, B.; Schwarz, U.; Zhang, L. Interface and electronic characterization of thin epitaxial Co3O4 films. Surf. Sci. 2009, 603, 291–297. [Google Scholar] [CrossRef]
- Klepper, K.B.; Nilsen, O.; Fjellvåg, H. Epitaxial growth of cobalt oxide by atomic layer deposition. J. Cryst. Growth 2007, 307, 457–465. [Google Scholar] [CrossRef]
- Alema, F.; Osinsky, A.; Mukhopadhyay, P.; Schoenfeld, W.V. Epitaxial growth of Co3O4 thin films using Co(dpm)3 by MOCVD. J. Cryst. Growth 2019, 525, 125207. [Google Scholar] [CrossRef]
- Prieto, P.; Marco, J.F.; Serrano, A.; Manso, M.; de la Figuera, J. Highly oriented (111) CoO and Co3O4 thin films grown by ion beam sputtering. J. Alloys Compd. 2019, 810, 151912. [Google Scholar] [CrossRef]
- Tanoue, H.; Takenouchi, M.; Yamashita, T.; Wada, S.; Yatabe, Z.; Nagaoka, S.; Naka, Y.; Nakamura, Y. Improvement of m-plane ZnO films formed on buffer layers on sapphire substrates by mist chemical vapor deposition. Phys. Status Solidi A 2017, 214, 1600603. [Google Scholar] [CrossRef]
- Park, S.-Y.; Ha, M.-T.; Kim, K.-H.; Van Lich, L.; Shin, Y.-J.; Jeong, S.-M.; Kwon, S.-H.; Bae, S.-Y. Enhanced thickness uniformity of large-scale α-Ga2O3 epilayers grown by vertical hot-wall mist chemical vapor deposition. Ceram. Int. 2021, 48, 5075–5082. [Google Scholar] [CrossRef]
- Oshima, T.; Niwa, M.; Mukai, A.; Nagami, T.; Suyama, T.; Ohtomo, A. Epitaxial growth of wide-band-gap ZnGa2O4 films by mist chemical vapor deposition. J. Cryst. Growth 2014, 386, 190–193. [Google Scholar] [CrossRef]
- Nishinaka, H.; Yoshimoto, M. Mist Chemical Vapor Deposition of Single-Phase Metastable Rhombohedral Indium Tin Oxide Epitaxial Thin Films with High Electrical Conductivity and Transparency on Various alpha-Al2O3 Substrates. Cryst. Growth Des. 2018, 18, 4022–4028. [Google Scholar] [CrossRef]
- Yatabe, Z.; Tsuda, T.; Matsushita, J.; Sato, T.; Otabe, T.; Sue, K.; Nagaoka, S.; Nakamura, Y. Single crystalline SnO2 thin films grown on m -plane sapphire substrate by mist chemical vapor deposition. Phys. Status Solidi C 2017, 14, 1600148. [Google Scholar] [CrossRef]
- Chen, H.-G.; Shih, Y.-H.; Wang, H.-S.; Jian, S.-R.; Yang, T.-Y.; Chuang, S.-C. Van der Waals Epitaxial Growth of ZnO Films on Mica Substrates in Low-Temperature Aqueous Solution. Coatings 2022, 12, 706. [Google Scholar] [CrossRef]
- Nishinaka, H.; Kamada, Y.; Kameyama, N.; Fujita, S. Epitaxial ZnO Thin Films on a-Plane Sapphire Substrates Grown by Ultrasonic Spray-Assisted Mist Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2009, 48, 121103. [Google Scholar] [CrossRef]
- Burriel, M.; Garcia, G.; Santiso, J.; Hansson, A.N.; Linderoth, S.; Figueras, A. Co3O4 protective coatings prepared by Pulsed Injection Metal Organic Chemical Vapour Deposition. Thin Solid Film. 2005, 473, 98–103. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; Morilla dos Santos, C.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. A 2016, 72, 385–390. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO–Co3O4 crossover using Raman spectroscopy in magnetic octahedron-shaped nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Grube, M. Raman and infrared spectromicroscopy of manganese oxides. J. Alloys Compd. 2009, 480, 97–99. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199. [Google Scholar] [CrossRef]
- Shalini, K.; Mane, A.U.; Shivashankar, S.A.; Rajeswari, M.; Choopun, S. Epitaxial growth of Co3O4 films by low temperature, low pressure chemical vapour deposition. J. Cryst. Growth 2001, 231, 242–247. [Google Scholar] [CrossRef]
- Forsythe, J.; Leiko, O.; Uvarov, V.; Škvára, J.; Mysliveček, J.; Mašek, K.; Johánek, V. CoO epitaxial growth on Cu(111) by reactive PVD and plasma oxidation. Surf. Interfaces 2022, 34, 102364. [Google Scholar] [CrossRef]
- Entani, S.; Kiguchi, M.; Saiki, K.; Koma, A. Epitaxial growth of CoO films on semiconductor and metal substrates by constructing a complex heterostructure. J. Cryst. Growth 2003, 247, 110–118. [Google Scholar] [CrossRef]
- Feeler, D.; Williams, R.S. Epitaxial Growth of CoO (001) and Co3O4 (001) Films on MgO (001) by Pulsed Laser Deposition. MRS Online Proc. Libr. 1994, 341, 29–34. [Google Scholar] [CrossRef]
- Ayers, J.E. The measurement of threading dislocation densities in semiconductor crystals by X-ray diffraction. J. Cryst. Growth 1994, 135, 71–77. [Google Scholar] [CrossRef]
- Amodeo, J.; Merkel, S.; Tromas, C.; Carrez, P.; Korte-Kerzel, S.; Cordier, P.; Chevalier, J. Dislocations and Plastic Deformation in MgO Crystals: A Review. Crystals 2018, 8, 240. [Google Scholar] [CrossRef]
- Mitchell, T.E.; Hwang, L.; Heuer, A.H. Deformation in spinel. J. Mater. Sci. 1976, 11, 264–272. [Google Scholar] [CrossRef]
- Patil, P.S.; Kadam, L.D.; Lokhande, C.D. Preparation and characterization of spray pyrolysed cobalt oxide thin films. Thin Solid Film. 1996, 272, 29–32. [Google Scholar] [CrossRef]
- Tsai, S.-H.; Basu, S.; Huang, C.-Y.; Hsu, L.-C.; Lin, Y.-G.; Horng, R.-H. Deep-Ultraviolet Photodetectors Based on Epitaxial ZnGa2O4 Thin Films. Sci. Rep. 2018, 8, 14056. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-G.; Hung, S.-P. Epitaxial growth of Sb-doped nonpolar a-plane ZnO thin films on r-plane sapphire substrates by RF magnetron sputtering. J. Alloys Compd. 2014, 586, S339–S342. [Google Scholar] [CrossRef]
- Wang, P.; Jin, C.; Zheng, D.; Yang, T.; Wang, Y.; Zheng, R.; Bai, H. Engineering Co Vacancies for Tuning Electrical Properties of p-Type Semiconducting Co3O4 Films. ACS Appl. Mater. Interfaces 2021, 13, 26621–26629. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, B.; Zhao, C.; Li, S.; Zhang, M.; Yan, L. Valence control of cobalt oxide thin films by annealing atmosphere. Appl. Surf. Sci. 2011, 257, 3358–3362. [Google Scholar] [CrossRef]
- Jena, A.; Penki, T.R.; Munichandraiah, N.; Shivashankar, S.A. Flower-like porous cobalt(II) monoxide nanostructures as anode material for Li-ion batteries. J. Electroanal. Chem. 2016, 761, 21–27. [Google Scholar] [CrossRef]
- Perekrestov, R.; Spesyvyi, A.; Maixner, J.; Mašek, K.; Leiko, O.; Khalakhan, I.; Maňák, J.; Kšírová, P.; Hubička, Z.; Čada, M. The comparative study of electrical, optical and catalytic properties of Co3O4 thin nanocrystalline films prepared by reactive high-power impulse and radio frequency magnetron sputtering. Thin Solid Film. 2019, 686, 137427. [Google Scholar] [CrossRef]
- Masaya, O.; Rie, T.; Hitoshi, K.; Tomochika, T.; Takahiro, S.; Toshimi, H. Schottky barrier diodes of corundum-structured gallium oxide showing on-resistance of 0.1 mΩ·cm2 grown by MIST EPITAXY®. Appl. Phys. Express 2016, 9, 021101. [Google Scholar]
- Carson, G.A.; Nassir, M.H.; Langell, M.A. Epitaxial growth of Co3O4 on CoO (100). J. Vac. Sci. Technol. A 1996, 14, 1637–1642. [Google Scholar] [CrossRef]
- Carson, G.A.; Nassir, M.H.; Langell, M.A. Co3O4 epitaxial formation on CoO (100). Mater. Res. Soc. 1995, 355, 163–168. [Google Scholar] [CrossRef]
- Żyła, M.; Smoła, G.; Knapik, A.; Rysz, J.; Sitarz, M.; Grzesik, Z. The formation of the Co3O4 cobalt oxide within CoO substrate. Corros. Sci. 2016, 112, 536–541. [Google Scholar] [CrossRef]
- Kaczmarska, A.; Grzesik, Z.; Mrowec, S. On the Defect Structure and Transport Properties of Co3O4 Spinel Oxide. High Temp. Mater. Process. 2012, 31, 371–379. [Google Scholar] [CrossRef]
- Przybylski, K.; Smeltzer, W.W. High Temperature Oxidation Mechanism of CoO to Co3O4. J. Electrochem. Soc. 1981, 128, 897–902. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-G.; Wang, H.-S.; Jian, S.-R.; Yeh, T.-L.; Feng, J.-Y. Epitaxial Growth of Cobalt Oxide Thin Films on Sapphire Substrates Using Atmospheric Pressure Mist Chemical Vapor Deposition. Coatings 2023, 13, 1878. https://doi.org/10.3390/coatings13111878
Chen H-G, Wang H-S, Jian S-R, Yeh T-L, Feng J-Y. Epitaxial Growth of Cobalt Oxide Thin Films on Sapphire Substrates Using Atmospheric Pressure Mist Chemical Vapor Deposition. Coatings. 2023; 13(11):1878. https://doi.org/10.3390/coatings13111878
Chicago/Turabian StyleChen, Hou-Guang, Huei-Sen Wang, Sheng-Rui Jian, Tung-Lun Yeh, and Jing-Yi Feng. 2023. "Epitaxial Growth of Cobalt Oxide Thin Films on Sapphire Substrates Using Atmospheric Pressure Mist Chemical Vapor Deposition" Coatings 13, no. 11: 1878. https://doi.org/10.3390/coatings13111878




