A Comparative Study on the Influence of Al2O3-ZnO ALD Nanolaminates on the Properties of CrCN PVD Sputtered Coatings with Distinctive Morphologies and Microstructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition of CrCN Coatings
2.2. PVD/ALD/PVD Duplex Coatings Deposition
2.3. Characterization
3. Results
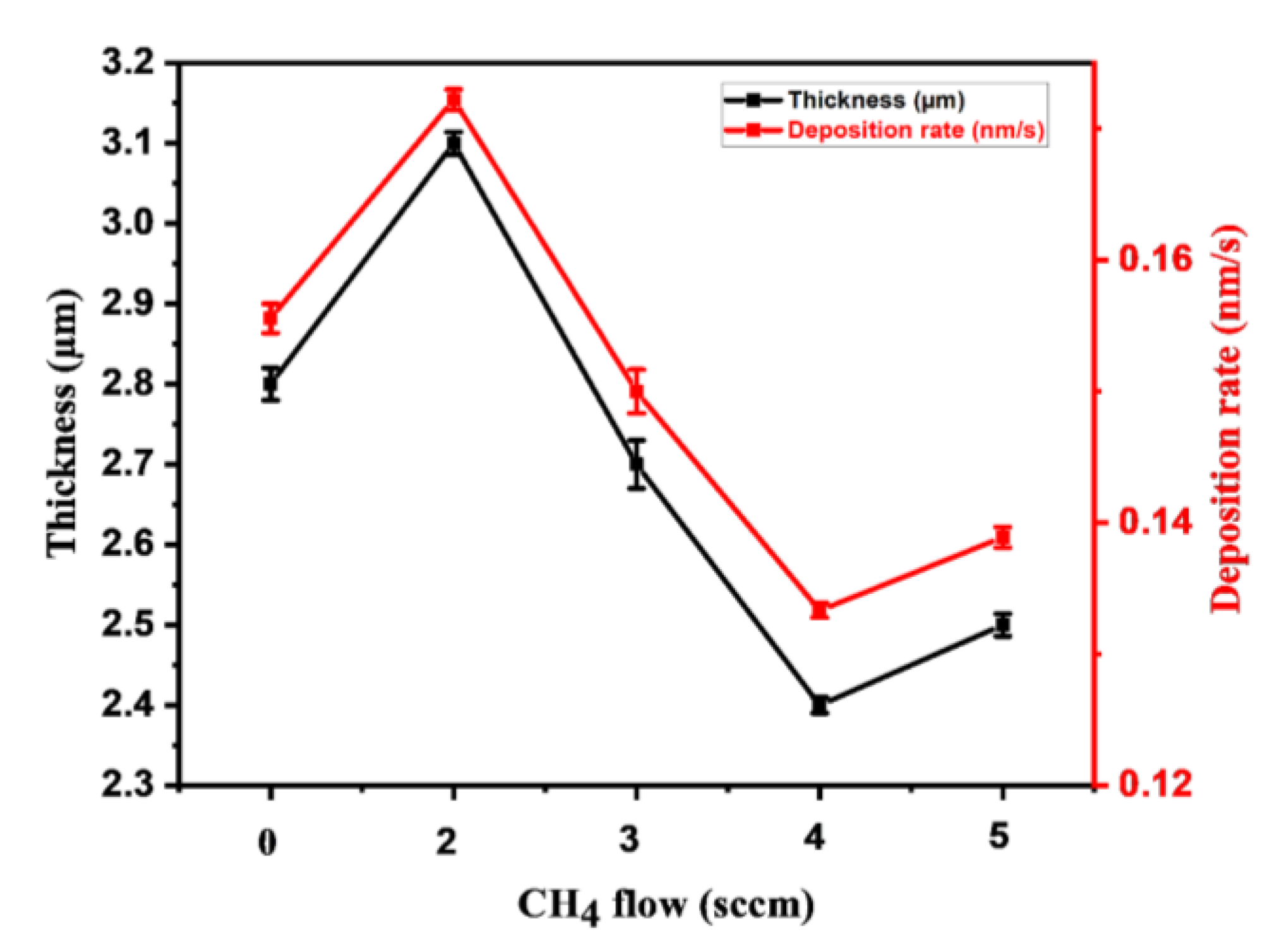
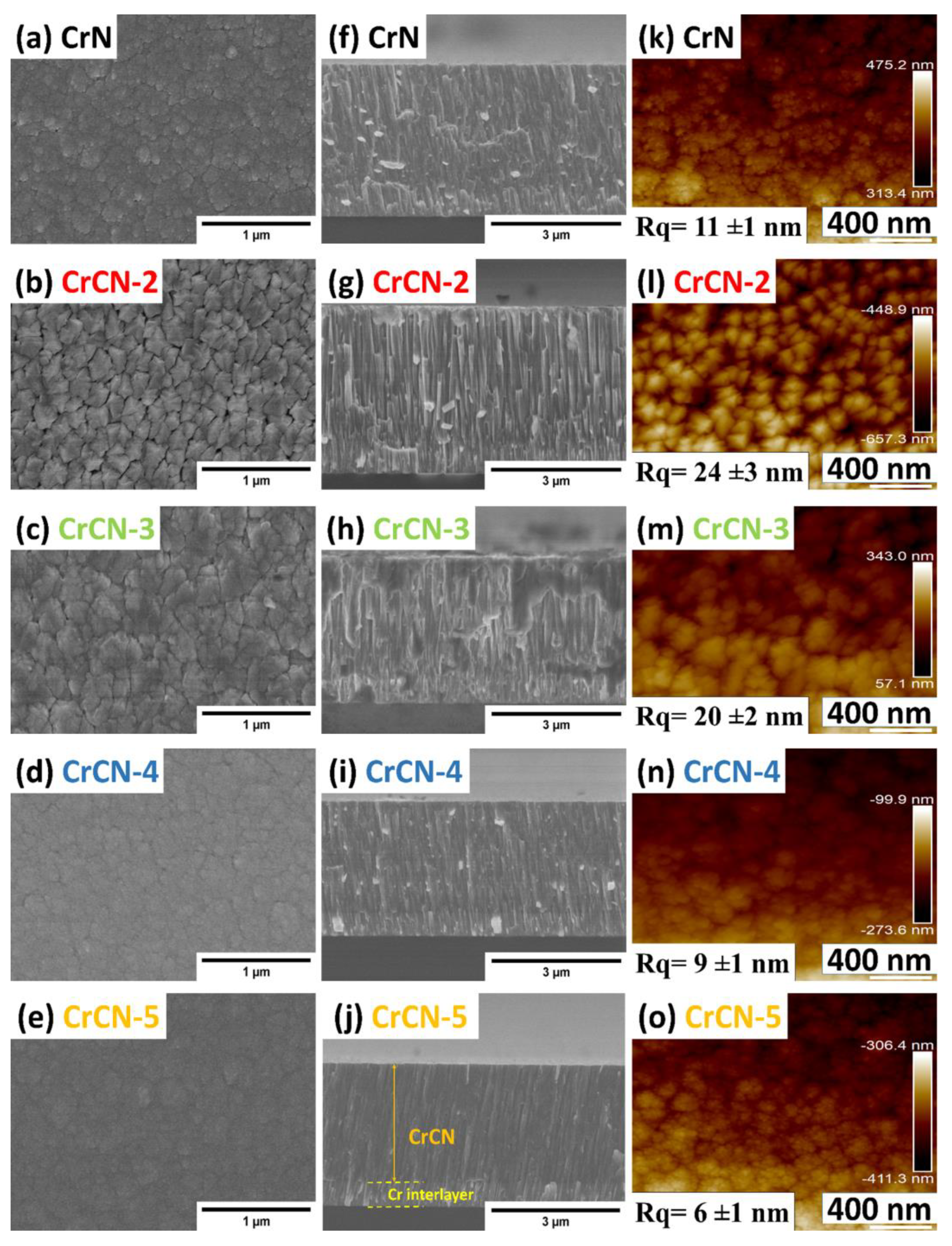
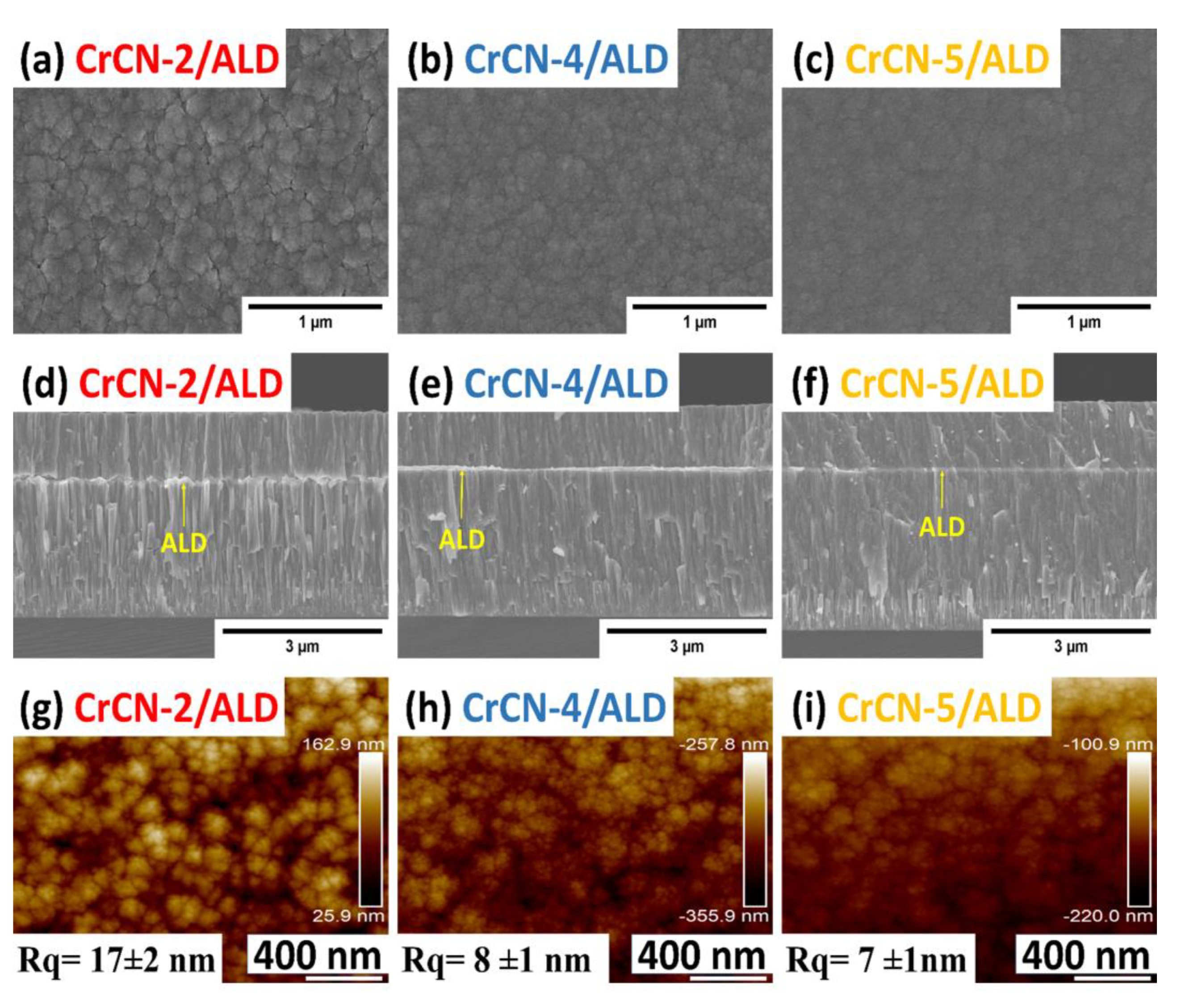
3.1. Coatings Morphology and Surface Topography
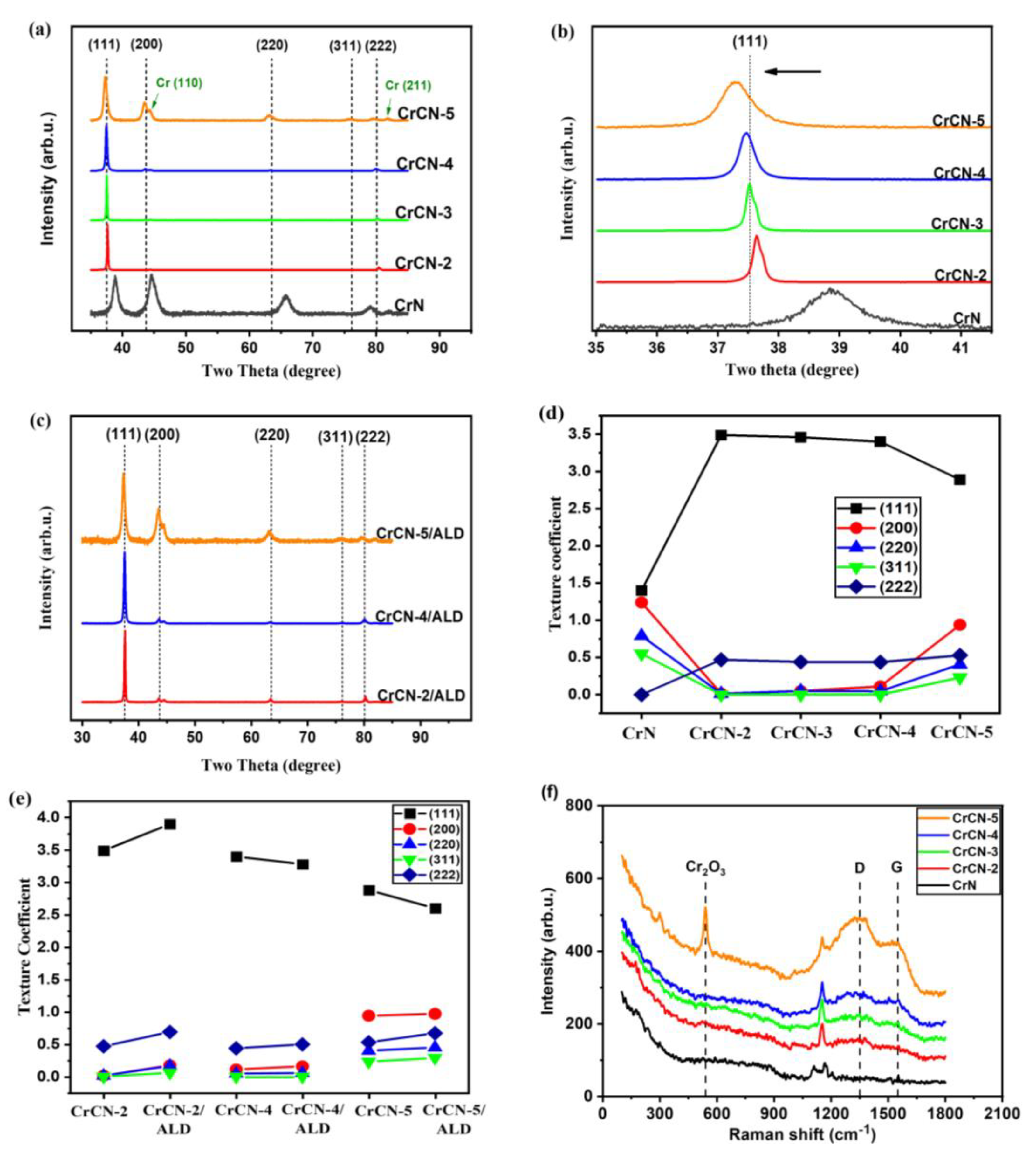
3.2. Phase Composition and Microstructure Analysis
3.3. Hardness and Young’s Modulus
3.4. Wettability Behavior
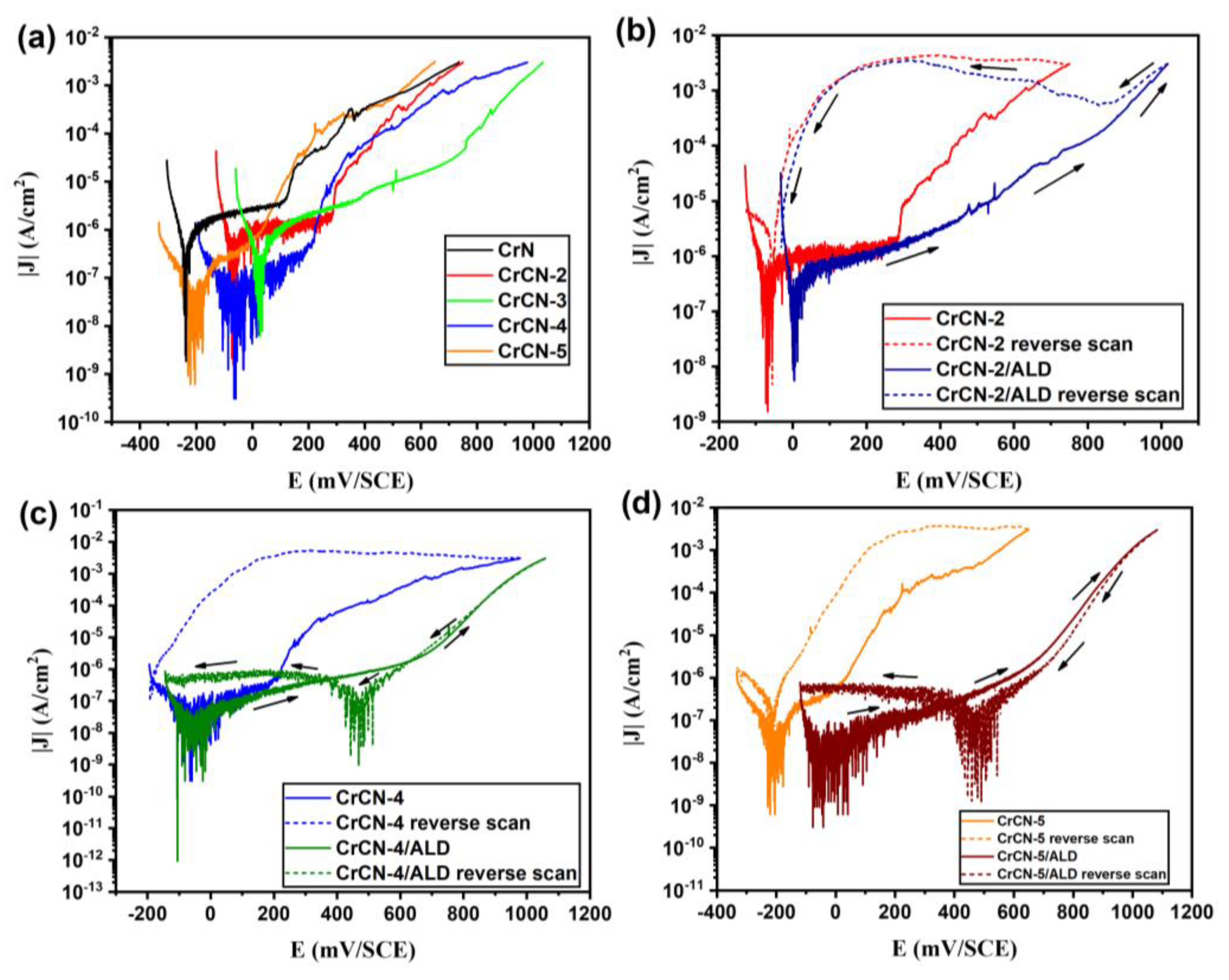
3.5. Corrosion Behavior of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, F.; Ma, Q.; Wang, Q.; Zhou, Z.; Li, L.K.-Y. Electrochemical and Tribological Properties of CrBCN Coatings with Various B Concentrations in Artificial Seawater. Tribol. Int. 2017, 116, 19–25. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, F.; Wang, Q.; Fu, Y.; Zhou, Z. Structural and Tribological Properties of CrMoCN Coatings with Various Mo Contents in Artificial Seawater. Appl. Surf. Sci. 2019, 493, 485–496. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Chen, H.; Li, J.; Yao, Y.; Wang, C. Doping Carbon to Improve the Tribological Performance of CrN Coatings in Seawater. Tribol. Int. 2015, 90, 362–371. [Google Scholar] [CrossRef]
- Zhang, J.; Su, X.; Shan, L.; Liu, Y.; Zhang, P.; Jia, Y. Preparation and Tribocorrosion Performance of CrCN Coatings in Artificial Seawater on Different Substrates with Different Bias Voltages. Ceram. Int. 2019, 45, 9901–9911. [Google Scholar] [CrossRef]
- Liu, C.; Ju, H.; Han, P.; Quan, C.; Mo, C.; Zhao, Y.; Geng, Y.; Xu, J.; Yu, L. The Influence of Carbon Content on the Microstructure, Mechanical and Frictional Property of Chromium Carbon Nitride Composite Films. Vacuum 2020, 178, 109368. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Wang, C.; Li, J.; Yao, Y. An Analysis on Tribological Performance of CrCN Coatings with Different Carbon Contents in Seawater. Tribol. Int. 2015, 91, 131–139. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Chen, H.; Li, J.; Zhou, S.; Xue, Q. Influences of Bias Voltage on the Microstructures and Tribological Performances of Cr–C–N Coatings in Seawater. Surf. Coatings Technol. 2015, 270, 305–313. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Xu, Z.; Li, Z.; Su, F. Microstructure, Electrochemical and Tribocorrosion Behaviors of CrCN Nanocomposite Coating with Various Carbon Content. Surf. Coatings Technol. 2021, 411, 126997. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhou, S.; Wang, Y.; Wang, C.; Wang, Y.; Sui, Y.; Lan, J.; Xue, Q. Improvement in the Tribocorrosion Performance of CrCN Coating by Multilayered Design for Marine Protective Application. Appl. Surf. Sci. 2020, 528, 147061. [Google Scholar] [CrossRef]
- Gunen, A.; Kalkandelen, M.; Karahan, I.H.; Kurt, B.; Kanca, E.; Sabri Gok, M.; Serdar Karakas, M. Properties and Corrosion Behavior of Chromium and Vanadium Carbide Composite Coatings Produced on Ductile Cast Iron by Thermoreactive Diffusion Technique. J. Eng. Mater. Technol. 2020, 142, 1–12. [Google Scholar] [CrossRef]
- Kim, Y.J.; Byun, T.J.; Han, J.G. Tribological and Mechanical Behaviors of CrN/a-CNx Superlattice Thin Films. Surf. Coatings Technol. 2008, 203, 790–793. [Google Scholar] [CrossRef]
- Pengfei, H.; Bailing, J. Study on Tribological Property of CrCN Coating Based on Magnetron Sputtering Plating Technique. Vacuum 2011, 85, 994–998. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.; Ding, X.; Zhou, Z.; Wang, C.; Zhang, W.; Li, L.K.-Y.; Lee, S.-T. Microstructure and Water-Lubricated Friction and Wear Properties of CrN(C) Coatings with Different Carbon Contents. Appl. Surf. Sci. 2013, 268, 579–587. [Google Scholar] [CrossRef]
- Gilewicz, A.; Chmielewska, P.; Murzynski, D.; Dobruchowska, E.; Warcholinski, B. Corrosion Resistance of CrN and CrCN/CrN Coatings Deposited Using Cathodic Arc Evaporation in Ringer’s and Hank’s Solutions. Surf. Coatings Technol. 2016, 299, 7–14. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Stueber, M.; Holleck, H.; Leiste, H.; Seemann, K.; Ulrich, S.; Ziebert, C. Concepts for the Design of Advanced Nanoscale PVD Multilayer Protective Thin Films. J. Alloys Compd. 2009, 483, 321–333. [Google Scholar] [CrossRef]
- Holleck, H.; Schier, V. Multilayer PVD Coatings for Wear Protection. Surf. Coatings Technol. 1995, 76–77, 328–336. [Google Scholar] [CrossRef]
- Kong, Y.; Tian, X.; Gong, C.; Chu, P.K. Enhancement of Toughness and Wear Resistance by CrN/CrCN Multilayered Coatings for Wood Processing. Surf. Coatings Technol. 2018, 344, 204–213. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Beresnev, V.M.; Bondar, O.V.; Postolnyi, B.O.; Zaleski, K.; Coy, E.; Jurga, S.; Lisovenko, M.O.; Konarski, P.; Rebouta, L.; et al. Superhard CrN/MoN Coatings with Multilayer Architecture. Mater. Des. 2018, 153, 47–59. [Google Scholar] [CrossRef]
- Dai, W.; Wang, Q.; Kim, K.-H.; Kwon, S.-H. Al2O3/CrAlSiN Multilayer Coating Deposited Using Hybrid Magnetron Sputtering and Atomic Layer Deposition. Ceram. Int. 2019, 45, 11335–11341. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, T.F.; Lee, H.-B.-R.; Yang, J.H.; Choi, W.C.; Han, B.; Kim, K.H.; Kwon, S.-H. Improved Corrosion Resistance and Mechanical Properties of CrN Hard Coatings with an Atomic Layer Deposited Al2O3 Interlayer. ACS Appl. Mater. Interfaces 2015, 7, 26716–26725. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Li, C.; Sun, X.; Xuan, Y.; Zhai, H.; Li, A.; Wang, Q.; Zhou, F. Improved Tribological Properties and Corrosion Protection of CrN Coating by Ultrathin Composite Oxide Interlayer. Appl. Surf. Sci. 2021, 541, 148606. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Xu, P.; Cao, Y.-Q.; Li, A.-D.; Wang, Q.-Z.; Zhou, F. Improved Corrosion Protection of CrN Hard Coating on Steel Sealed with TiOxNy-TiN Composite Layers. Surf. Coatings Technol. 2020, 381, 125108. [Google Scholar] [CrossRef]
- Kaady, E.; Habchi, R.; Bechelany, M.; Zgheib, E.; Alhussein, A. Effect of Al2O3, ZnO and TiO2 Atomic Layer Deposition Grown Thin Films on the Electrochemical and Mechanical Properties of Sputtered Al-Zr Coating. Coatings 2023, 13, 65. [Google Scholar] [CrossRef]
- Cremers, V.; Puurunen, R.L.; Dendooven, J. Conformality in Atomic Layer Deposition : Current Status Overview of Analysis and Modelling. Appl. Phys. Rev. 2019, 6, 021302. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Graniel, O.; Weber, M.; Balme, S.; Miele, P.; Bechelany, M. Atomic Layer Deposition for Biosensing Applications. Biosens. Bioelectron. 2018, 122, 147–159. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Ayral, A.; Miele, P.; Bechelany, M. Atomic Layer Deposition for Membranes: Basics, Challenges, and Opportunities. Chem. Mater. 2018, 30, 7368–7390. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Paussa, L.; Guzman, L.; Fedrizzi, L. Long Term Performance of Atomic Layer Deposition Coatings for Corrosion Protection of Stainless Steel. Mater. Corros. 2015, 66, 907–914. [Google Scholar] [CrossRef]
- Marin, E.; Guzman, L.; Lanzutti, A.; Fedrizzi, L.; Saikkonen, M. Chemical and Electrochemical Characterization of Hybrid PVD+ALD Hard Coatings on Tool Steel. Electrochem. Commun. 2009, 11, 2060–2063. [Google Scholar] [CrossRef]
- Potts, S.E.; Schmalz, L.; Fenker, M.; Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Radnóczi, G.; Tóth, L.; et al. Ultra-Thin Aluminium Oxide Films Deposited by Plasma-Enhanced Atomic Layer Deposition for Corrosion Protection. J. Electrochem. Soc. 2011, 158, C132–C138. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Światowska, J.; Seyeux, A.; Maurice, V.; Ritala, M.; Marcus, P. Corrosion Properties of Steel Protected by Nanometre-Thick Oxide Coatings. Corros. Sci. 2014, 82, 208–217. [Google Scholar] [CrossRef]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Zanna, S.; Salmi, E.; Ritala, M.; Marcus, P. Corrosion Protection of Aluminium by Ultra-Thin Atomic Layer Deposited Alumina Coatings. Corros. Sci. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion Protection of Copper Using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Díaz, B.; Härkönen, E.; Maurice, V.; Światowska, J.; Seyeux, A.; Ritala, M.; Marcus, P. Failure Mechanism of Thin Al2O3 Coatings Grown by Atomic Layer Deposition for Corrosion Protection of Carbon Steel. Electrochim. Acta 2011, 56, 9609–9618. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, T.F.; Ding, J.C.; Kim, C.-M.; Park, S.-W.; Yang, Y.; Kim, K.-H.; Kwon, S.-H. Enhanced Corrosion Resistance of PVD-CrN Coatings by ALD Sealing Layers. Nanoscale Res. Lett. 2017, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Leppäniemi, J.; Sippola, P.; Broas, M.; Aromaa, J.; Lipsanen, H.; Koskinen, J. Corrosion Protection of Steel with Multilayer Coatings: Improving the Sealing Properties of Physical Vapor Deposition CrN Coatings with Al2O3/TiO2 Atomic Layer Deposition Nanolaminates. Thin Solid Films 2017, 627, 59–68. [Google Scholar] [CrossRef]
- Härkönen, E.; Kolev, I.; Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Fenker, M.; Toth, L.; Radnoczi, G.; et al. Sealing of Hard CrN and DLC Coatings with Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2014, 6, 1893–1901. [Google Scholar] [CrossRef]
- Kaady, E.; Alhussein, A.; Bechelany, M.; Habchi, R. Al2O3-ZnO Atomic Layer Deposited Nanolaminates for Improving Mechanical and Corrosion Properties of Sputtered CrN Coatings. Thin Solid Films 2022, 759, 139476. [Google Scholar] [CrossRef]
- Leppäniemi, J.; Sippola, P.; Peltonen, A.; Aromaa, J.J.; Lipsanen, H.; Koskinen, J. Effect of Surface Wear on Corrosion Protection of Steel by CrN Coatings Sealed with Atomic Layer Deposition. ACS Omega 2018, 3, 1791–1800. [Google Scholar] [CrossRef]
- Staszuk, M.; Pakuła, D.; Reimann, Ł.; Kloc-Ptaszna, A.; Pawlyta, M.; Kříž, A. Structure and Properties of TiO2/NanoTiO2 Bimodal Coatings Obtained by a Hybrid PVD/ALD Method on 316L Steel Substrate. Materials 2021, 14, 4369. [Google Scholar] [CrossRef] [PubMed]
- Billard, A.; Perry, F. Pulvérisation Cathodique Magnétron. In Matériaux|Trait. des Métaux; Techniques de l’ingénieur: Paris, France, 2005. [Google Scholar]
- Gautier, C.; Machet, J. Study of the Growth Mechanisms of Chromium Nitride Films Deposited by Vacuum ARC Evaporation. Thin Solid Films 1997, 295, 43–52. [Google Scholar] [CrossRef]
- Guan, J.J.; Wang, H.Q.; Qin, L.Z.; Liao, B.; Liang, H.; Li, B. Phase Transitions of Doped Carbon in CrCN Coatings with Modified Mechanical and Tribological Properties via Filtered Cathodic Vacuum Arc Deposition. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 397, 86–91. [Google Scholar] [CrossRef]
- Lasalmonie, A.; Strudel, J.L. Influence of Grain Size on the Mechanical Behaviour of Some High Strength Materials. J. Mater. Sci. 1986, 21, 1837–1852. [Google Scholar] [CrossRef]
- Tong, C.-Y.; Lee, J.-W.; Kuo, C.-C.; Huang, S.-H.; Chan, Y.-C.; Chen, H.-W.; Duh, J.-G. Effects of Carbon Content on the Microstructure and Mechanical Property of Cathodic Arc Evaporation Deposited CrCN Thin Films. Surf. Coatings Technol. 2013, 231, 482–486. [Google Scholar] [CrossRef]
- Ruden, A.; Restrepo-Parra, E.; Paladines, A.U.; Sequeda, F. Corrosion Resistance of CrN Thin Films Produced by Dc Magnetron Sputtering. Appl. Surf. Sci. 2013, 270, 150–156. [Google Scholar] [CrossRef]
- Liao, B.; Luo, Z.; Wan, S.; Chen, L. Insight into the Anti-Corrosion Performance of Acanthopanax Senticosus Leaf Extract as Eco-Friendly Corrosion Inhibitor for Carbon Steel in Acidic Medium. J. Ind. Eng. Chem. 2023, 117, 238–246. [Google Scholar] [CrossRef]


| Coatings | CH4 Flow (sccm) | Elemental Composition (at.%) Er (C, N, O) ~ 5% Er (Cr) ~ 3% | C/N | (C+N)/Cr | |||
|---|---|---|---|---|---|---|---|
| Cr | N | C | O | ||||
| CrN | - | 58 | 38 | - | 4 | - | 0.7 |
| CrCN-2 | 2 | 43 | 36 | 17 | 4 | 0.5 | 1.3 |
| CrCN-3 | 3 | 36 | 36 | 21 | 7 | 0.6 | 1.6 |
| CrCN-4 | 4 | 34 | 34 | 27 | 5 | 0.8 | 1.8 |
| CrCN-5 | 5 | 30 | 38 | 28 | 4 | 0.7 | 2.2 |
| CrCN-2/ALD | 2 | 22 | 45 | 22 | 11 | 0.5 | 3.1 |
| CrCN-4/ALD | 4 | 24 | 44 | 24 | 8 | 0.5 | 2.8 |
| CrCN-5/ALD | 5 | 28 | 38 | 29 | 5 | 0.8 | 2.3 |
| Sample | CrN | CrCN-2 | CrCN-3 | CrCN-4 | CrCN-5 | CrCN-2/ALD | CrCN-4/ALD | CrCN-5/ALD |
|---|---|---|---|---|---|---|---|---|
| Crystallite Size (nm) | 9 ± 1 | 46 ± 1 | 49 ± 1 | 30 ± 1 | 14 ± 1 | 39 ± 1 | 30 ± 1 | 15 ± 1 |
| Lattice parameter (Å) | 4.011 | 4.137 | 4.150 | 4.157 | 4.174 | 4.148 | 4.152 | 4.171 |
| Sample | CrN | CrCN-2 | CrCN-3 | CrCN-4 | CrCN-5 | CrCN-2/ALD | CrCN-4/ALD | CrCN-5/ALD |
|---|---|---|---|---|---|---|---|---|
| H (GPa) | 19 ± 1 | 11 ± 1 | 15 ± 2 | 20 ± 2 | 19 ± 1 | 14 ± 1 | 19 ± 1 | 19 ± 1 |
| Er (GPa) | 220 ± 5 | 172 ± 8 | 160 ± 10 | 181 ± 8 | 167 ± 3 | 158 ± 4 | 192 ± 8 | 170 ± 5 |
| Sample | CrN | CrCN-2 | CrCN-3 | CrCN-4 | CrCN-5 | CrCN-2/ALD | CrCN-4/ALD | CrCN-5/ALD |
|---|---|---|---|---|---|---|---|---|
| Ecorr (mV/SCE) | −238 | −8 | 26 | −59 | −207 | 4 | −56 | −50 |
| Jcorr (×10−8 A·cm−2) | 79 | 60 | 48 | 2.5 | 5 | 43 | 1.5 | 1.2 |
| βc (mV/decade) | 28 | 23 | 56 | 83 | 119 | 12 | 56 | 38 |
| βa (mV/decade) | 300 | 385 | 234 | 190 | 130 | 500 | 148 | 370 |
| Rp (kΩ·cm2) | 32 | 36 | 95 | 2283 | 1230 | 27 | 2655 | 2945 |
| Epit (mV/SCE) | 122 | 285 | 763 | 224 | 8 | 918 | 666 | 662 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaady, E.; Sayegh, S.; Bechelany, M.; Habchi, R.; Alhussein, A. A Comparative Study on the Influence of Al2O3-ZnO ALD Nanolaminates on the Properties of CrCN PVD Sputtered Coatings with Distinctive Morphologies and Microstructures. Coatings 2023, 13, 1017. https://doi.org/10.3390/coatings13061017
Kaady E, Sayegh S, Bechelany M, Habchi R, Alhussein A. A Comparative Study on the Influence of Al2O3-ZnO ALD Nanolaminates on the Properties of CrCN PVD Sputtered Coatings with Distinctive Morphologies and Microstructures. Coatings. 2023; 13(6):1017. https://doi.org/10.3390/coatings13061017
Chicago/Turabian StyleKaady, Elias, Syreina Sayegh, Mikhael Bechelany, Roland Habchi, and Akram Alhussein. 2023. "A Comparative Study on the Influence of Al2O3-ZnO ALD Nanolaminates on the Properties of CrCN PVD Sputtered Coatings with Distinctive Morphologies and Microstructures" Coatings 13, no. 6: 1017. https://doi.org/10.3390/coatings13061017





