Relationship between Osteoblast Proliferation and the Surface Properties of Polymer-like Carbon Films Deposited at Different Ar/CH4 Mixed-Gas Ratios in the Radio-Frequency Plasma CVD Process
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Deposition Conditions of the PLC Film
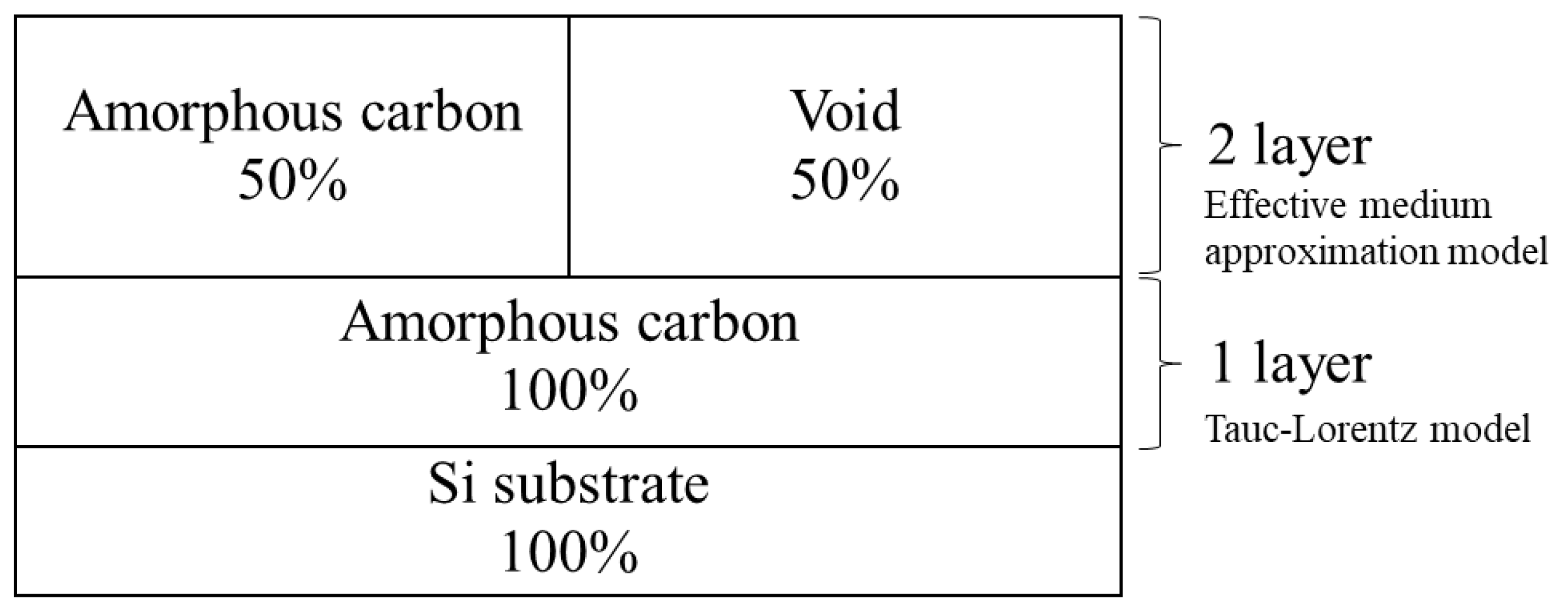
2.2. Spectroscopic Ellipsometer Analysis
2.3. Structural Analysis
2.4. Cell Culture Test
2.5. Statistical Analysis
2.6. Cell Morphology
2.7. Surface Analysis
3. Results and Discussion
3.1. Confirmation of Amorphous Carbon-Type Based on n-k Plots
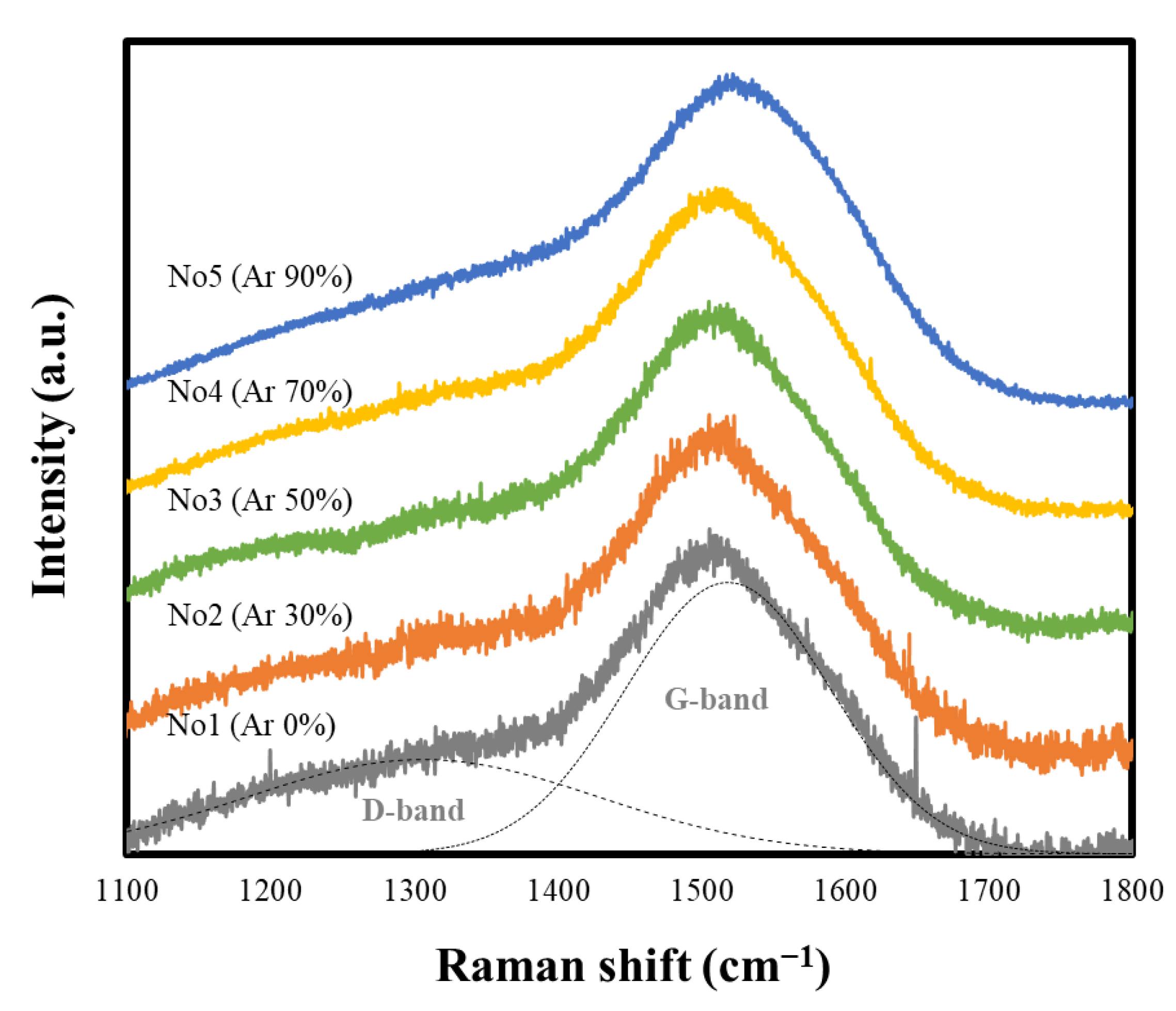
3.2. Structural Changes of the PLC Film with Ar/CH4 Gas Mixture Ratios
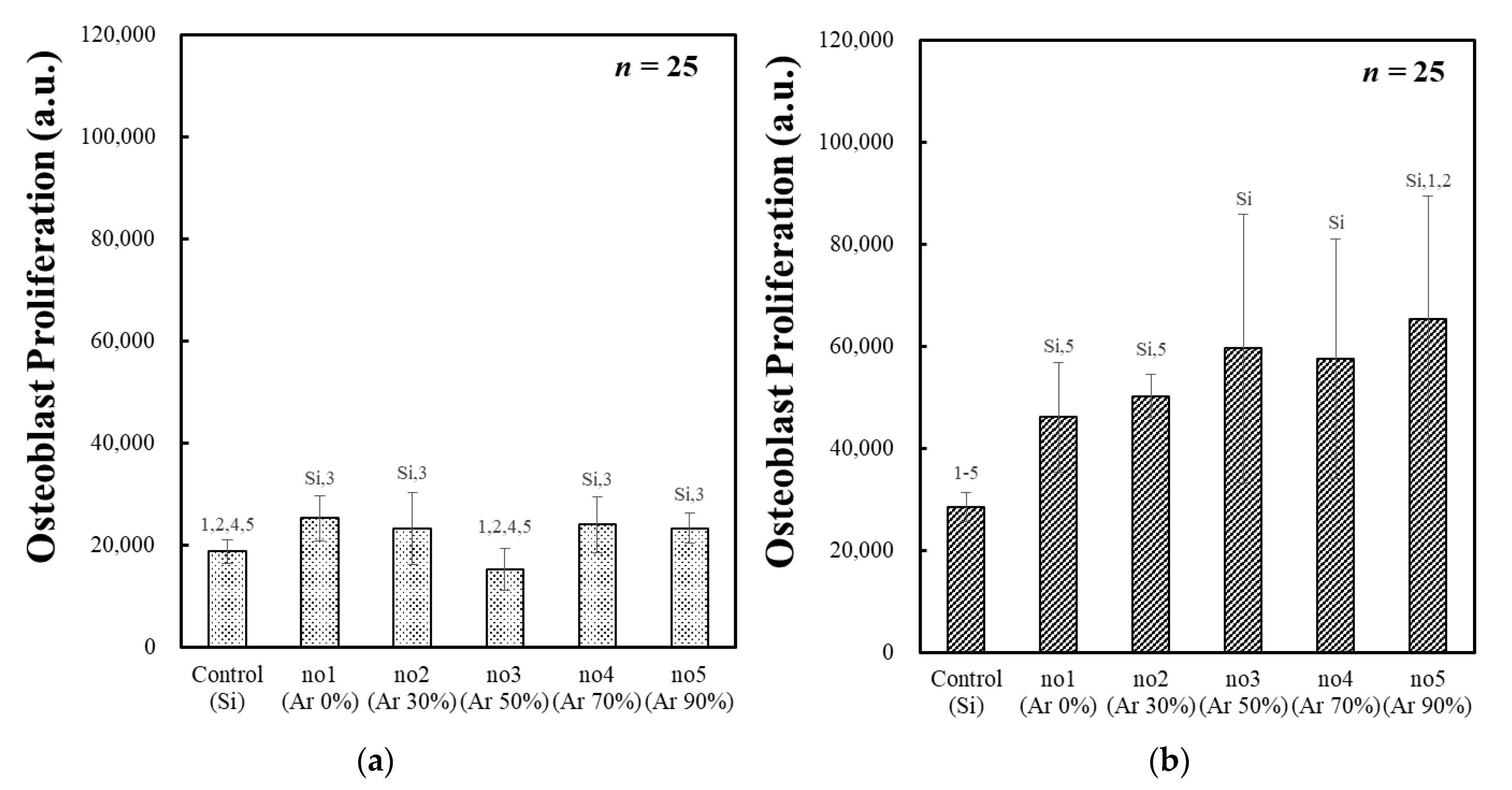
3.3. Osteoblast Proliferation of the PLC Film with Ar/CH4 Gas Mixture Ratio
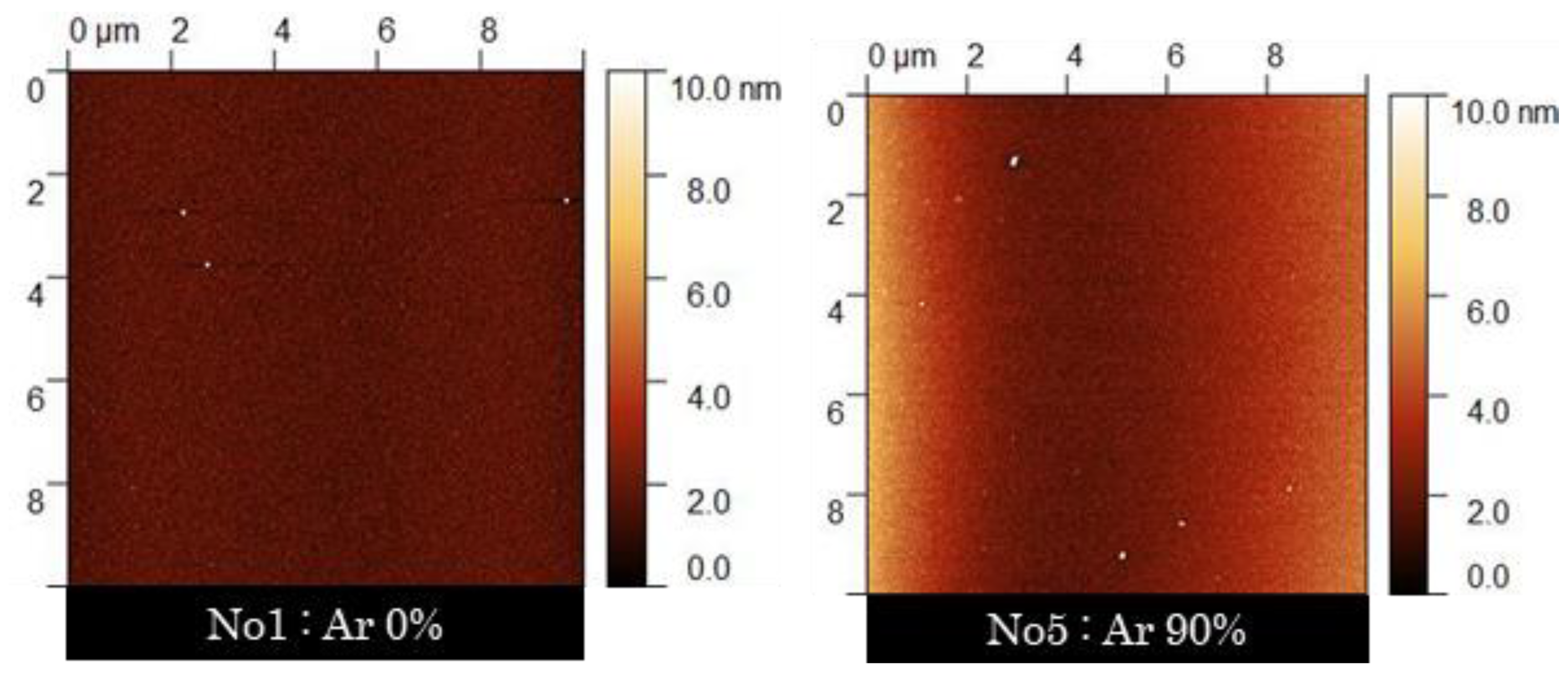
3.4. Change in Surface Properties of the PLC Film with Ar/CH4 Mixed-Gas Ratio
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K.A. Multifunctional Coatings on Implant Materials—A Systematic Review of the Current Scenario. Coatings 2022, 13, 69. [Google Scholar] [CrossRef]
- Filip, N.; Radu, I.; Veliceasa, B.; Filip, C.; Pertea, M.; Clim, A.; Pinzariu, A.C.; Drochioi, I.C.; Hilitanu, R.L.; Serban, I.L. Biomaterials in Orthopedic Devices: Current Issues and Future Perspectives. Coatings 2022, 12, 1544. [Google Scholar] [CrossRef]
- Abdudeen, A.; Abu Qudeiri, J.E.; Kareem, A.; Valappil, A.K. Latest Developments and Insights of Orthopedic Implants in Biomaterials Using Additive Manufacturing Technologies. J. Manuf. Mater. Process. 2022, 6, 162. [Google Scholar] [CrossRef]
- Peng, Y.; Peng, J.; Wang, Z.; Xiao, Y.; Qiu, X. Diamond-like Carbon Coatings in the Biomedical Field: Properties, Applications and Future Development. Coatings 2022, 12, 1088. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.-R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 72–84. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef]
- ISO 23216:2021; Carbon-Based Films—Determination of Optical Properties of Amorphous Carbon Films by Spectroscopic Ellipsometry. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/74875.html (accessed on 22 August 2022).
- Tsubone, D.; Hasebe, T.; Kamijo, A.; Hotta, A. Fracture mechanics of diamond-like carbon (DLC) films coated on flexible polymer substrates. Surf. Coat. Technol. 2007, 201, 6423–6430. [Google Scholar] [CrossRef]
- Shirakura, A.; Nakaya, M.; Koga, Y.; Kodama, H.; Hasebe, T.; Suzuki, T. Diamond-like carbon films for PET bottles and medical applications. Thin Solid Films 2006, 494, 84–91. [Google Scholar] [CrossRef]
- Matsuo, H.; Kanasugi, K.; Ohgoe, Y.; Hirakuri, K.K.; Fukui, Y. Biocompatibility of a-C:H film coating for synthetic vascular graft. Diam. Relat. Mater. 2009, 18, 1031–1034. [Google Scholar] [CrossRef]
- As, A. Medical Application of Diamond-Like Carbon (DLC) Coating-A Review. J. Clin. Med Images 2021, 05. [Google Scholar] [CrossRef]
- Kanasugi, K.; Eguchi, H.; Ohgoe, Y.; Manome, Y.; Alanazi, A.; Hirakuri, K. Correlation of Cell Proliferation with Surface Properties of Polymer-like Carbon Films of Different Thicknesses Prepared by a Radio-Frequency Plasma CVD Process. Materials 2022, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Cho, S.M.; Choi, W.S.; Hong, B.; Yoon, D.H. Dependence of the bonding structure of DLC thin films on the deposition conditions of PECVD method. Surf. Coat. Technol. 2003, 169-170, 291–294. [Google Scholar] [CrossRef]
- Caschera, D.; Cossari, P.; Federici, F.; Kaciulis, S.; Mezzi, A.; Padeletti, G.; Trucchi, D.M. Influence of PECVD parameters on the properties of diamond-like carbon films. Thin Solid Films 2011, 519, 4087–4091. [Google Scholar] [CrossRef]
- Varade, A.; Reddy, K.N.; Sasen, D.; Krishna, A.; Chellamalai, M.; Shashikumar, P. Detailed Raman Study of DLC Coating on Si (100) Made by RF-PECVD. Procedia Eng. 2014, 97, 1452–1456. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, D.Y.; Bae, I.H.; Kim, G.-H.; Kang, S.-K.; Ban, S.I.; Lee, J.I.; Kim, J.S.; Lee, S.-H.; Choi, H.K.; et al. Growth of Room-Temperature 1.32-mum-Emitting InAs Quantum Dots Using GaAs/InAs Quasi-Monolayers. J. Korean Phys. Soc. 2003, 42, 237. [Google Scholar] [CrossRef]
- Samadi, M.; Eshaghi, A.; Bakhshi, S.R.; Aghaei, A.A. The influence of gas flow rate on the structural, mechanical, optical and wettability of diamond-like carbon thin films. Opt. Quantum Electron. 2018, 50, 193. [Google Scholar] [CrossRef]
- Toro, R.; Calandra, P.; Cortese, B.; de Caro, T.; Brucale, M.; Mezzi, A.; Federici, F.; Caschera, D. Argon and hydrogen plasma influence on the protective properties of diamond-like carbon films as barrier coating. Surf. Interfaces 2017, 6, 60–71. [Google Scholar] [CrossRef]
- Liao, T.T.; Zhang, T.F.; Li, S.S.; Deng, Q.Y.; Wu, B.J.; Zhang, Y.Z.; Zhou, Y.J.; Guo, Y.B.; Leng, Y.X.; Huang, N. Biological responses of diamond-like carbon (DLC) films with different structures in biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 751–759. [Google Scholar] [CrossRef]
- Manome, Y.; Tachibana, T.; Hiratsuka, M.; Sato, K.; Ohgoe, Y.; Hirakuri, K. Osteoblastic MC3T3-E1 cells on diamond-like carbon-coated silicon plates: Field emission scanning electron microscopy data. Data Brief 2021, 38, 107385. [Google Scholar] [CrossRef]
- Fernández-Lizárraga, M.; García-López, J.; Rodil, S.E.; Ribas-Aparicio, R.M.; Silva-Bermudez, P. Evaluation of the Biocompatibility and Osteogenic Properties of Metal Oxide Coatings Applied by Magnetron Sputtering as Potential Biofunctional Surface Modifications for Orthopedic Implants. Materials 2022, 15, 5240. [Google Scholar] [CrossRef]
- Kanasugi, K.; Arimura, K.; Alanazi, A.; Ohgoe, Y.; Manome, Y.; Hiratsuka, M.; Hirakuri, K. UV Sterilization Effects and Osteoblast Proliferation on Amorphous Carbon Films Classified Based on Optical Constants. Bioengineering 2022, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Kanasugi, K.; Ohgoe, Y.; Hiratsuka, M.; Nakamori, H.; Homma, A.; Hirakuri, K. Classification of DLC films for cell proliferation based on optical constants. Diam. Relat. Mater. 2021, 113, 108266. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Hardjawinata, K.; Setiawati, R.; Dewi, W. Bactericidal Efficacy of Ultraviolet Irradiation on Staphylococcus aureus. Asian J. Oral Maxillofac. Surg. 2005, 17, 157–161. [Google Scholar] [CrossRef]
- Kanasugi, K.; Ohgoe, Y.; Hirakuri, K.K.; Fukui, Y. Cytocompatibility of modified a-C:H film deposited on complicated polymeric medical apparatus. J. Appl. Phys. 2009, 105, 094702. [Google Scholar] [CrossRef]
- Marciano, F.R.; Marcuzzo, J.S.; Bonetti, L.F.; Corat, E.J.; Trava-Airoldi, V.J. Use of near atmospheric pressure and low pressure techniques to modification DLC film surface. Surf. Coat. Technol. 2009, 204, 64–68. [Google Scholar] [CrossRef]
- Nitta, Y.; Okamoto, K.; Nakatani, T.; Hoshi, H.; Homma, A.; Tatsumi, E.; Taenaka, Y. Diamond-like carbon thin film with controlled zeta potential for medical material application. Diam. Relat. Mater. 2008, 17, 1972–1976. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Huang, G.; Xi, L.; Qin, G.; Zhu, M.; Chu, H. Fabrication and applications of the optical diamond-like carbon films: A review. J. Mater. Sci. 2022, 57, 3971–3992. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Nakamori, H.; Kogo, Y.; Sakurai, M.; Ohtake, N.; Saitoh, H. Correlation between Optical Properties and Hardness of Diamond-Like Carbon Films. J. Solid Mech. Mater. Eng. 2013, 7, 187–198. [Google Scholar] [CrossRef]
- Safaie, P.; Eshaghi, A.; Bakhshi, S.R. Oxygen doping effect on the wettability of diamond-like carbon thin films. J. Non-Cryst. Solids 2017, 471, 410–414. [Google Scholar] [CrossRef]
- Ohgoe, Y.; Hirakuri, K.K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC films in terms of biological response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]
- Bachmann, S.; Schulze, M.; Morasch, J.; Hesse, S.; Hussein, L.; Krell, L.; Schnagl, J.; Stark, R.W.; Narayan, S. Aging of oxygen and hydrogen plasma discharge treated a-C:H and ta-C coatings. Appl. Surf. Sci. 2016, 371, 613–623. [Google Scholar] [CrossRef]
- Harigai, T.; Yasuoka, Y.; Nitta, N.; Furuta, H.; Hatta, A. X-ray reflectivity analysis on initial stage of diamond-like carbon film deposition on Si substrate by RF plasma CVD and on removal of the sub-surface layer by oxygen plasma etching. Diam. Relat. Mater. 2013, 38, 36–40. [Google Scholar] [CrossRef]
- Washburn, N.R.; Yamada, K.M.; Simon, C.G., Jr.; Kennedy, S.B.; Amis, E.J. High-throughput investigation of osteoblast response to polymer crystallinity: Influence of nanometer-scale roughness on proliferation. Biomaterials 2004, 25, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wan, Y.; Ren, B.; Liu, Z. Fabrication of an orderly micro/nanostructure on titanium surface and its effect on cell proliferation. Mater. Lett. 2018, 212, 247–250. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, C.; Luo, J. Effect of substrate morphology on the roughness evolution of ultra thin DLC films. Appl. Surf. Sci. 2008, 254, 6742–6748. [Google Scholar] [CrossRef]
- Al-Azizi, A.A.; Eryilmaz, O.; Erdemir, A.; Kim, S.H. Nano-texture for a wear-resistant and near-frictionless diamond-like carbon. Carbon 2014, 73, 403–412. [Google Scholar] [CrossRef]


| Sample No. | Deposition Method | Power (W) | Source Gas | Gas Pressure (Pa) | ||
|---|---|---|---|---|---|---|
| CH4 (sccm) | Ar (sccm) | Ar Gas Ratio (%) | ||||
| 1 | 13.56 MHz RF-PCVD | 200 | 100 | 0 | 0 | 50 |
| 2 | 70 | 30 | 30 | |||
| 3 | 50 | 50 | 50 | |||
| 4 | 30 | 70 | 70 | |||
| 5 | 10 | 90 | 90 | |||
| Cell | Osteoblast (MC-3T3) |
|---|---|
| Seeding density | 1.0 × 104 cells/cm2 |
| Medium | MEM-α |
| CO2 concentration | 5.0% |
| Temperature | 37.0 °C |
| Incubation time | 24 h, 72 h |
| pH | 6.8–7.2 |
| Sample No. | Ar Gas Ratio (%) | χ2 | 1 Layer | 2 Layer | ||
|---|---|---|---|---|---|---|
| Film Thickness (nm) | n | k | Optical Surface Roughness (nm) | |||
| λ = 550 nm | ||||||
| 1 | 0 | 2.47 | 192.5 ± 0.2 | 1.796 | 0.020 | 0.7 ± 0.3 |
| 2 | 30 | 2.17 | 190.0 ± 0.2 | 1.796 | 0.019 | 0.8 ± 0.3 |
| 3 | 50 | 1.98 | 259.6 ± 0.3 | 1.885 | 0.054 | 1.3 ± 0.3 |
| 4 | 70 | 1.81 | 251.2 ± 0.3 | 1.941 | 0.079 | 2.1 ± 0.3 |
| 5 | 90 | 1.85 | 238.8 ± 0.4 | 2.018 | 0.135 | 2.2 ± 0.3 |
| Sample No. | Ar Gas Ratio (%) | D-Peak Position (cm−1) | G-Peak Position (cm−1) | ID/IG Intensity Ratio |
|---|---|---|---|---|
| 1 | 0 | 1306 ± 2.3 | 1519 ± 0.4 | 0.35 |
| 2 | 30 | 1322 ± 5.3 | 1520 ± 0.3 | 0.37 |
| 3 | 50 | 1331 ± 2.8 | 1520 ± 0.2 | 0.45 |
| 4 | 70 | 1323 ± 1.4 | 1524 ± 0.2 | 0.41 |
| 5 | 90 | 1355 ± 1.8 | 1538 ± 0.1 | 0.49 |
| Sample No. | Ar Gas Ratio (%) | 24 h Cell Culture | 72 h Cell Culture | ||
|---|---|---|---|---|---|
| Initial Cell Adhesion | p < 0.05 | Osteoblast Proliferation | p < 0.05 | ||
| 1 | 0 | 25,351 ± 4399 | Si,3 | 46,075 ± 10,781 | Si,5 |
| 2 | 30 | 23,274 ± 7091 | Si,3 | 50,266 ± 4236 | Si,5 |
| 3 | 50 | 15,317 ± 4163 | 1,2,4,5 | 59,585 ± 26,347 | Si |
| 4 | 70 | 24,081 ± 5454 | Si,3 | 57,486 ± 23,450 | Si |
| 5 | 90 | 23,370 ± 3012 | Si,3 | 65,307 ± 24,052 | Si,1,2 |
| Si substrate (Control) | - | 18,750 ± 2241 | 1,2,4,5 | 28,546 ± 2863 | 1–5 |
| Sample No. | Ar Gas Ratio (%) | Contact Angle (°) | Surface Free Energy (mJ/m2) | Surface Roughness (nm) (n = 3) | |||
|---|---|---|---|---|---|---|---|
| Pure Water (n = 10) | Methylene Iodide (n = 10) | Dispersive | Polar | Total | |||
| 1 | 0 | 79.5 ± 2.2 | 37.3 ± 2.1 | 38 | 4 | 42 | 0.95 ± 0.36 |
| 2 | 30 | 77.7 ± 1.5 | 37.8 ± 1.2 | 37 | 5 | 42 | 1.19 ± 0.15 |
| 3 | 50 | 76.0 ± 0.8 | 37.3 ± 1.6 | 37 | 6 | 43 | 1.38 ± 0.10 |
| 4 | 70 | 74.9 ± 1.1 | 34.4 ± 3.2 | 39 | 7 | 44 | 1.43 ± 0.09 |
| 5 | 90 | 70.2 ± 1.5 | 31.6 ± 1.0 | 39 | 8 | 46 | 1.61 ± 0.63 |
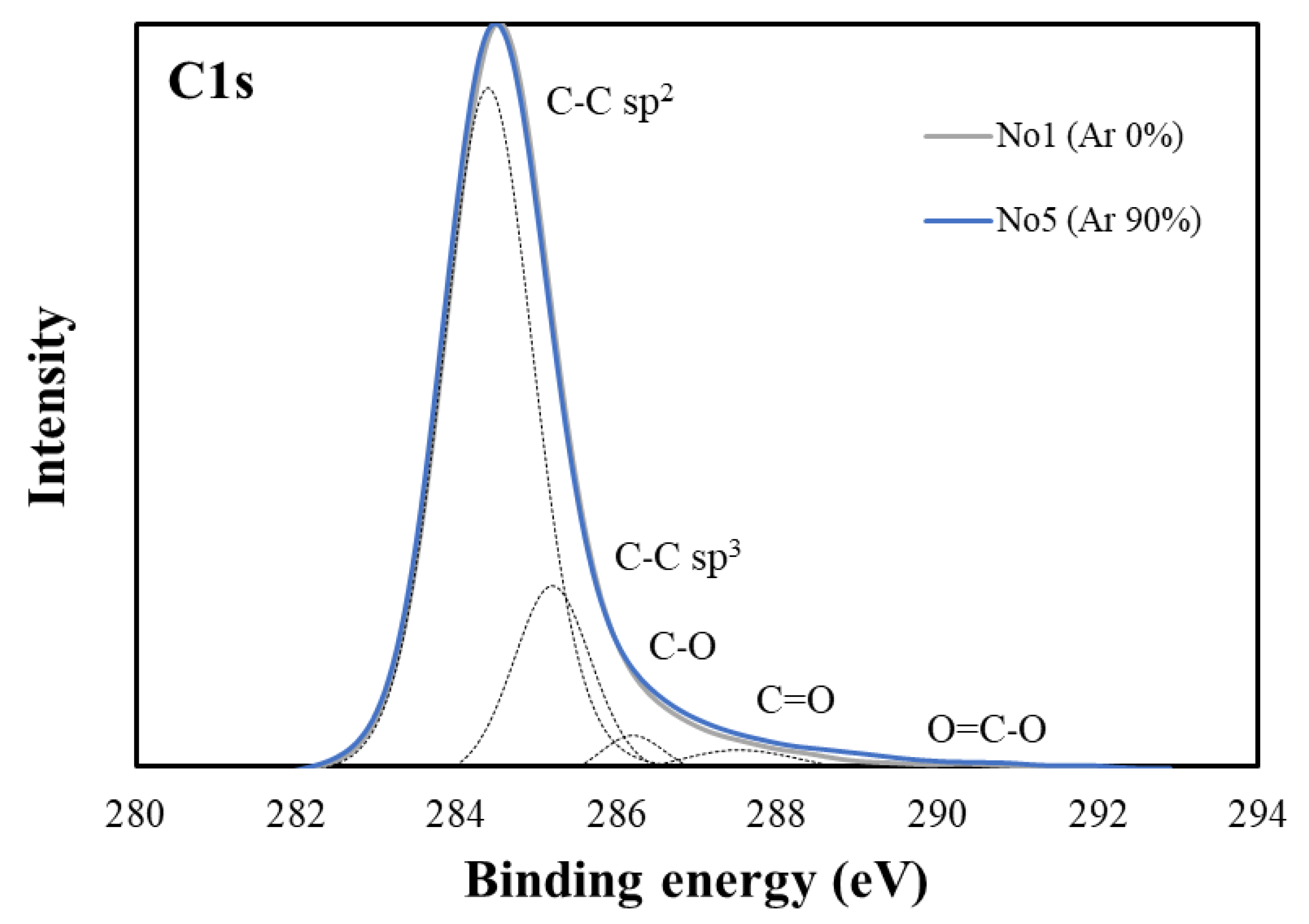
| Sample No. | Ar Gas Ratio (%) | Compositional Bond Ratio of C1s Peaks | ||||
|---|---|---|---|---|---|---|
| C-Csp2 | C-Csp3 | C-O | C=O | O=C-O | ||
| 1 | 0 | 0.74 | 0.18 | 0.03 | 0.04 | 0.01 |
| 2 | 30 | 0.76 | 0.20 | 0.02 | 0.03 | 0.00 |
| 3 | 50 | 0.78 | 0.14 | 0.04 | 0.03 | 0.01 |
| 4 | 70 | 0.81 | 0.11 | 0.05 | 0.03 | 0.01 |
| 5 | 90 | 0.81 | 0.12 | 0.04 | 0.03 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.; Kanasugi, K.; Eguchi, H.; Manome, Y.; Ohgoe, Y.; Hirakuri, K. Relationship between Osteoblast Proliferation and the Surface Properties of Polymer-like Carbon Films Deposited at Different Ar/CH4 Mixed-Gas Ratios in the Radio-Frequency Plasma CVD Process. Coatings 2023, 13, 983. https://doi.org/10.3390/coatings13060983
Alanazi A, Kanasugi K, Eguchi H, Manome Y, Ohgoe Y, Hirakuri K. Relationship between Osteoblast Proliferation and the Surface Properties of Polymer-like Carbon Films Deposited at Different Ar/CH4 Mixed-Gas Ratios in the Radio-Frequency Plasma CVD Process. Coatings. 2023; 13(6):983. https://doi.org/10.3390/coatings13060983
Chicago/Turabian StyleAlanazi, Ali, Kazuya Kanasugi, Hiroaki Eguchi, Yoshinobu Manome, Yasuharu Ohgoe, and Kenji Hirakuri. 2023. "Relationship between Osteoblast Proliferation and the Surface Properties of Polymer-like Carbon Films Deposited at Different Ar/CH4 Mixed-Gas Ratios in the Radio-Frequency Plasma CVD Process" Coatings 13, no. 6: 983. https://doi.org/10.3390/coatings13060983








