Insights into the Ligand Effect in β-CD@Fe3O4 Composites to Activate Peroxymonosulfate for Efficient Degradation of Pharmaceutical Contaminants: A Study Employing Density Functional Theory
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of β-CD@Fe3O4 Linked with CIT, PEI, and CTAB
2.3. Experimental Procedures and Chemical Analysis
2.4. Density Functional Theory (DFT) Calculations
3. Results and Discussion
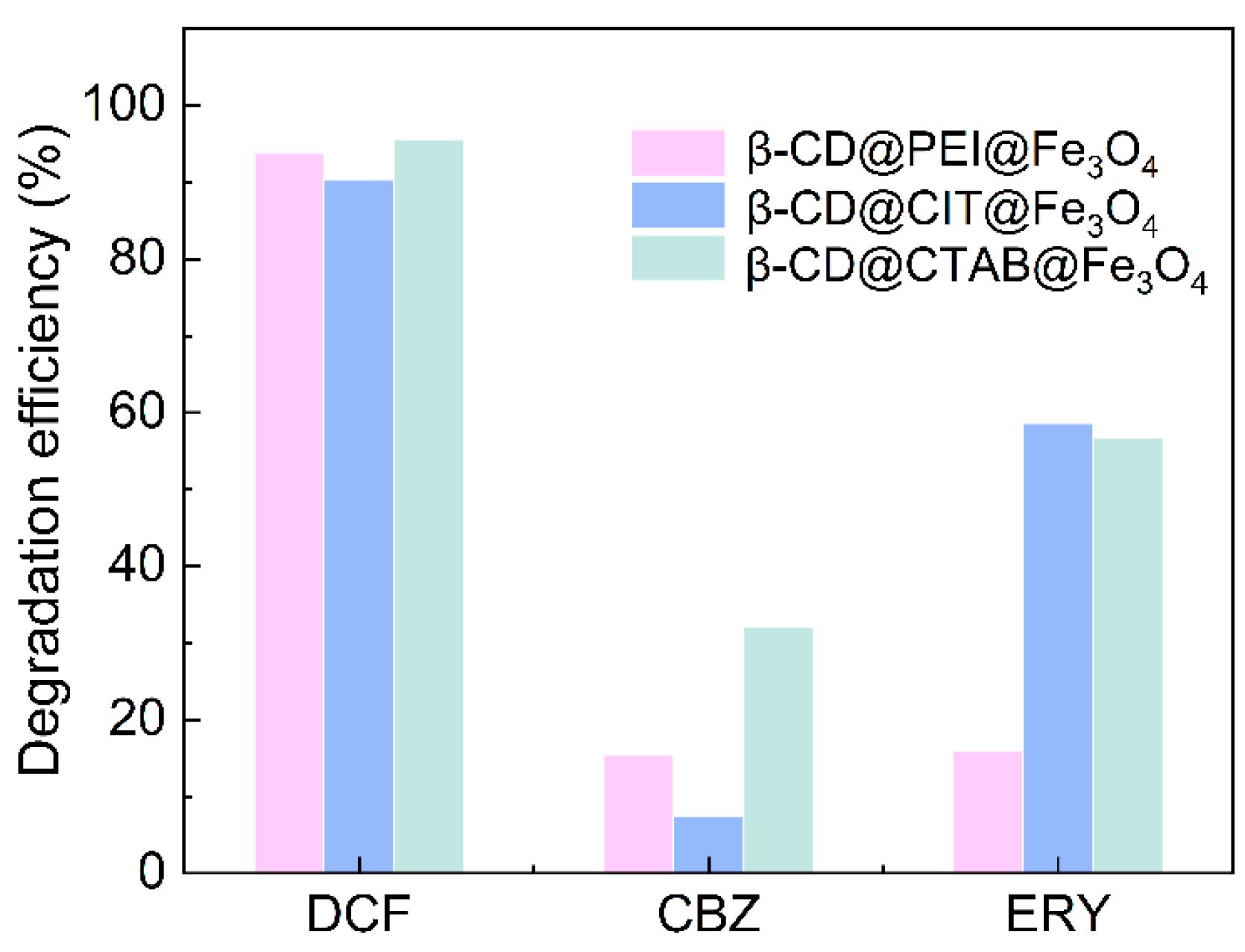
3.1. PMS Activation via β-CD@Fe3O4 towards DCF, CBZ and ERY Degradation
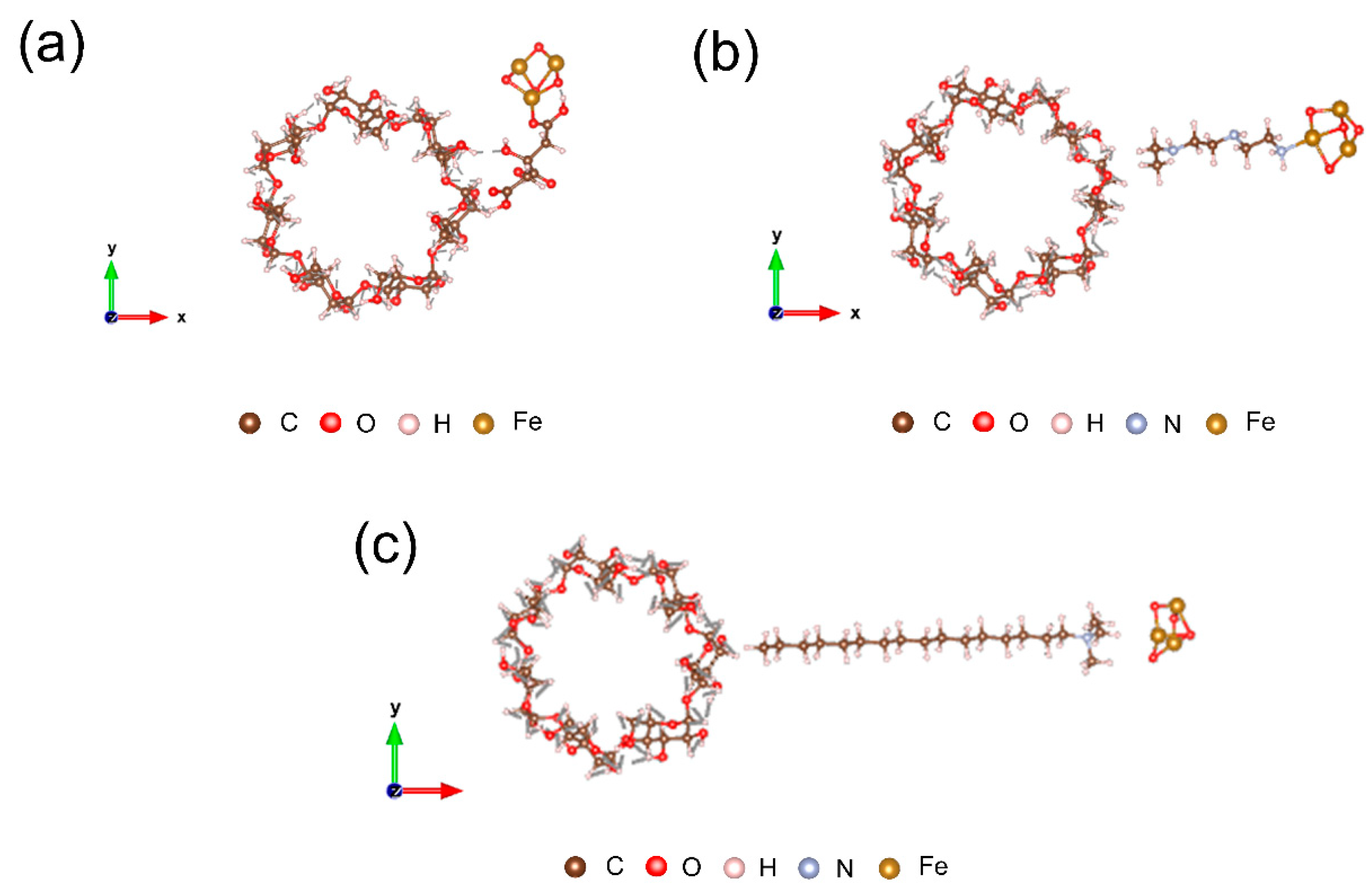
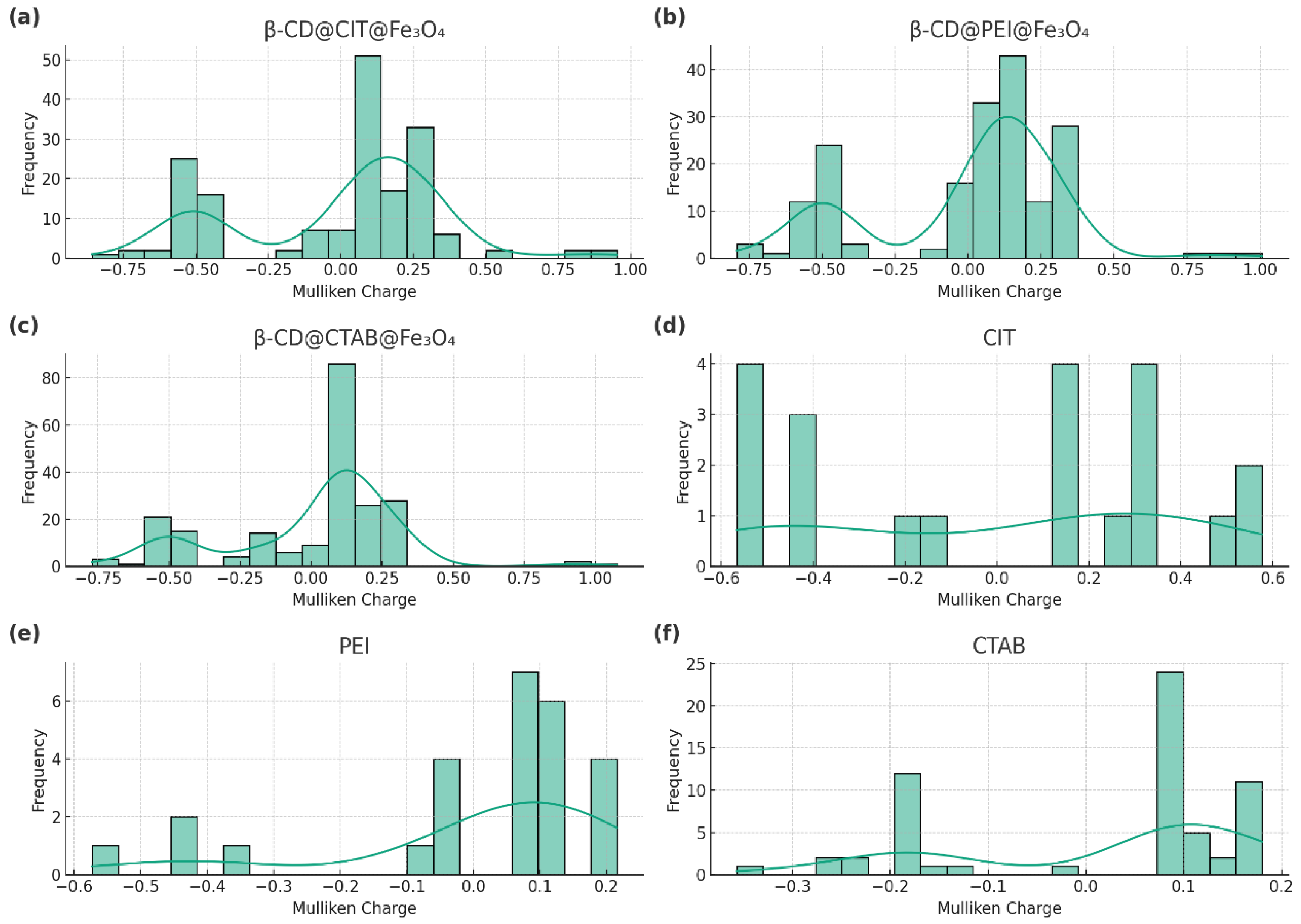
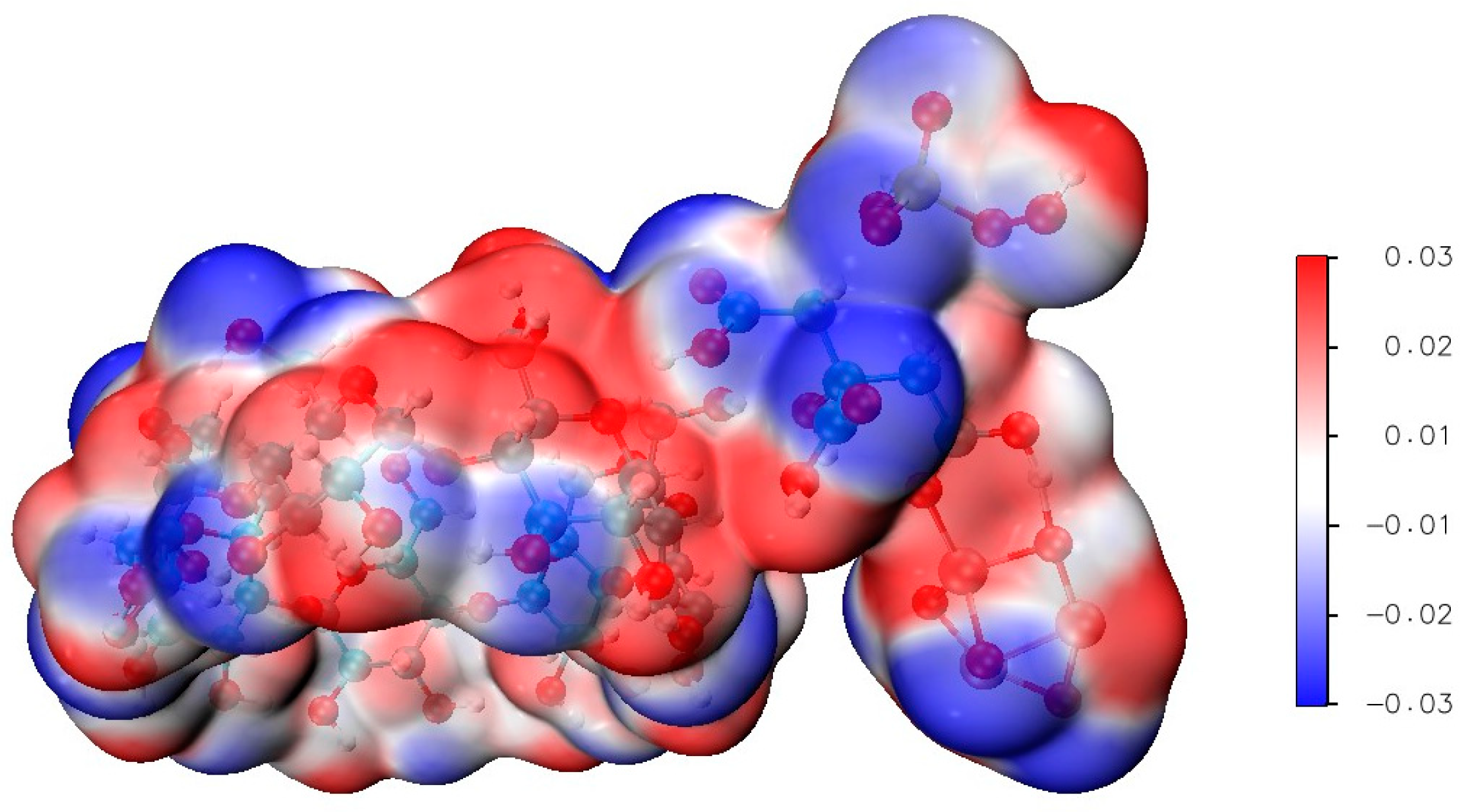
3.2. Electrostatic Properties of β-CD@Fe3O4 Nanocomposites
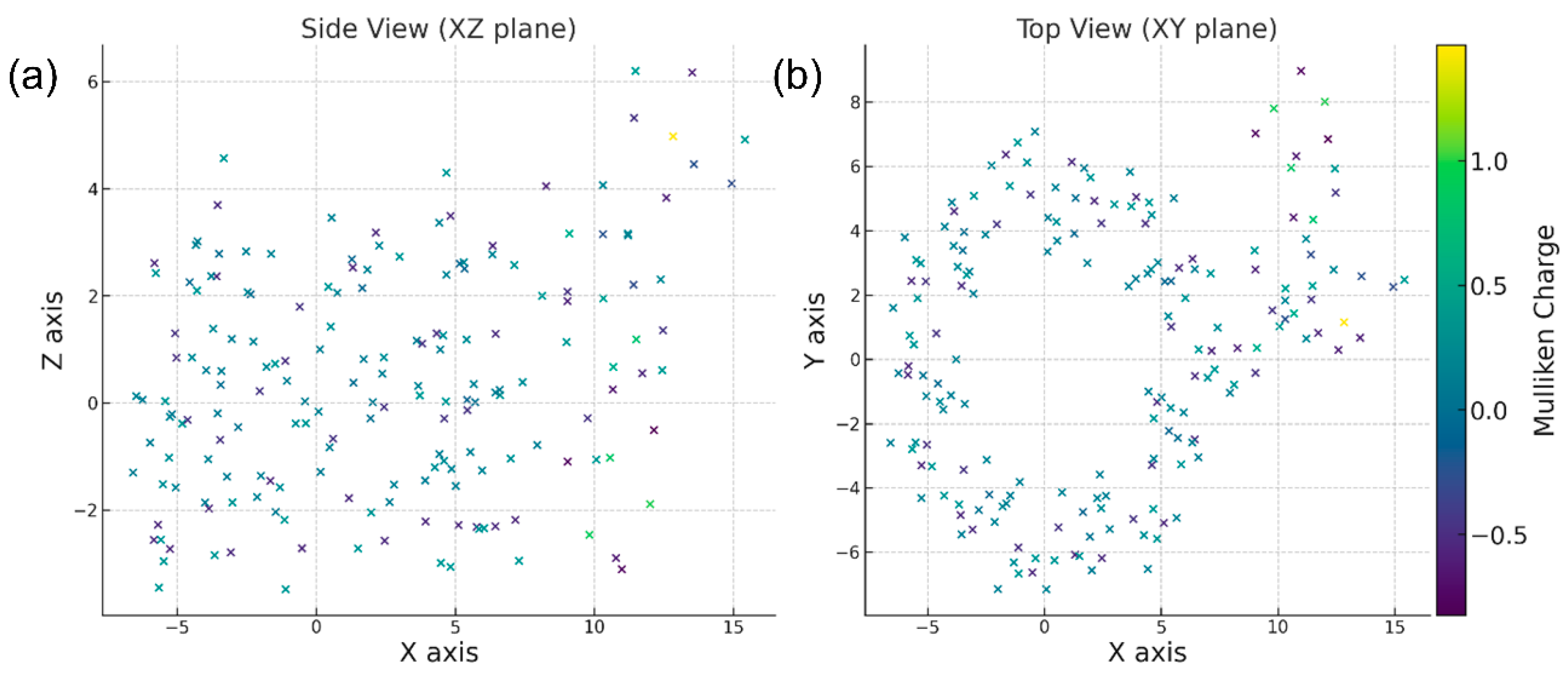
3.3. Molecular Dynamics and Electrostatic Potential Mapping of PMS Adsorption on β-CD@CTAB@Fe3O4
3.4. Electrostatic and Molecular Interactions in Pollutant Adsorption on β-CD@CTAB@Fe3O4
3.5. Diclofenac Degradation Pathways in the β-CD@CTAB@Fe3O4/PMS System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan., D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Koskue, V.; Monetti, J.; Rossi, N.; Nieradzik, L.; Freguia, S.; Kokko, M.; Ledezma, P. Fate of pharmaceuticals and PFASs during the electrochemical generation of a nitrogen-rich nutrient product from real reject water. J. Environ. Chem. Eng. 2022, 10, 107284. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; del Solar, M.R.; Levchuk, I. Photocatalytic degradation of pharmaceutically active compounds (PhACs) in urban wastewater treatment plants effluents under controlled and natural solar irradiation using immobilized TiO2. Sol. Energy 2020, 208, 480–492. [Google Scholar] [CrossRef]
- Sendra, M.; Damián-Serrano, A.; Araújo, C.V.M.; Moreno-Garrido, I.; Blasco, J. Erythromycin sensitivity across different taxa of marine phytoplankton. A novel approach to sensitivity of microalgae and the evolutionary history of the 23S gene. Aquat. Toxicol. 2018, 204, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Xie, Q.; Cai, X. Developments of cyclodextrin-coated Fe3O4@Fe0 nanoparticles to both efficiently activate persulfates and rapidly access hydrophobic PAHs in water. Chem. Eng. J. 2023, 468, 143510. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Ling, L.; Zhou, Y. Enhanced activation of PMS by a novel Fenton-like composite Fe3O4/S-WO3 for rapid chloroxylenol degradation. Chem. Eng. J. 2022, 446, 137067. [Google Scholar] [CrossRef] [PubMed]
- Janjhi, F.A.; Ihsanullah, I.; Bilal, M.; Castro-Muñoz, R.; Boczkaj, G.; Gallucci, F. MXene-based materials for removal of antibiotics and heavy metals from wastewater-a review. Water Resour. Ind. 2023, 29, 100202. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yukselkadag, A.; Ağtaş, M.; Mehrabi, M.; Salehi, E.; Castro-Muñoz, R.; Koyuncu, I. Zeolitic imidazolate framework (ZIF-8) modified cellulose acetate NF membranes for potential water treatment application. Carbohydr. Polym. 2023, 299, 120230. [Google Scholar] [CrossRef]
- Wu, G.; Tu, H.; Niu, F.; Lu, S.; Liu, Y.; Gao, K.; Chen, Z.; Wang, P.; Li, Z. Synthesis of polymer-functionalized β-cyclodextrin, Mg2+ doped, coating magnetic Fe3O4 nanoparticle carriers for penicillin G acylase immobilization. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130609. [Google Scholar] [CrossRef]
- Qin, Y.; Ahmed, A.; Iqbal, S.; Usman, M. Construction of β-cyclodextrin modified Fe3O4 composites with enhanced peroxymonosulfate activation towards efficient degradation of norfloxacin. Mol. Catal. 2023, 547, 113368. [Google Scholar] [CrossRef]
- Yan, H.; Yu, X.; Dong, G.; Zhang, Z.; Ding, K.; Yang, H.; Su, G. Functionalized β-cyclodextrin with polyethyleneimine-coated Fe3O4 as a recyclable demulsifier for the efficient treatment of oily wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130877. [Google Scholar] [CrossRef]
- Jaina, Y.; Kumaria, M.; Agarwalc, M.; Gupta, R. Robust synthesis of sugar-coumarin based fluorescent 1,4-disubstituted-1,2,3-triazoles using highly efficient recyclable citrate grafted β-cyclodextrin@magnetite nano phase transfer catalyst in aqueous media. Carbohydr. Res. 2019, 482, 107736. [Google Scholar] [CrossRef] [PubMed]
- Sathyan, M.; Jandas, P.J.; Venkatesan, M.; Pillai, S.C.; John, H. Electrode material for high performance symmetric supercapacitors based on superparamagnetic Fe3O4 nanoparticles modified with cetyltrimetylammonium bromide. Synth. Met. 2022, 287, 117080. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Ayalew, Z.M.; Guo, X.; Zhang, X. Synthesis and application of polyethyleneimine (PEI)-based composite/nanocomposite material for heavy metals removal from wastewater: A critical review. J. Hazard. Mater. Adv. 2022, 8, 100158. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Wu, T.; Huang, W.; Ma, T.; Zhou, X. A novel adaptive multi-scale Rényi transfer entropy based on kernel density estimation. Chaos Solitons Fractals 2023, 175, 113972. [Google Scholar] [CrossRef]
- Miao, M.; Lu, Q.; Wang, X.; Zhang, Y.; Wong, N.H.; Sunarso, J.; Xiao, C.; Li, N. Removal of micro-organic contaminants from wastewater: A critical review of treatment technology. Next Mater. 2023, 1, 100016. [Google Scholar] [CrossRef]
- Grape, E.S.; Chacón-García, A.J.; Rojas, S.; Pérez, Y.; Jaworski, A.; Nero, M.; Åhlén, M.; Martínez-Ahumada, E.; Feindt, A.E.G.; Pepillo, M.; et al. Removal of pharmaceutical pollutants from effluent by a plant-based metal-organic framework. Nat. Water 2023, 1, 433–442. [Google Scholar] [CrossRef]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. npj Clean Water 2022, 5, 12. [Google Scholar] [CrossRef]
- Azhagiri, S.; Jayakumar, S.; Gunasekaran, S.; Srinivasan, S. Molecular structure, Mulliken charge, frontier molecular orbital and first hyperpolarizability analysis on 2-nitroaniline and 4-methoxy-2-nitroaniline using density functional theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tang, L.; Ding, M. Electrostatic polarization in single-atom catalysis. Cell Rep. Phys. Sci. 2023, 4, 101417. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Xie, S.; Sun, Q.; Zhang, Z.-X.; Zeng, G. Adsorption behavior of carbamazepine on Zn-MOFs derived nanoporous carbons: Defect enhancement, role of N doping and adsorption mechanism. J. Environ. Chem. Eng. 2022, 10, 107660. [Google Scholar] [CrossRef]
- Veclani, D.; Tolazzi, M.; Fogolari, F.; Melchior, A. Mechanism and thermodynamics of adsorption of diclofenac on graphene-based nanomaterials. J. Environ. Chem. Eng. 2022, 10, 108789. [Google Scholar] [CrossRef]
- Albornoz, L.L.; da Silva, S.W.; Bortolozzi, J.P.; Banús, E.D.; Brussino, P.; Ulla, M.A.; Bernardes, A.M. Degradation and mineralization of erythromycin by heterogeneous photocatalysis using SnO2-doped TiO2 structured catalysts: Activity and stability. Chemosphere 2021, 268, 128858. [Google Scholar] [CrossRef]
- Long, H.; Chen, T.-S.; Song, J.; Zhu, S.; Xu, H.-C. Electrochemical aromatic C-H hydroxylation in continuous flow. Nat. Commun. 2022, 13, 3945. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, X.; Lv, P.; Yin, L.; Zuo, W.; Tian, Y.; Zhang, J. Insights into the Ligand Effect in β-CD@Fe3O4 Composites to Activate Peroxymonosulfate for Efficient Degradation of Pharmaceutical Contaminants: A Study Employing Density Functional Theory. Coatings 2024, 14, 439. https://doi.org/10.3390/coatings14040439
Quan X, Lv P, Yin L, Zuo W, Tian Y, Zhang J. Insights into the Ligand Effect in β-CD@Fe3O4 Composites to Activate Peroxymonosulfate for Efficient Degradation of Pharmaceutical Contaminants: A Study Employing Density Functional Theory. Coatings. 2024; 14(4):439. https://doi.org/10.3390/coatings14040439
Chicago/Turabian StyleQuan, Xi, Pengzhao Lv, Linlin Yin, Wei Zuo, Yu Tian, and Jun Zhang. 2024. "Insights into the Ligand Effect in β-CD@Fe3O4 Composites to Activate Peroxymonosulfate for Efficient Degradation of Pharmaceutical Contaminants: A Study Employing Density Functional Theory" Coatings 14, no. 4: 439. https://doi.org/10.3390/coatings14040439
APA StyleQuan, X., Lv, P., Yin, L., Zuo, W., Tian, Y., & Zhang, J. (2024). Insights into the Ligand Effect in β-CD@Fe3O4 Composites to Activate Peroxymonosulfate for Efficient Degradation of Pharmaceutical Contaminants: A Study Employing Density Functional Theory. Coatings, 14(4), 439. https://doi.org/10.3390/coatings14040439







