Application of Secondary Ion Mass Spectrometry for Analysis of Decorative Coatings on Fancy Goods Accessories
Abstract
1. Introduction
2. Materials and Methods
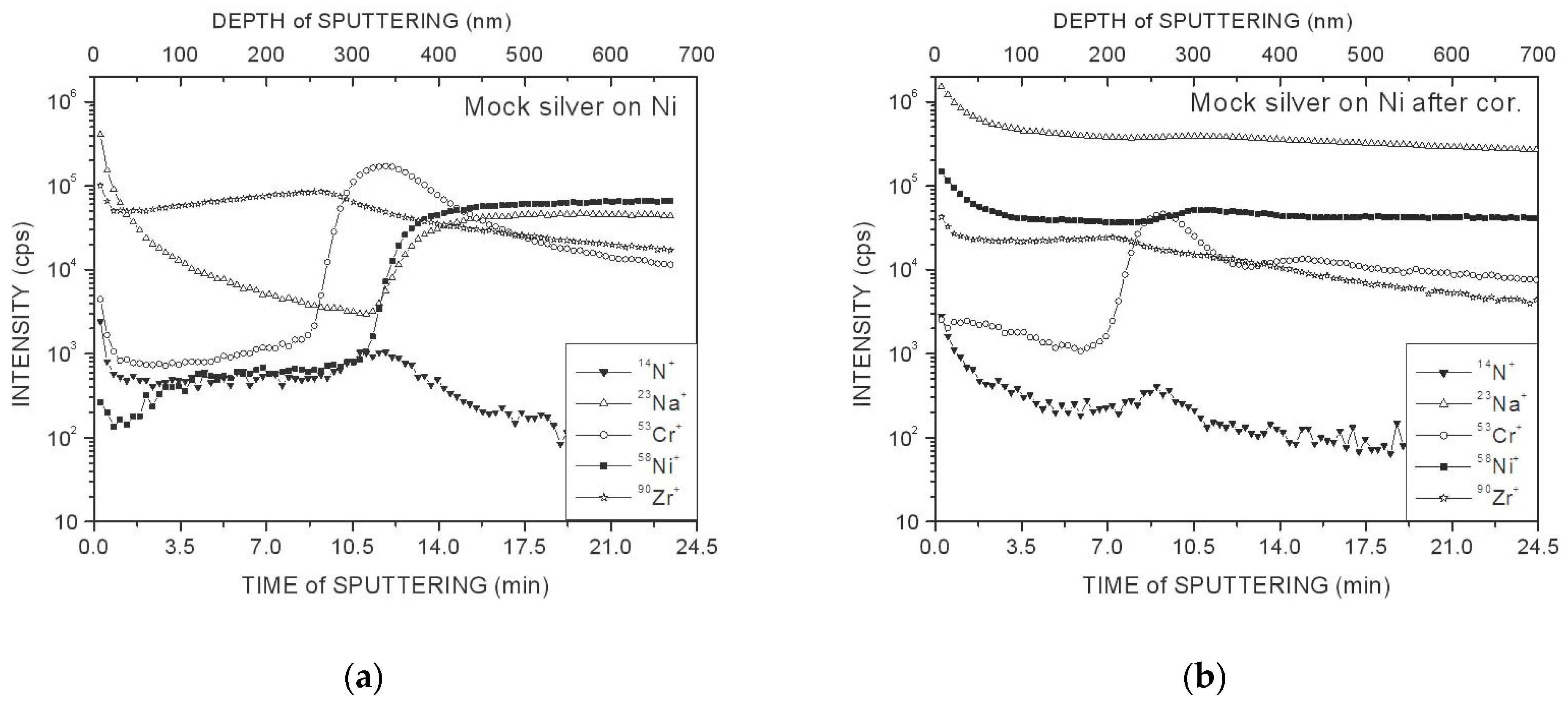
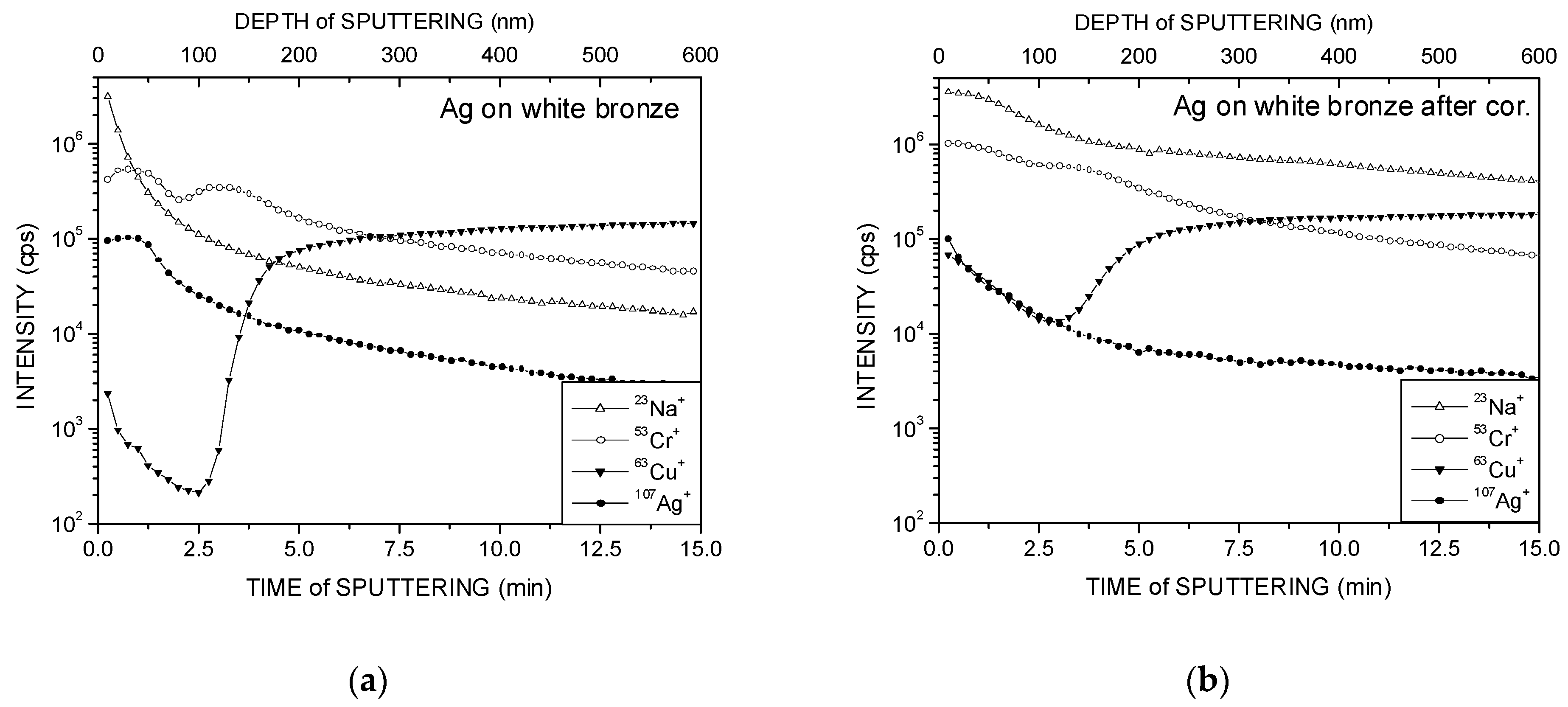
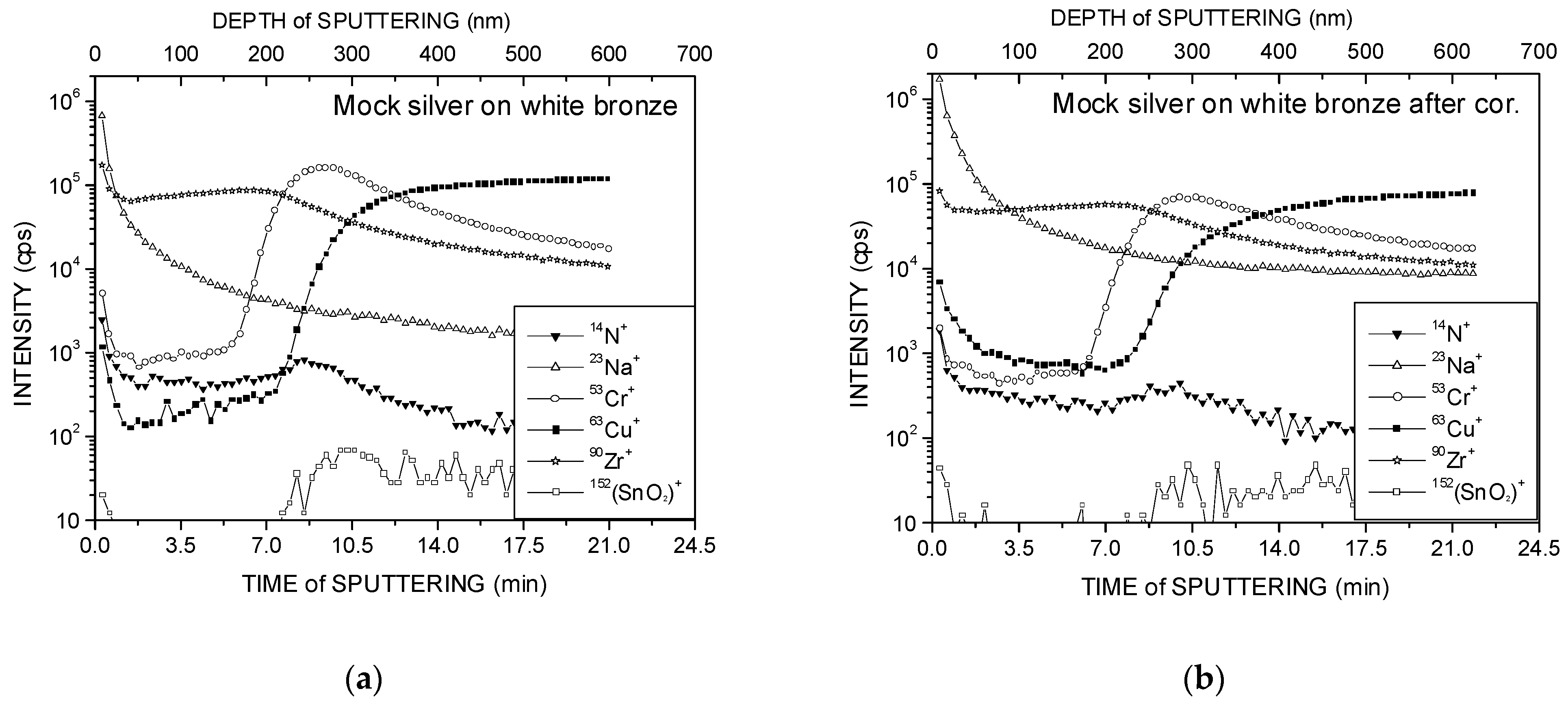
3. Results and Discussion
4. Conclusions
- Ag coatings on Ni and white bronze substrates, even for the initial samples, contain a large amount of Cr, and the degradation of these coatings progresses after the corrosion test.
- Initial ZrN coatings on Ni and white bronze substrates were less broken due to Cr diffusion from the intermediate adhesion layer placed between the coating and the substrate than in the case of Ag coatings with the same adhesion layer on the same substrates.
- ZrN coatings on a white bronze substrate turned out to be the most resistant to the corrosion test.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fashion Metal. Available online: https://www.fashionmetalacc.com/ (accessed on 10 March 2024).
- Giurlani, W.; Zangari, G.; Gambinossi, F.; Passaponti, M.; Salvietti, E.; Di Benedetto, F.; Caporali, S.; Innocenti, M. Electroplating for Decorative Applications: Recent Trends in Research and Development. Coatings 2018, 8, 260. [Google Scholar] [CrossRef]
- Vorobyova, M.; Biffoli, F.; Giurlani, W.; Martinuzzi, S.M.; Linser, M.; Caneschi, A.; Innocenti, M. PVD for Decorative Applications: A Review. Materials 2023, 16, 4919. [Google Scholar] [CrossRef] [PubMed]
- Benninghoven, A.; Rüdenauer, F.G.; Werner, H.W. Secondary Ion Mass Spectrometry: Basic Concepts, Instrumental Aspects, Applications, and Trends; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Wilson, R.G.; Stevie, F.A.; Magee, C.W. Secondary Ion Mass Spectrometry: Practical Handbook for Depth Profiling and Bulk Impurity Analysis; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Standard ISO 9227:2022. Available online: https://www.iso.org/standard/81744.html/ (accessed on 5 April 2024).
- Tolstogouzov, A.; Daolio, S.; Pagura, C.; Greenwood, C.L. Energy distributions of secondary ions sputtered from aluminium and magnesium by Ne+, Ar+ and O2+: A comprehensive study. Int. J. Mass Specrom. 2002, 214, 327–337. [Google Scholar] [CrossRef]
- Bardi, U.; Caporali, S.; Chenakin, S.P.; Lavacchi, A.; Miorin, E.; Pagura, C.; Tolstogouzov, A. Characterization of electrodeposited metal coatings by secondary ion mass spectrometry. Surf. Coat. Technol. 2006, 200, 2870–2874. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (USA). Available online: https://pubchem.ncbi.nlm.nih.gov/ptable/ionization-energy/ (accessed on 4 April 2024).
- Wilson, R.G. SIMS quantification in Si, GaAs, and diamond—An update. Int. J. Mass Spectrom. Ion Process 1995, 143, 43–49. [Google Scholar] [CrossRef]
- Drozdov, M.N.; Drozdov, Y.N.; Csik, A.; Novikov, A.V.; Vad, K.; Yunin, P.A.; Yurasov, D.V.; Belykh, S.F.; Gololobov, G.P.; Suvorov, D.V.; et al. Quantitative depth profiling of Si1–xGex structures by time-of-flight secondary ion mass spectrometry and secondary neutral mass spectrometry. Thin Solid Film. 2016, 607, 25–31. [Google Scholar] [CrossRef]

| Ag | Cr | Ni | Zr | Cu | Sn |
|---|---|---|---|---|---|
| 500 | 65 | 370 | 40 | 310 | 300 |
| Ag on Ni | Ag on wb | ZrN on Ni | ZrN on wb | |
|---|---|---|---|---|
| Initial sample | Ag 20, Cr 80 | Ag 10, Cr 90 | Zr 83, Cr 11, Ni 6 | Zr 88, Cr 12 |
| After corrosion test | Ag 2, Cr 89, Ni 9 | Ag 8, Cr 88, Cu 3, Sn 1 | Zr 6, Cr 2, Ni 92 | Zr 84, Cr 5, Cu 8, Sn 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abudouwufu, T.; Lan, Y.; Han, B.; Fu, D.; Tolstoguzov, A. Application of Secondary Ion Mass Spectrometry for Analysis of Decorative Coatings on Fancy Goods Accessories. Coatings 2024, 14, 469. https://doi.org/10.3390/coatings14040469
Abudouwufu T, Lan Y, Han B, Fu D, Tolstoguzov A. Application of Secondary Ion Mass Spectrometry for Analysis of Decorative Coatings on Fancy Goods Accessories. Coatings. 2024; 14(4):469. https://doi.org/10.3390/coatings14040469
Chicago/Turabian StyleAbudouwufu, Tushagu, Yueqiang Lan, Bin Han, Dejun Fu, and Alexander Tolstoguzov. 2024. "Application of Secondary Ion Mass Spectrometry for Analysis of Decorative Coatings on Fancy Goods Accessories" Coatings 14, no. 4: 469. https://doi.org/10.3390/coatings14040469
APA StyleAbudouwufu, T., Lan, Y., Han, B., Fu, D., & Tolstoguzov, A. (2024). Application of Secondary Ion Mass Spectrometry for Analysis of Decorative Coatings on Fancy Goods Accessories. Coatings, 14(4), 469. https://doi.org/10.3390/coatings14040469








