Development of pH Indicator Composite Films Based on Anthocyanins and Neutral Red for Monitoring Minced Meat and Fish in Modified Gas Atmosphere (MAP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of pH Indicator Films
2.3. Characterization of Indicator Films
2.3.1. Moisture Content
2.3.2. Solubility in Water
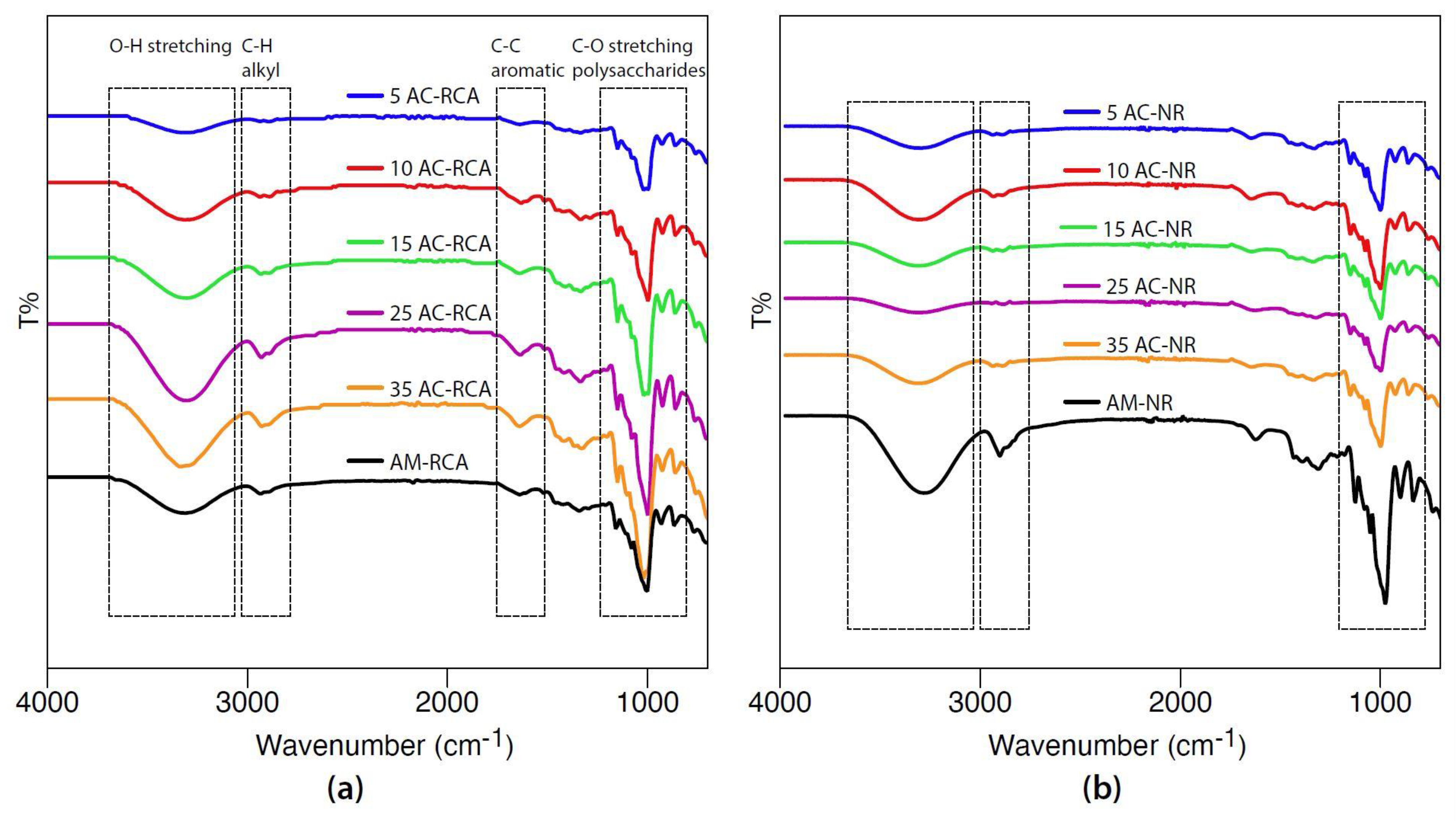
2.3.3. Fourier Transform Infra-Red (FT-IR) Spectroscopy
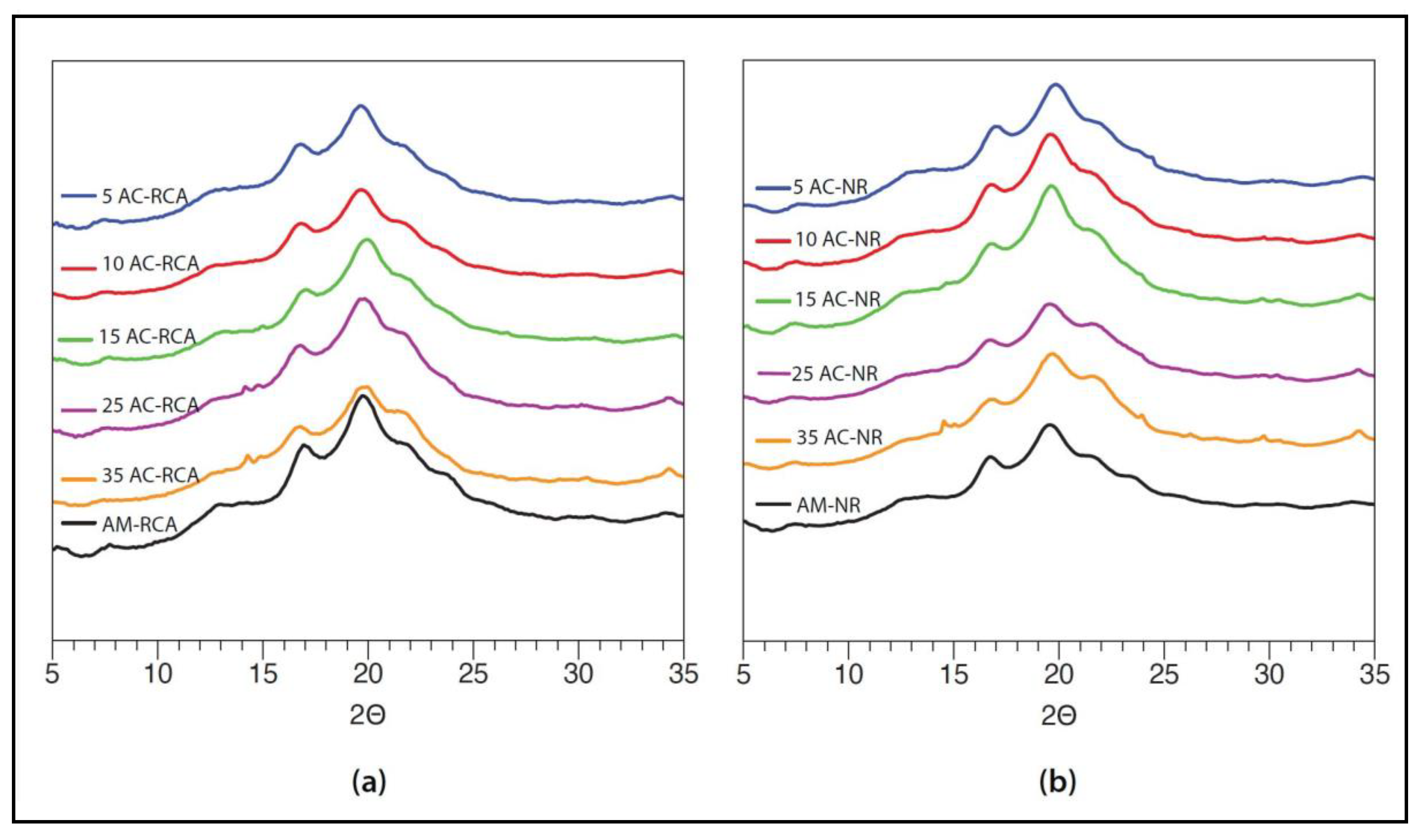
2.3.4. Wide-Angle X-ray Scattering (WAXS)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Permeability
2.3.7. Mechanical Measurements
2.3.8. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3.9. Opacity and Light Barrier Properties
2.4. Monitoring Minced Meat and Minced Fish Films
2.4.1. Sample Preparation
2.4.2. Microbial Analysis
2.4.3. pH Measurements
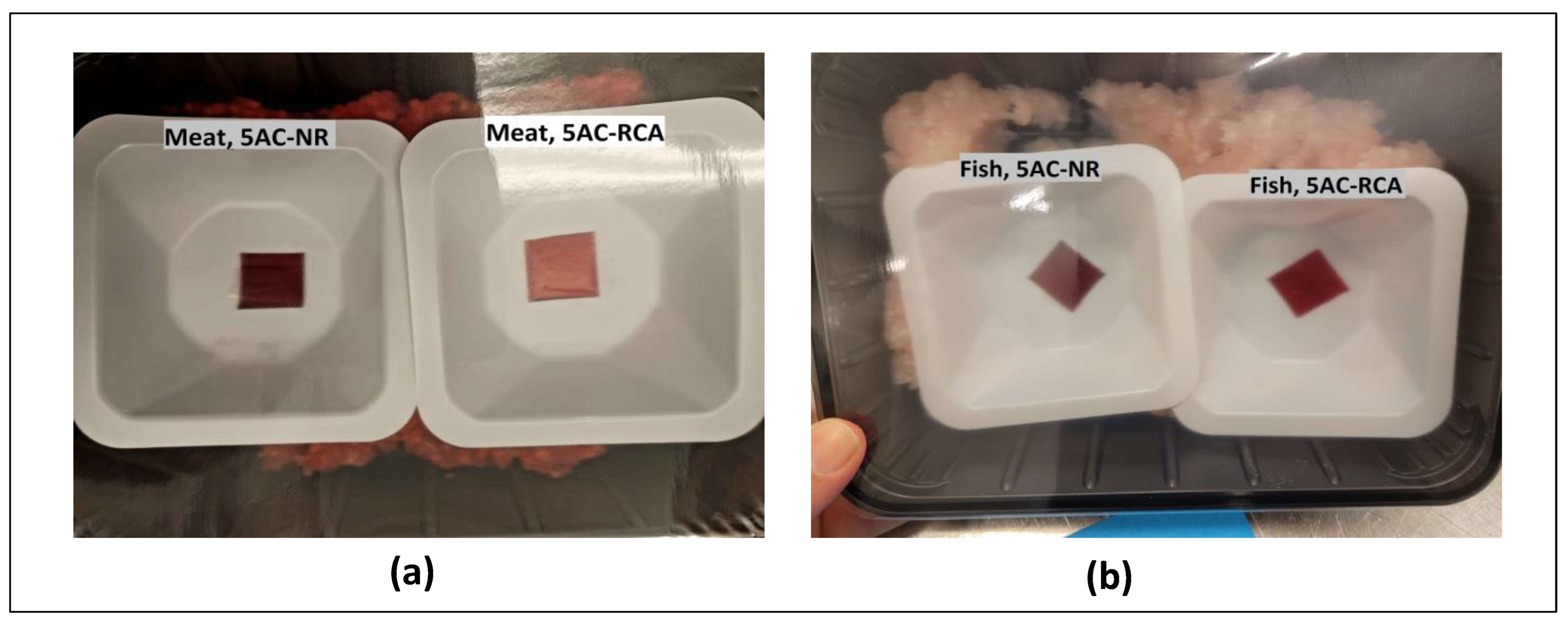
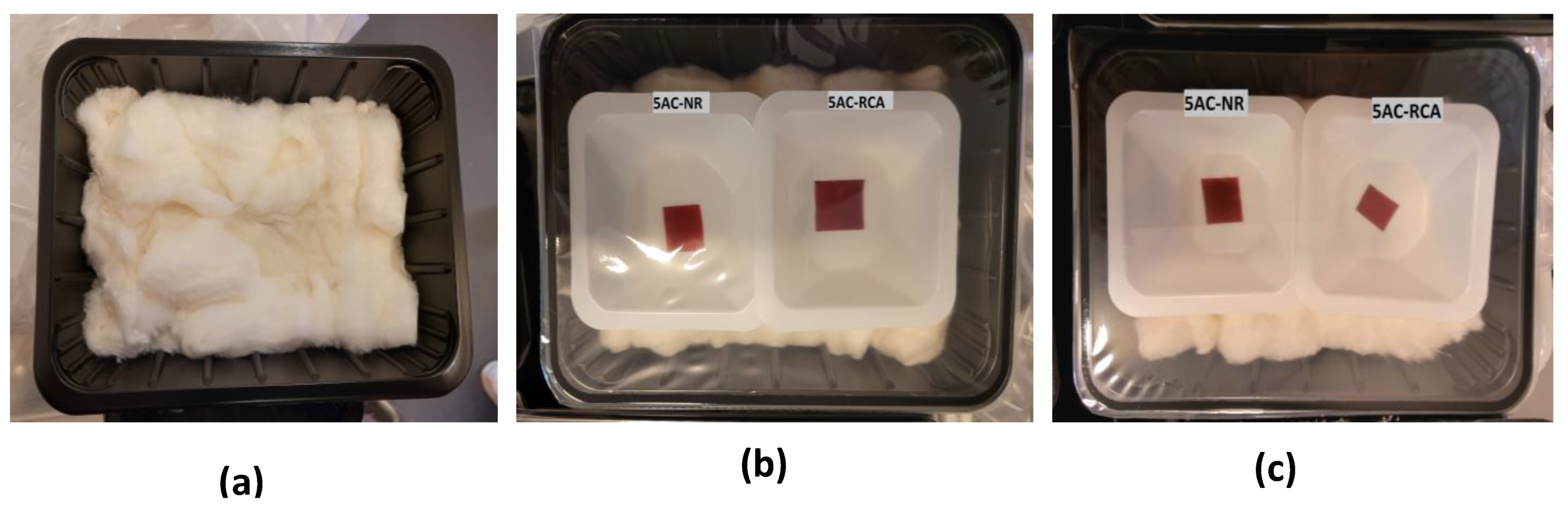
2.4.4. Color Response of Indicator Films during Meat/Fish Packaging
2.4.5. Gas Measurement
2.4.6. Sensory Evaluation
2.4.7. Statistical Analysis
3. Results and Discussion
3.1. Moisture Content (MC) and Water Solubility (WS)
3.2. FT-IR
3.3. Wide-Angle X-ray Scattering (WAXS)
3.4. Thickness and Gas Permeability
3.5. Thermogravimetric Analysis (TGA)
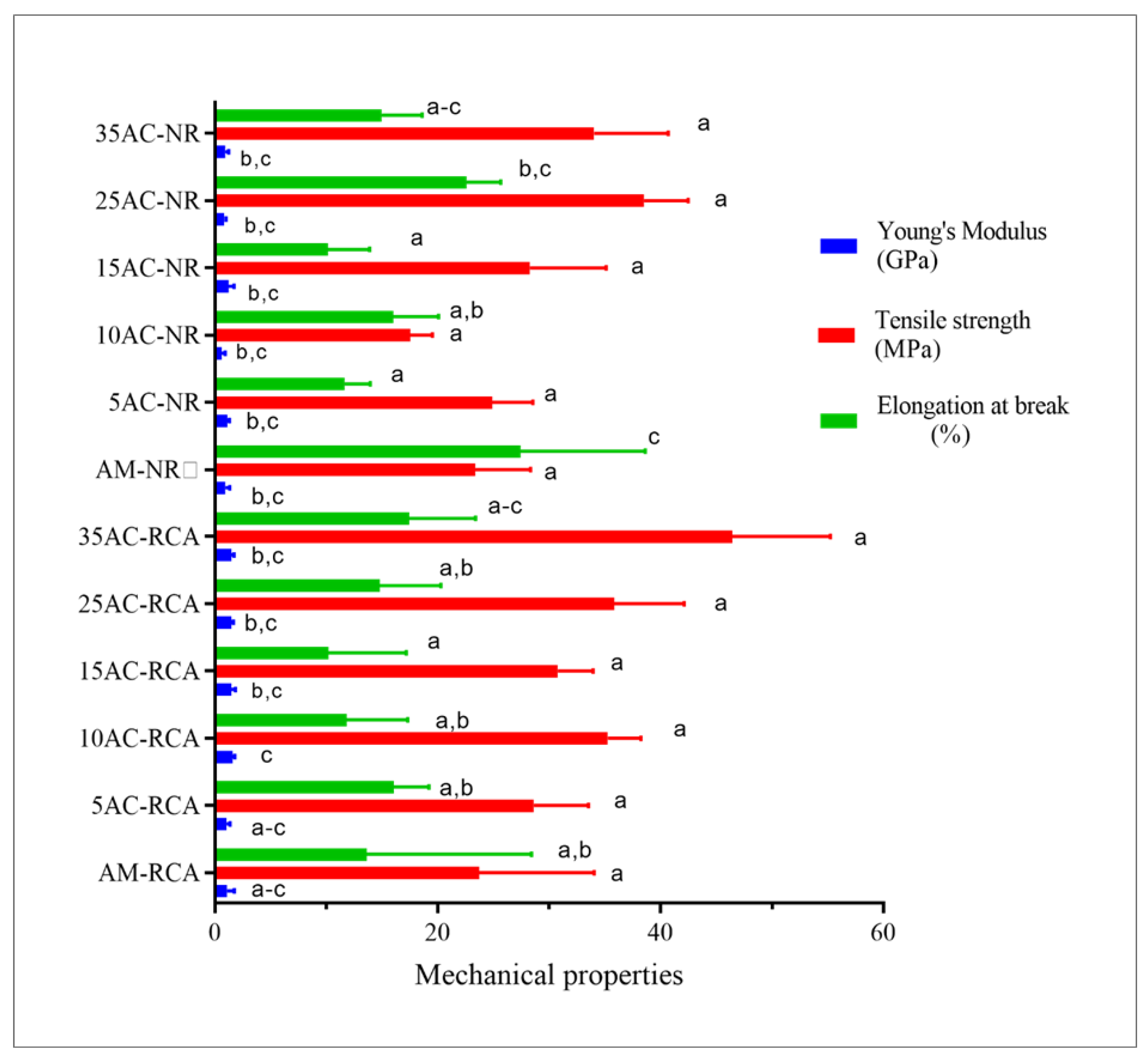
3.6. Mechanical Properties
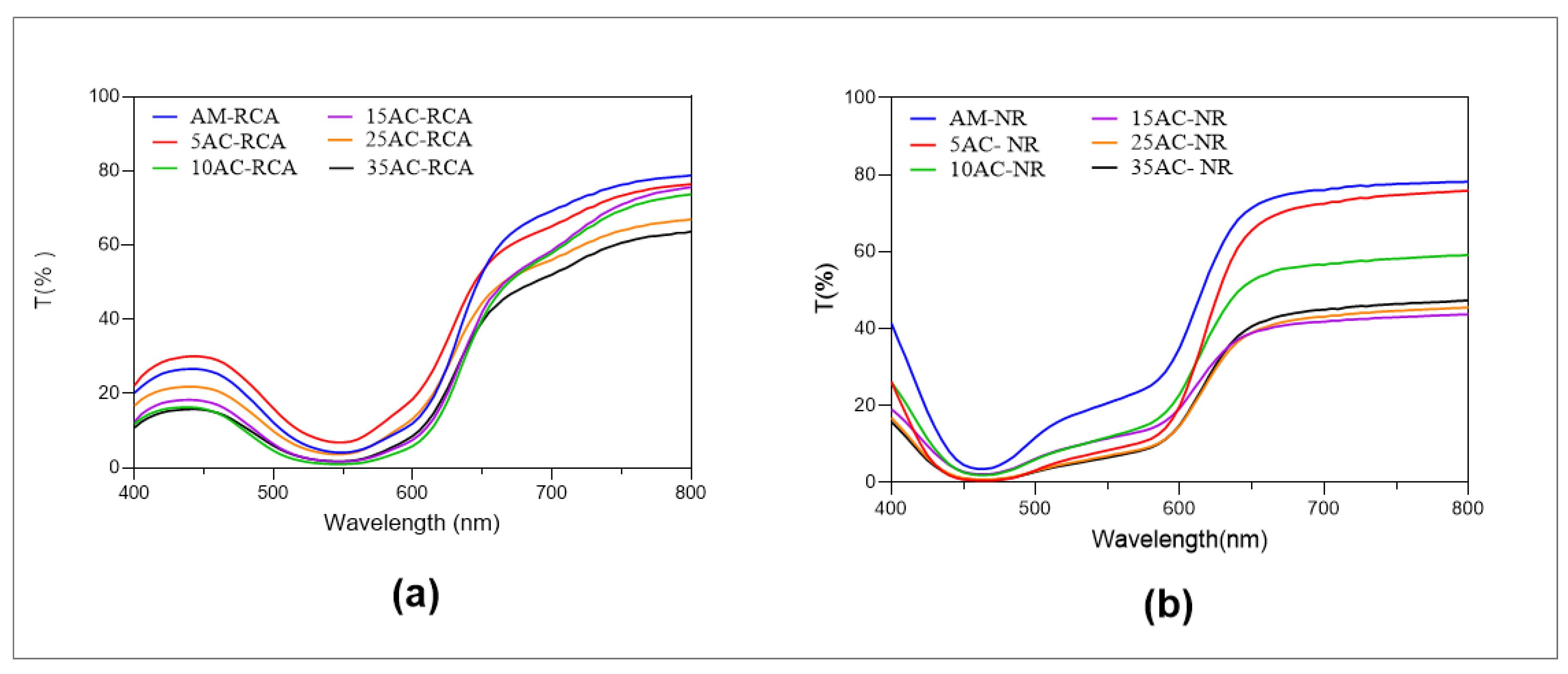
3.7. Optical and Barrier Properties
3.8. Microstructural Analysis
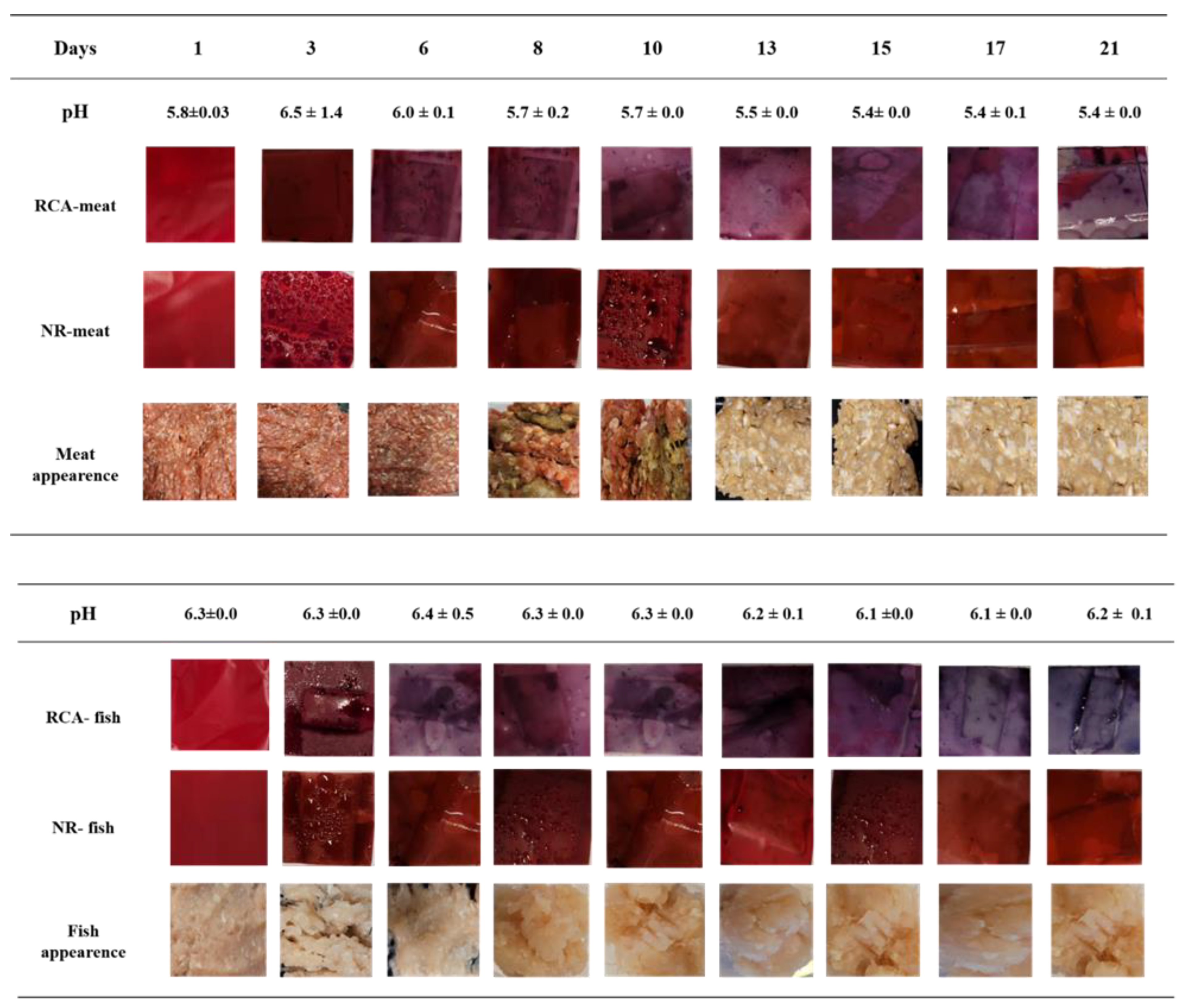
3.9. Microbial Counts and pH Measurements
3.10. Color Changes of Indicator Films of Minced Meat and Minced Fish
3.11. Sensory Scores
3.12. Correlation between Quality Indices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, M.; Bose, I.; Nousheen; Roy, S. Development of Intelligent Indicators Based on Cellulose and Prunus Domestica Extracted Anthocyanins for Monitoring the Freshness of Packaged Chicken. Int. J. Biomater. 2024, 2024, 7949258. [Google Scholar] [CrossRef] [PubMed]
- Ajaykumar, V.J.; Mandal, P.K. Modern Concept and Detection of Spoilage in Meat and Meat Products; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128192337. [Google Scholar]
- Akhila, K.; Ramakanth, D. Plant Phytochemicals as Bio-Responsive Compounds in Smart Packaging for Food Spoilage Detection: A Comprehensive Review. Sustain. Food Technol. 2024. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent Poly (Vinyl Alcohol)-Chitosan Nanoparticles-Mulberry Extracts Films Capable of Monitoring PH Variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Sperotto, N.; Spada, J.C.; Tessaro, I.C. Effect of Blueberry Agro-Industrial Waste Addition to Corn Starch-Based Films for the Production of a PH-Indicator Film. Int. J. Biol. Macromol. 2017, 104, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel Colorimetric Films Based on Starch/Polyvinyl Alcohol Incorporated with Roselle Anthocyanins for Fish Freshness Monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef]
- Lee, E.J.; Shin, H.S. Development of a Freshness Indicator for Monitoring the Quality of Beef during Storage. Food Sci. Biotechnol. 2019, 28, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Muthukumarappan, K.; Wei, L. A Biopolymer-Based PH Indicator Film for Visually Monitoring Beef and Fish Spoilage. Food Biosci. 2022, 46, 101523. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Gelatin Based Films Capable of Modifying Its Color against Environmental PH Changes. Food Hydrocoll. 2016, 61, 523–530. [Google Scholar] [CrossRef]
- Păușescu, I.; Dreavă, D.M.; Bîtcan, I.; Argetoianu, R.; Dăescu, D.; Medeleanu, M. Bio-Based PH Indicator Films for Intelligent Food Packaging Applications. Polymers 2022, 14, 3622. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials Chemistry and the Futurist Eco-Friendly Applications of Nanocellulose: Status and Prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Carciofi, M.; Blennow, A.; Jensen, S.L.; Shaik, S.S.; Henriksen, A.; Buléon, A.; Holm, P.B.; Hebelstrup, K.H. Concerted Suppression of All Starch Branching Enzyme Genes in Barley Produces Amylose-Only Starch Granules. BMC Plant Biol. 2012, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, D.; Hooshmand, K.; Kemmer, G.C.; Kirkensgaard, J.J.K.; Mortensen, K.; Giosafatto, C.V.L.; Holse, M.; Hebelstrup, K.H.; Bao, J.; Stelte, W.; et al. Cross-Linked Amylose Bio-Plastic: A Transgenic-Based Compostable Plastic Alternative. Int. J. Mol. Sci. 2017, 18, 2075. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Bevilacqua, M.; Bro, R.; Bordallo, H.N.; Judas, J.; Kirkensgaard, K.; Hebelstrup, K.H.; Blennow, A. International Journal of Biological Macromolecules Colorimetric PH Indicators Based on Well-Defined Amylose and Amylopectin Matrices Enriched with Anthocyanins from Red Cabbage. Int. J. Biol. Macromol. 2023, 250, 126250. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sagnelli, D.; Faisal, M.; Perzon, A.; Taresco, V.; Mais, M.; Giosafatto, C.V.L.; Hebelstrup, K.H.; Ulvskov, P.; Jørgensen, B.; et al. Amylose/Cellulose Nanofiber Composites for All-Natural, Fully Biodegradable and Flexible Bioplastics. Carbohydr. Polym. 2021, 253, 117277. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a Natural Source for Groundbreaking Applications in Materials Science: Today’s State. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Holland, C.; Perzon, A.; Cassland, P.R.C.; Jensen, J.P.; Langebeck, B.; Sørensen, O.B.; Whale, E.; Hepworth, D.; Plaice-Inglis, R.; Moestrup, Ø.; et al. Nanofibers Produced from Agro-Industrial Plant Waste Using Entirely Enzymatic Pretreatments. Biomacromolecules 2019, 20, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent PH-Sensitive Indicator Based on Starch-Cellulose and Alizarin Dye to Track Freshness of Rainbow Trout Fillet. Int. J. Biol. Macromol. 2019, 132, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Mohanty, B. Modified Atmosphere Packaging of Fish and Fishery Products: A Review. J. Entomol. Zool. Stud. 2020, 8, 651–659. [Google Scholar]
- Perzon, A.; Jørgensen, B.; Ulvskov, P. Sustainable Production of Cellulose Nanofiber Gels and Paper from Sugar Beet Waste Using Enzymatic Pre-Treatment. Carbohydr. Polym. 2020, 230, 115581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Dhamodharan, R. Tannic Acid Crosslinked Chitosan-Guar Gum Composite Films for Packaging Application. Int. J. Biol. Macromol. 2024, 260, 129317. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango Leaf Extract Incorporated Chitosan Antioxidant Film for Active Food Packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Elhadef, K.; Chaari, M.; Akermi, S.; Ben Hlima, H.; Ennouri, M.; Abdelkafi, S.; Agriopoulou, S.; Ali, D.S.; Boulekbache-Makhlouf, L.; Mellouli, L.; et al. PH-Sensitive Films Based on Carboxymethyl Cellulose/Date Pits Anthocyanins: A Convenient Colorimetric Indicator for Beef Meat Freshness Tracking. Food Biosci. 2024, 57, 103508. [Google Scholar] [CrossRef]
- Faisal, M.; Žmirić, M.; Kim, N.; Bruun, S.; Mariniello, L.; Famiglietti, M.; Bordallo, H.; Kirkensgaard, J.; Jørgensen, B.; Ulvskov, P.; et al. A Comparison of Cellulose Nanocrystals and Nanofibers as Reinforcements to Amylose-Based Composite Bioplastics. Coatings 2023, 13, 1573. [Google Scholar] [CrossRef]
- Tian, Y.; Petersen, B.L.; Liu, X.; Li, H.; Kirkensgaard, J.J.K.; Enemark-Rasmussen, K.; Khakimov, B.; Hebelstrup, K.H.; Zhong, Y.; Blennow, A. Characterization of Different High Amylose Starch Granules. Part Ⅱ: Structure Evolution during Digestion and Distinct Digestion Mechanisms. Food Hydrocoll. 2024, 149, 109593. [Google Scholar] [CrossRef]
- Jessen, C.H.; Bendix, J.; Nannestad, T.B.; Bordallo, H.N.; Pedersen, M.J.; Pedersen, C.M.; Bols, M. Methane Capture with α-Cyclodextrins. New J. Chem. 2023, 47, 14624–14629. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.; Liu, S.; Kan, J.; Jin, C. hai Preparation and Characterization of Protocatechuic Acid Grafted Chitosan Films with Antioxidant Activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Babic Milijasevic, J.; Milijasevic, M.; Djordjevic, V. Modified Atmosphere Packaging of Fish-An Impact on Shelf Life. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012028. [Google Scholar] [CrossRef]
- Heir, E.; Solberg, L.E.; Jensen, M.R.; Skaret, J.; Grøvlen, M.S.; Holck, A.L. Improved Microbial and Sensory Quality of Chicken Meat by Treatment with Lactic Acid, Organic Acid Salts and Modified Atmosphere Packaging. Int. J. Food Microbiol. 2022, 362, 109498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Li, N.; Li, M.; Xu, X. High CO2-Modified Atmosphere Packaging for Extension of Shelf-Life of Chilled Yellow-Feather Broiler Meat: A Special Breed in Asia. LWT 2015, 64, 1123–1129. [Google Scholar] [CrossRef]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A Colorimetric Film Based on Polyvinyl Alcohol/Sodium Carboxymethyl Cellulose Incorporated with Red Cabbage Anthocyanin for Monitoring Pork Freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a Colorimetric PH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica oleraceae) Extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Gillgren, T.; Blennow, A.; Pettersson, A.J.; Stading, M. Modulating Rheo-Kinetics of Native Starch Films towards Improved Wet-Strength. Carbohydr. Polym. 2011, 83, 383–391. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the Properties of Natural Fibers-Reinforced Thermoplastic Starch Composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and Characterization of Active and Intelligent Packaging Films Based on Cassava Starch and Anthocyanins from Lycium Ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Versino, F.; García, M.A. Cassava (Manihot esculenta) Starch Films Reinforced with Natural Fibrous Filler. Ind. Crops Prod. 2014, 58, 305–314. [Google Scholar] [CrossRef]
- Garcia, P.S.; Grossmann, M.V.E.; Shirai, M.A.; Lazaretti, M.M.; Yamashita, F.; Muller, C.M.O.; Mali, S. Improving Action of Citric Acid as Compatibiliser in Starch/Polyester Blown Films. Ind. Crops Prod. 2014, 52, 305–312. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent Gelatin/Oxidized Chitin Nanocrystals Nanocomposite Films Containing Black Rice Bran Anthocyanins for Fish Freshness Monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Prachayawarakorn, J.; Ruttanabus, P.; Boonsom, P. Effect of Cotton Fiber Contents and Lengths on Properties of Thermoplastic Starch Composites Prepared from Rice and Waxy Rice Starches. J. Polym. Environ. 2011, 19, 274–282. [Google Scholar] [CrossRef]
- Prietto, L.; Correa, T.; Fernanda, J.; Levien, N.; Lim, L.; Renato, A.; Dias, G.; Zavareze, R. PH-Sensitive Fi Lms Containing Anthocyanins Extracted from Black Bean Seed Coat and Red Cabbage. LWT 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Shalwan, A.; Yousif, B.F. In State of Art: Mechanical and Tribological Behaviour of Polymeric Composites Based on Natural Fibres. Mater. Des. 2013, 48, 14–24. [Google Scholar] [CrossRef]
- Mikołajczyk-Bator, K.; Pawlak, S. The Effect of Thermal Treatment on Antioxidant Capacity and Pigment Contents in Separated Betalain Fractions. Acta Sci. Pol. Technol. Aliment. 2016, 15, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, X.; Guo, F.; Yu, Y.; Lu, J.; Li, Y.; Cheng, Q.; Peng, J.; Yu, G. Biodegradable Cellulose/Curcumin Films with Janus Structure for Food Packaging and Freshness Monitoring. Carbohydr. Polym. 2024, 324, 121516. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. International Journal of Biological Macromolecules Utilization of Flax (Linum usitatissimum) Cellulose Nanocrystals as Reinforcing Material for Chitosan Films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Gram, L. How to Meet an FSO: Control of Listeria Monocytogenes in the Smoked Fish Industry. Mitteilungen Leb. Hyg. 2004, 95, 59–67. [Google Scholar]
- Chen, M.; Yan, T.; Huang, J.; Zhou, Y.; Hu, Y. Fabrication of Halochromic Smart Films by Immobilizing Red Cabbage Anthocyanins into Chitosan/Oxidized-Chitin Nanocrystals Composites for Real-Time Hairtail and Shrimp Freshness Monitoring. Int. J. Biol. Macromol. 2021, 179, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Opara, U.L.; Fadiji, T.; Caleb, O.J.; Oluwole, A.O. Effects of Modified Atmosphere Packaging, Storage Temperature, and Absorbent Pads on the Quality of Fresh Cape Hake Fish Fillets. Coatings 2022, 12, 310. [Google Scholar] [CrossRef]
- Chen, S.H.; Fegan, N.; Kocharunchitt, C.; Bowman, J.P.; Duffy, L.L. Changes of the Bacterial Community Diversity on Chicken Carcasses through an Australian Poultry Processing Line. Food Microbiol. 2020, 86, 103350. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yuan, C.; Cui, B.; Sha, H.; Liu, P.; Lu, L.; Wu, Z.; Yuan, C. High-Amylose Corn Starch/Konjac Glucomannan Composite Film: Reinforced by Incorporating β-Cyclodextrin. J. Agric. Food Chem. 2021, 69, 2493–2500. [Google Scholar] [CrossRef]



| Films | MC% | WS% | Relative Crystallinity (RC)% | TGA (°C) |
|---|---|---|---|---|
| AM-RCA | 8.7 ± 2.9 a | 16.7 ± 6.9 a | 19.2 ± 0.1 a | 309.5 ± 3.5 d |
| 5AC-RCA | 18.4 ± 7.0 a,b | 14.5 ± 9.5 a | 15.8 ± 0.1 a | 309.0 ± 1.4 c,d |
| 10AC-RCA | 12.9 ± 1.7 a,b | 24.6 ± 17.3 a | 14.8 ± 0.1 a | 306.0 ± 1.4 b–d |
| 15AC-RCA | 19.5 ± 6.2 a,b | 16.8 ± 3.0 a | 15.0 ± 0.1 a | 307.0 ± 2.1 b–d |
| 25AC-RCA | 16.9 ± 7.4 a,b | 21.4 ± 6.3 a | 14.1 ± 0.1 a | 308.0 ± 2.8 c,d |
| 35AM-RCA | 18.5 ± 2.9 a,b | 11.3 ± 3.8 a | 20.7 ± 0.1 a | 311.0 ± 2.8 d |
| AM-NR | 19.4 ± 1.9 a,b | 24.2 ± 7.1 a | 21.2 ± 0.1 a | 310.5 ± 0.7 d |
| 5AC-NR | 23.4 ± 2.0 b | 15.9 ± 0.8 a | 20.1 ± 0.1 a | 310.8 ± 3.5 d |
| 10AC-NR | 19.3 ± 2.7 a,b | 19.5 ± 5.1 a | 18.7 ± 0.1 a | 301.5 ± 2.8 a–c |
| 15AC-NR | 17.2 ± 2.0 a,b | 14.6 ± 2.0 a | 18.0 ± 0.1 a | 299.5 ± 0.7 a,b |
| 25AC-NR | 18.2 ± 2.7 a,b | 14.6 ± 6.3 a | 14.9 ± 0.1 a | 298.3 ± 0.3 a |
| 35AC-NR | 20.8 ± 3.9 b | 16.1 ± 5.1 a | 19.6 ± 0.1 a | 299.3 ± 1.4 a,b |
| Films | Thickness (mm) | Permeability (cm3 × mm/(m2 × h × kPa)) | ||
|---|---|---|---|---|
| O2 | CO2 | H2O | ||
| AM-RCA | 0.07 ± 0.01 c | 4.28 ± 1.04 b | 0.14 ± 0.00 a | 4.48 ± 0.64 a |
| 5AC-RCA | 0.06 ± 0.01 b-c | ND | ND | ND |
| 10AC-RCA | 0.04 ± 0.01 a–c | 6.57 ± 0.96 c | 0.12 ± 0.00 a | 4.45 ±0.38 a |
| 15AC-RCA | 0.06 ± 0.01 b,c | ND | ND | ND |
| 25AC-RCA | 0.05 ± 0.01 a–c | ND | ND | ND |
| 35AC-RCA | 0.04 ± 0.01 a–c | 0.03 ± 0.00 a | 0.52 ± 0.11 c | 8.99 ± 0.28 c |
| AM-NR | 0.05 ± 0.01 a-c | ND | ND | ND |
| 5AC-NR | 0.05 ± 0.01 b,c | ND | ND | ND |
| 10AC-NR | 0.06 ± 0.01 b,c | ND | ND | ND |
| 15AC-NR | 0.07 ± 0.01 b,c | 0.86 ± 0.11 a | 0.23 ± 0.01 a | 6.91 ± 0.55 b |
| 25AC-NR | 0.06 ± 0.01 b,c | 0.01 ±0.00 a | 0.55 ±0.00 c | 11.11 ± 0.32 d |
| 35AC-NR | 0.05 ± 0.01 a–c | 0.05 ± 0.01 a | 0.36 ± 0.01 | 8.73 ± 0.44 c |
| Days | Microbial Count (Meat) | pH (Meat) | Microbial Count (Fish) | pH (Fish) |
|---|---|---|---|---|
| 1 | 4.46 ± 0.17 a | 5.81 ± 0.03 a,b | 2.35 ± 0.07 a | 6.16 ± 0.02 a–c |
| 3 | 5.42 ± 0.10 b | 6.49 ± 1.35 b | 2.28 ± 0.32 a | 6.26 ± 0.03 c,d |
| 6 | 7.40 ± 0.18 c | 5.96 ± 0.06 a,b | 4.56 ± 0.30 b | 6.44 ± 0.05 e |
| 8 | 8.02 ± 0.05 d | 5.72 ± 0.15 a,b | 5.85 ± 0.36 c | 6.26 ± 0.04 b,c |
| 10 | 8.2 ± 0.2 d,e | 6.00 ± 0.03 a | 6.99 ± 0.36 d | 6.32 ± 0.04 d |
| 13 | 8.22 ± 0.02 d,e | 5.45 ± 0.02 a | 8.23 ± 0.20 e | 6.15 ± 0.09 a,b |
| 15 | 8.16 ± 0.05 d,e | 5.44 ± 0.02 a | 8.23 ± 0.05 e | 6.11 ± 0.04 a |
| 17 | 8.26 ± 0.13 e | 5.39 ± 0.12 a | 8.37 ± 0.07 e | 6.07 ± 0.04 a |
| 20 | 8.13 ± 0.04 d,e | 5.48 ± 0.04 a | 8.45 ± 0.06 e | 6.24 ± 0.09 b–d |
| Days | Meat Appearance | Meat Odor | Fish Appearance | Fish Odor |
|---|---|---|---|---|
| 0 | 1 ± 0.0 a | 1 ± 0.0 a | 1 ± 0.0 a | 1.0 ± 0.1 a |
| 3 | 1.3 ± 0.1 a,b | 1.4 ± 0.3 a,b | 1.4 ± 0.2 a,b | 1.2 ± 0.3 a,b |
| 6 | 1.5 ± 0.4 b | 2.1 ± 0.6 b | 1.7 ± 0.4 a–c | 1.7 ± 0. 3 a–c |
| 8 | 2.7 ± 0.9 c | 3.2 ± 1.1 c | 1.8 ± 0. 5 a–c | 1.8 ± 0.6 b,c |
| 10 | 3.3 ± 1.1 d | 3.2 ± 1.1 c | 1.7 ± 0. 3 a–c | 2.0 ± 0.6 c,d |
| 13 | ND | ND | 2.3 ± 0.7 c,d | 2.5 ± 1.0 d,e |
| 15 | ND | ND | 2.4 ± 0.7 d | 2.7 ± 0.9 e |
| 17 | ND | ND | 2.9 ± 1.0 d | 3.1 ± 1.1 e |
| 20 | ND | ND | 2.6 ± 0. 8 d | 3.2 ± 1.17 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Jacobson, T.; Meineret, L.; Vorup, P.; Bordallo, H.N.; Kirkensgaard, J.J.K.; Ulvskov, P.; Blennow, A. Development of pH Indicator Composite Films Based on Anthocyanins and Neutral Red for Monitoring Minced Meat and Fish in Modified Gas Atmosphere (MAP). Coatings 2024, 14, 725. https://doi.org/10.3390/coatings14060725
Faisal M, Jacobson T, Meineret L, Vorup P, Bordallo HN, Kirkensgaard JJK, Ulvskov P, Blennow A. Development of pH Indicator Composite Films Based on Anthocyanins and Neutral Red for Monitoring Minced Meat and Fish in Modified Gas Atmosphere (MAP). Coatings. 2024; 14(6):725. https://doi.org/10.3390/coatings14060725
Chicago/Turabian StyleFaisal, Marwa, Tomas Jacobson, Lene Meineret, Peter Vorup, Heloisa N. Bordallo, Jacob Judas Kain Kirkensgaard, Peter Ulvskov, and Andreas Blennow. 2024. "Development of pH Indicator Composite Films Based on Anthocyanins and Neutral Red for Monitoring Minced Meat and Fish in Modified Gas Atmosphere (MAP)" Coatings 14, no. 6: 725. https://doi.org/10.3390/coatings14060725







