Preparation of Porous Ni-W Alloys Electrodeposited by Dynamic Hydrogen Bubble Template and Their Alkaline HER Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Electrodeposition
2.2. Characterization
2.3. Electrocatalytic Evaluation
3. Results and Discussion
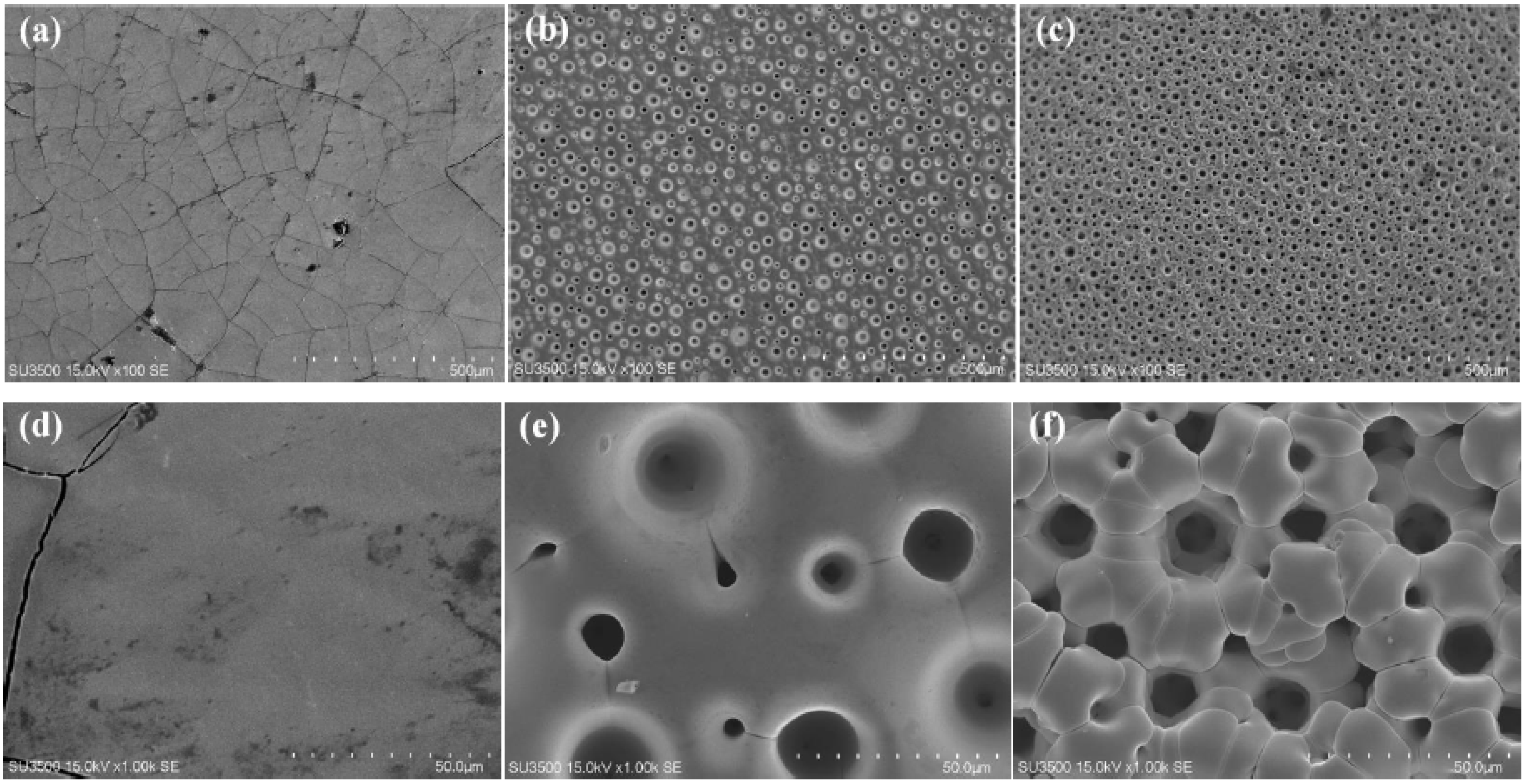
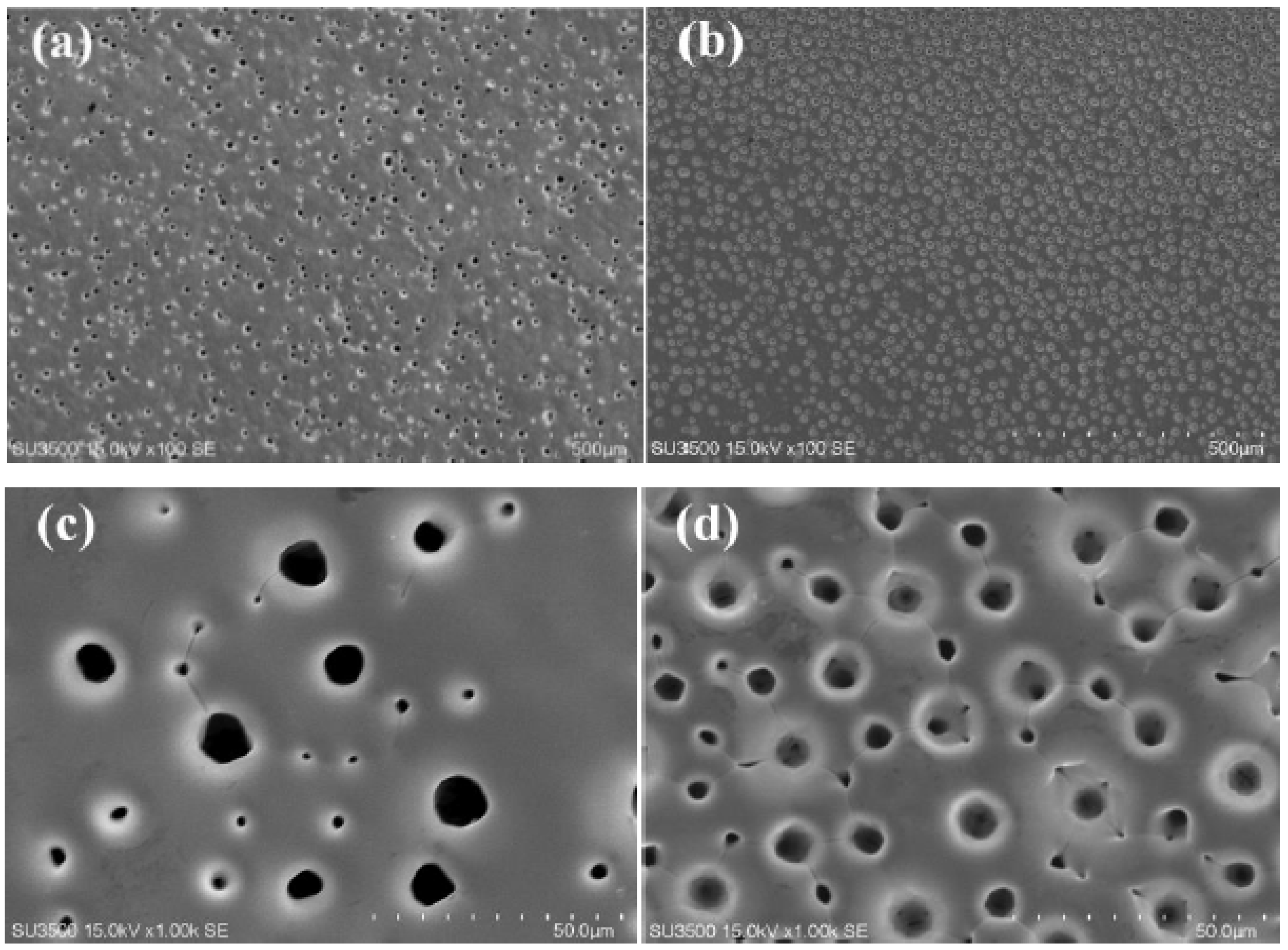
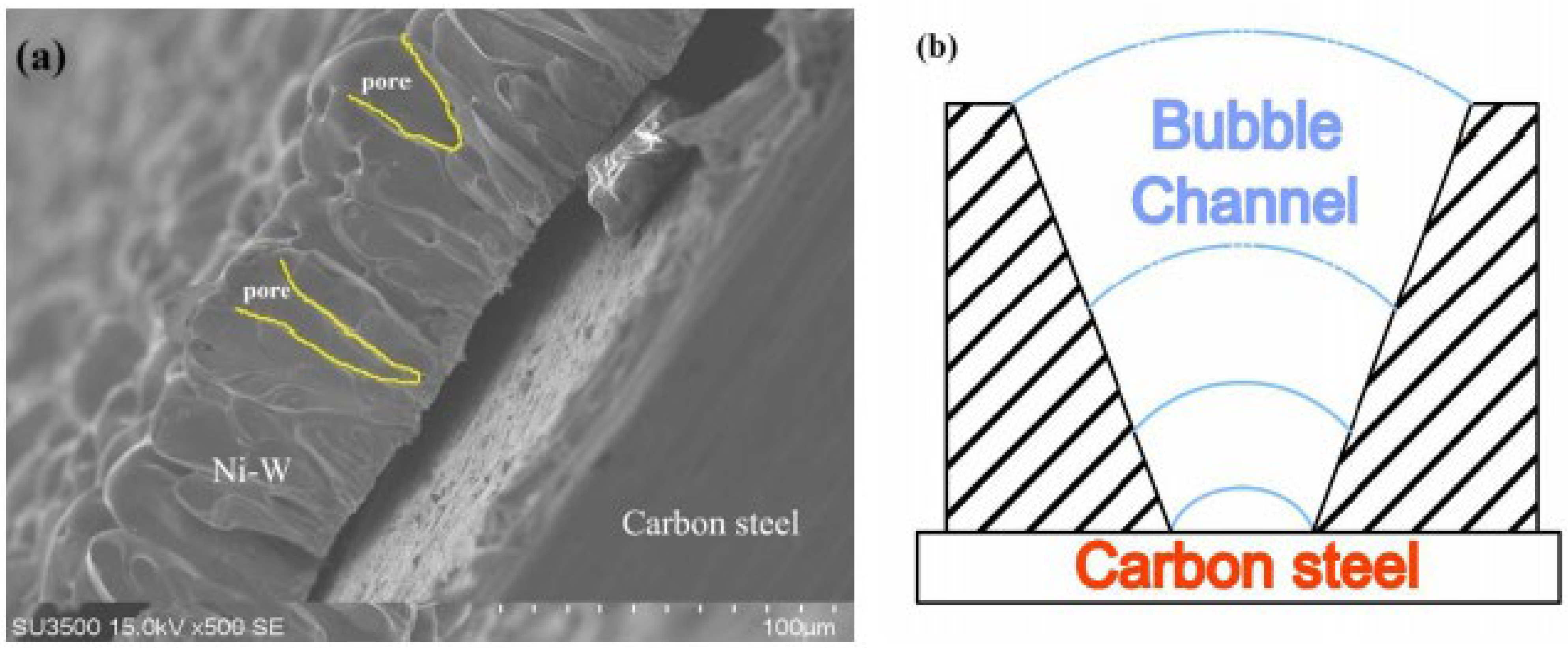
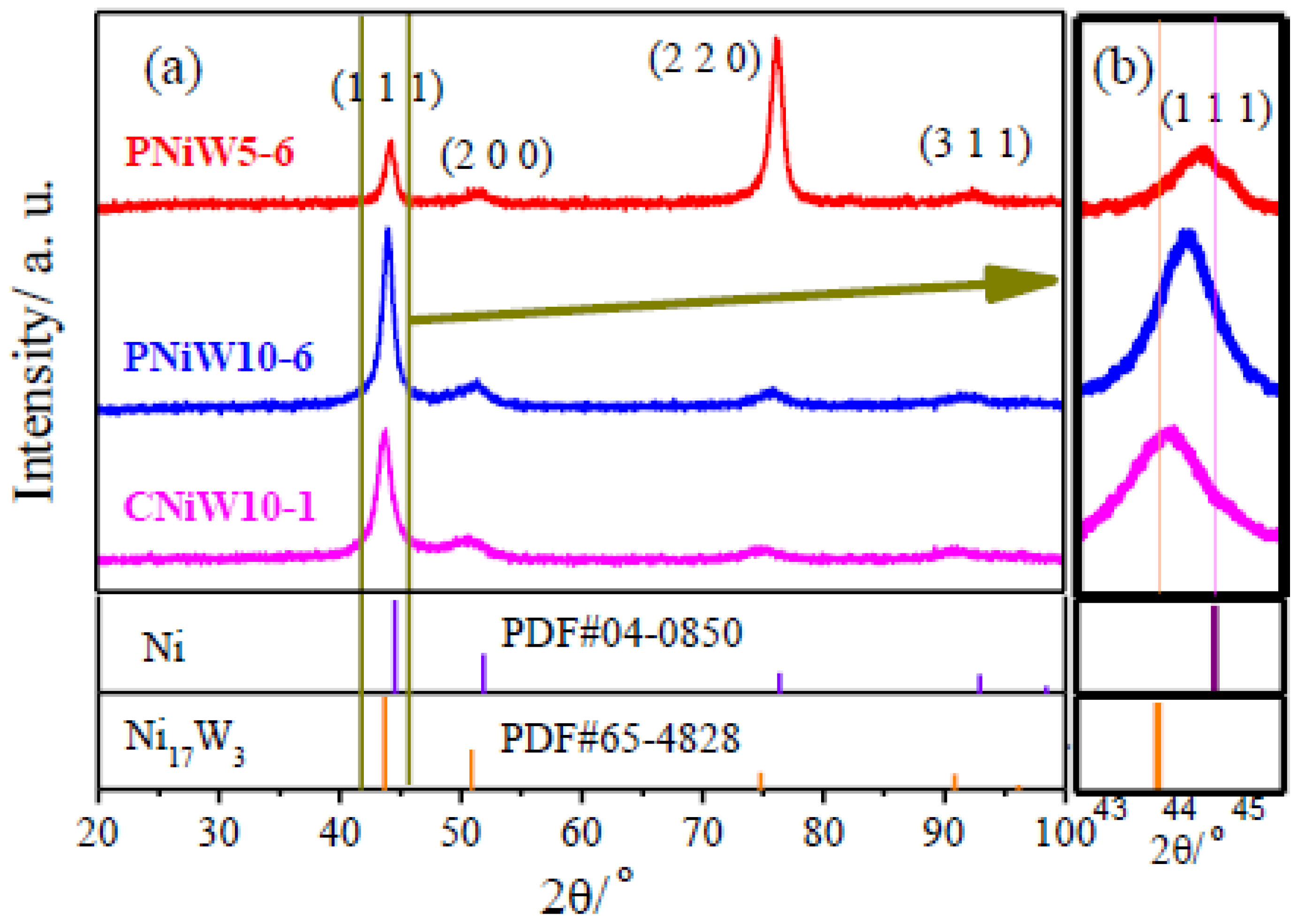
3.1. Characterization of Ni-W Alloys
3.2. Electrocatalytic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, Y.; Li, Z.; Deng, B.; Ye, C.; Zhang, L.; Wang, Y.; Wang, Y.; Li, T.; Liu, Q.; Cui, G.; et al. Superior hydrogen evolution electrocatalysis enabled by CoP nanowire array on graphite felt. Int. J. Hydrogen Energy 2022, 47, 3580–3586. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, H.; Hou, G.R.; Zhang, L.Z.; Li, Q.L.; Wu, Y.K.; Xu, M.; Bao, S.J. Puzzle-inspired carbon dots coupled with cobalt phosphide for constructing a highly-effective overall water splitting interface. Chem. Commun. 2020, 56, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Gou, L.; Wei, S.; Hou, X.; Wu, L. High-performance methanol electrolysis towards energy-saving hydrogen production: Using Cu2O-Cu decorated Ni2P nanoarray as bifunctional monolithic catalyst. Chem. Eng. J. 2023, 454, 140292. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Liang, J.; Fan, X.; He, X.; Chen, J.; Li, J.; Li, Z.; Cai, Z.; Sun, Z.; et al. Highly efficient and stable oxygen evolution from seawater enabled by a hierarchical NiMoSx microcolumn@ NiFe-layered double hydroxide nanosheet array. Inorg. Chem. Front. 2023, 10, 2766–2775. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Xu, S.; Liu, Q.; Li, T.; Luo, Y.; Gao, H.; Shi, X.; Asiri, A.; Sun, X. Recent Advances in 1D ElectrospunNanocatalysts for Electrochemical Water Splitting. Small Struct. 2022, 2, 2000048. [Google Scholar] [CrossRef]
- Jiang, H.; Cong, N.; Jiang, H.; Tian, M.; Xie, Z.; Fang, H.; Han, J.; Ren, Z.; Zhu, Y. Dynamic hydrogen bubble template electrodeposition of Ru on amorphous Co support for electrochemical hydrogen evolution. Int. J. Hydrogen Energy 2023, 48, 21599–21609. [Google Scholar] [CrossRef]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for electrochemical hydrogen evolution reaction: A review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Vernickaite, E.; Tsyntsaru, N.; Sobczak, K.; Cesiulis, H. Electrodeposited tungsten-rich Ni-W, Co-W and Fe-W cathodes for efficient hydrogen evolution in alkaline medium. Electrochim. Acta 2019, 318, 597–606. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Sui, Z.; Wang, M. Effects of ultrasonic field on structure evolution of Ni film electrodeposited by bubble template method for hydrogen evolution electrocatalysis. J. Solid State Electrochem. 2021, 25, 2201–2212. [Google Scholar] [CrossRef]
- González-Buch, C.; Herraiz-Cardona, I.; Ortega, E.; García-Antón, J.; Pérez-Herranz, V. Synthesis and characterization of macroporous Ni, Co and Ni-Co electrocatalytic deposits for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2013, 38, 10157–10169. [Google Scholar] [CrossRef]
- Wang, J.; Shao, H.; Ren, S.; Hu, A.; Li, M. Fabrication of porous Ni-Co catalytic electrode with high performance in hydrogen evolution reaction. Appl. Surf. Sci. 2021, 539, 148045. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Sabour Rouhaghdam, A.; Kiani, M.A. Three-dimensional Ni-Co alloy hierarchical nanostructure as efficient non-noble-metal electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 2019, 465, 846–862. [Google Scholar] [CrossRef]
- Reda, Y.; Abdel-Karim, R.; Zohdy, K.M.; El-Raghy, S. Electrochemical behavior of Ni-Cu foams fabricated by dynamic hydrogen bubble template electrodeposition used for energy applications. Ain Shams Eng. J. 2022, 13, 101532. [Google Scholar] [CrossRef]
- Han, Q.; Cui, S.; Pu, N.; Chen, J.; Liu, K.; Wei, X. A study on pulse plating amorphous Ni-Mo alloy coating used as HER cathode in alkaline medium. Int. J. Hydrogen Energy 2010, 35, 5194–5201. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Yu, X.; Guo, Z. Facile one-step electrodeposition preparation of porous Ni-Mo film as electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2015, 40, 2173–2181. [Google Scholar] [CrossRef]
- Raveendran, M.; Neethu, A.; Chitharanjan, H. Development of Ni-W alloy coatings and their electrocatalytic activity for water splitting reaction. Phys. B Condens. Matter 2020, 597, 412359. [Google Scholar] [CrossRef]
- Jameei Rad, P.; Aliofkhazraei, M.; Barati Darband, G. Ni-W nanostructure well-marked by Ni selective etching for enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 880–894. [Google Scholar] [CrossRef]
- Hong, S.H.; Ahn, S.H.; Choi, J.; Kim, J.Y.; Kim, H.Y.; Kim, H.J.; Jang, J.H.; Kim, H.; Kim, S.K. High-activity electrodeposited Ni-W catalysts for hydrogen evolution in alkaline water electrolysis. Appl. Surf. Sci. 2015, 349, 629–635. [Google Scholar] [CrossRef]
- Tang, J.; Niu, J.; Yang, C.; Rajendran, S.; Lei, Y.; Sawangphruk, M.; Zhang, X.; Qin, J. Twin boundaries boost the hydrogen evolution reaction on the solid solution of nickel and tungsten. Fuel 2022, 330, 125510. [Google Scholar] [CrossRef]
- Gao, D.; Guo, J.; Cui, X.; Yang, L.; Yang, Y.; He, H.; Xiao, P.; Zhang, Y. Three-Dimensional Dendritic Structures of Ni-Co-Mo as Efficient Electrocatalysts for the Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 22420–22431. [Google Scholar] [CrossRef]
- Vijayakumar, J.; Mohan, S.; Anand Kumar, S.; Suseendiran, S.R.; Pavithra, S. Electrodeposition of Ni-Co-Sn alloy from choline chloride-based deep eutectic solvent and characterization as cathode for hydrogen evolution in alkaline solution. Int. J. Hydrogen Energy 2013, 38, 10208–10214. [Google Scholar] [CrossRef]
- Lotfi, N.; Barati Darband, G.H. Energy-Saving Electrochemical Hydrogen Production on Dynamic Hydrogen Bubble-Template Electrodeposited Ni-Cu-Mn Nano-Micro Dendrite. J. Electrochem. Soc. 2022, 169, 096508. [Google Scholar] [CrossRef]
- Lu, S.S.; Shang, X.; Zhang, L.M.; Dong, B.; Gao, W.K.; Dai, F.N.; Liu, B.; Chai, Y.M.; Liu, C.G. Heterostructured binary Ni-W sulfides nanosheets as pH-universal electrocatalyst for hydrogen evolution. Appl. Surf. Sci. 2018, 445, 445–453. [Google Scholar] [CrossRef]
- Machado Oliveira, J.A.; Filgueirade Almeida, A.; Nascimento Campos, A.R.; Prasad, S.; Nicacio Alves, J.J.; Costa de Santana, R.A. Effect of current density, temperature and bath pH on properties of Ni-W-Co alloys obtained by electrodeposition. J. Alloys Compd. 2021, 853, 157104. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.H.; Aliofkhazraei, M.; Rezvanian, A.R.; Torabinejad, V.; Sabour Rouhaghdam, A.R. Ni-W electrodeposited coatings: Characterization, properties and applications. Surf. Coat. Technol. 2016, 307, 978–1010. [Google Scholar] [CrossRef]
- Bahari Mollamahale, Y.; Jafari, N.; Hosseini, D. Electrodeposited Ni-W nanoparticles: Enhanced catalytic activity toward hydrogen evolution reaction in acidic media. Mater. Lett. 2018, 213, 15–18. [Google Scholar] [CrossRef]
- Zhang, Y.; Bilan, H.K.; Podlaha, E. Enhancing the hydrogen evolution reaction with Ni-W-TiO2 composites. Electrochem. Commun. 2018, 96, 108–112. [Google Scholar] [CrossRef]
- Su, C.; Sa, Z.; Liu, Y.; Zhao, L.; Wu, F.; Bai, W. Excellent Properties of Ni-15 wt.% W Alloy Electrodeposited from a Low-Temperature Pyrophosphate System. Coatings 2021, 11, 1262. [Google Scholar] [CrossRef]
- Juškėnas, R.; Valsiūnas, I.; Pakštas, V.; Selskis, A.; Jasulaitienė, V.; Karpavičienė, V.; Kapočius, V. XRD, XPS and AFM studies of the unknown phase formed on the surface during electrodeposition of Ni–W alloy. Appl. Surf. Sci. 2006, 253, 1435–1442. [Google Scholar] [CrossRef]
- Chianpairot, A.; Lothongkum, G.; Schuh, C.A.; Boonyongmaneerat, Y. Corrosion of nanocrystalline Ni-W alloys in alkaline and acidic 3.5wt.% NaCl solutions. Corros. Sci. 2011, 53, 1066–1071. [Google Scholar] [CrossRef]
- Rosalbino, F.; Delsante, S.; Borzone, G.; Angelini, E.M.M.A. Correlation of microstructure and catalytic activity of crystalline Ni–Co–Y alloy electrode for the hydrogen evolution reaction in alkaline solution. J. Alloys Compd. 2007, 429, 270–275. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]





| Chemicals/Parameters | Values |
|---|---|
| NiSO4·6H2O | 52 g L−1 |
| NaWO4·2H2O | 50, 100 gL−1 |
| Na4P2O7·10H2O | 89 gL−1 |
| NH3 H2O | ~20 mL |
| H3BO3 | 120 gL−1 |
| Bath temperature | 55 °C |
| Current density | 0.1–0.6 A cm−2 |
| pH | 8.8–9.2 |
| Samples | Na2WO4·2H2O/g L−1 | Current Density/A cm−2 | Time/min |
|---|---|---|---|
| CNiW10-1 | 100 | 0.1 | 30 |
| PNiW5-6 | 50 | 0.6 | 5 |
| PNiW10-6 | 100 | 0.6 | 5 |
| Catalysts Material | Overpotential of Current Density at 10 mA cm−2 (mV) | Tafel Slope (mV per Decade) | pH | Refs. |
|---|---|---|---|---|
| T-Ru/a-Cu | 49 | 46.4 | 14 | [6] |
| Ni-Co | 197 | 92 | 14 | [11] |
| Ni-Co | 107 | 118 | 14 | [12] |
| Ni-Mo | 62 (200 mA cm−2 and 80 °C) | 20.1 | 14.8 | [14] |
| Ni-Mo | 47 (100 mA cm−2) | NA | 14 | [15] |
| Ni-W | 169 | 130 | 14 | [17] |
| Ni-Co-Mo | 132 | 108 | 14 | [20] |
| Ni-Co-Sn | NA | 122 | 14 | [21] |
| Ni-Cu-Mn | 63 | 111 | 14 | [31] |
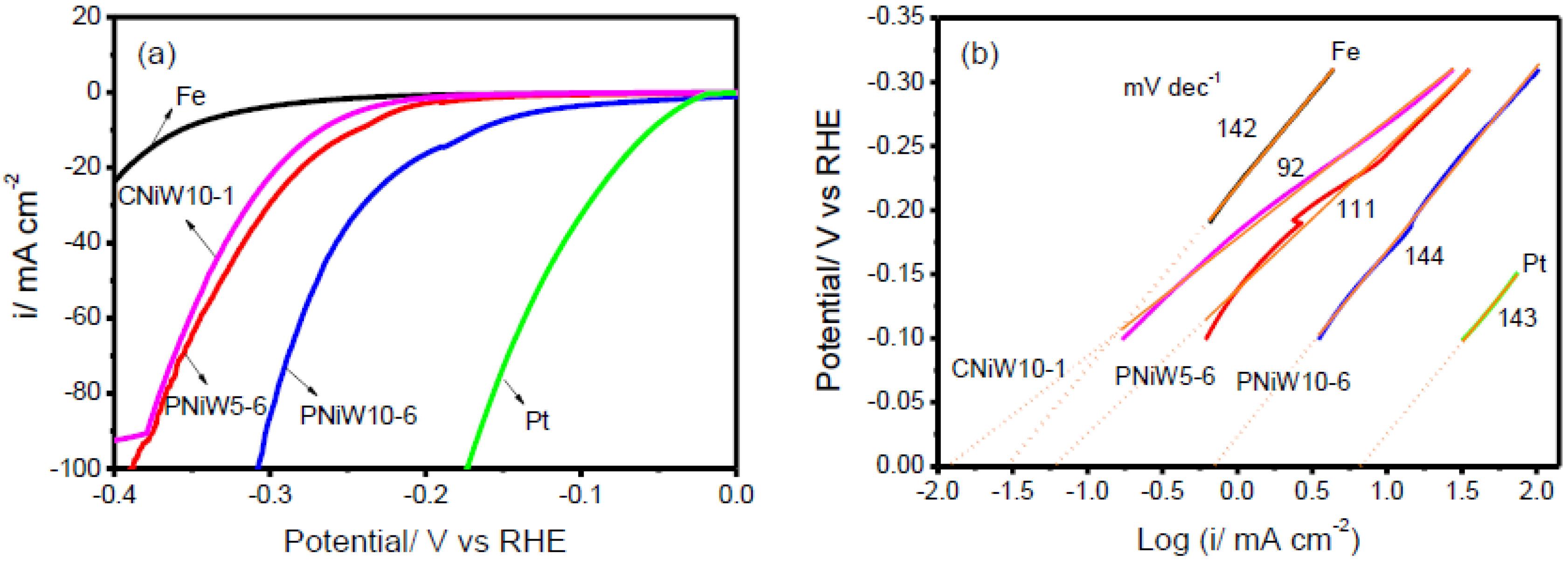
| Samples | b/mV dec−1 | i0/mA cm−2 | η10 (mV) | η20 (mV) | H50 (mV) | η100 (mV) |
|---|---|---|---|---|---|---|
| Fe | 142 | 0.030 | 357 | 391 | - | - |
| CNiW10-1 | 92 | 0.012 | 267 | 295 | 341 | - |
| PNiW5-6 | 111 | 0.062 | 243 | 273 | 331 | 388 |
| PNiW10-6 | 144 | 0.741 | 166 | 213 | 269 | 308 |
| Pt | 143 | 7.079 | 55 | 77 | 124 | 173 |
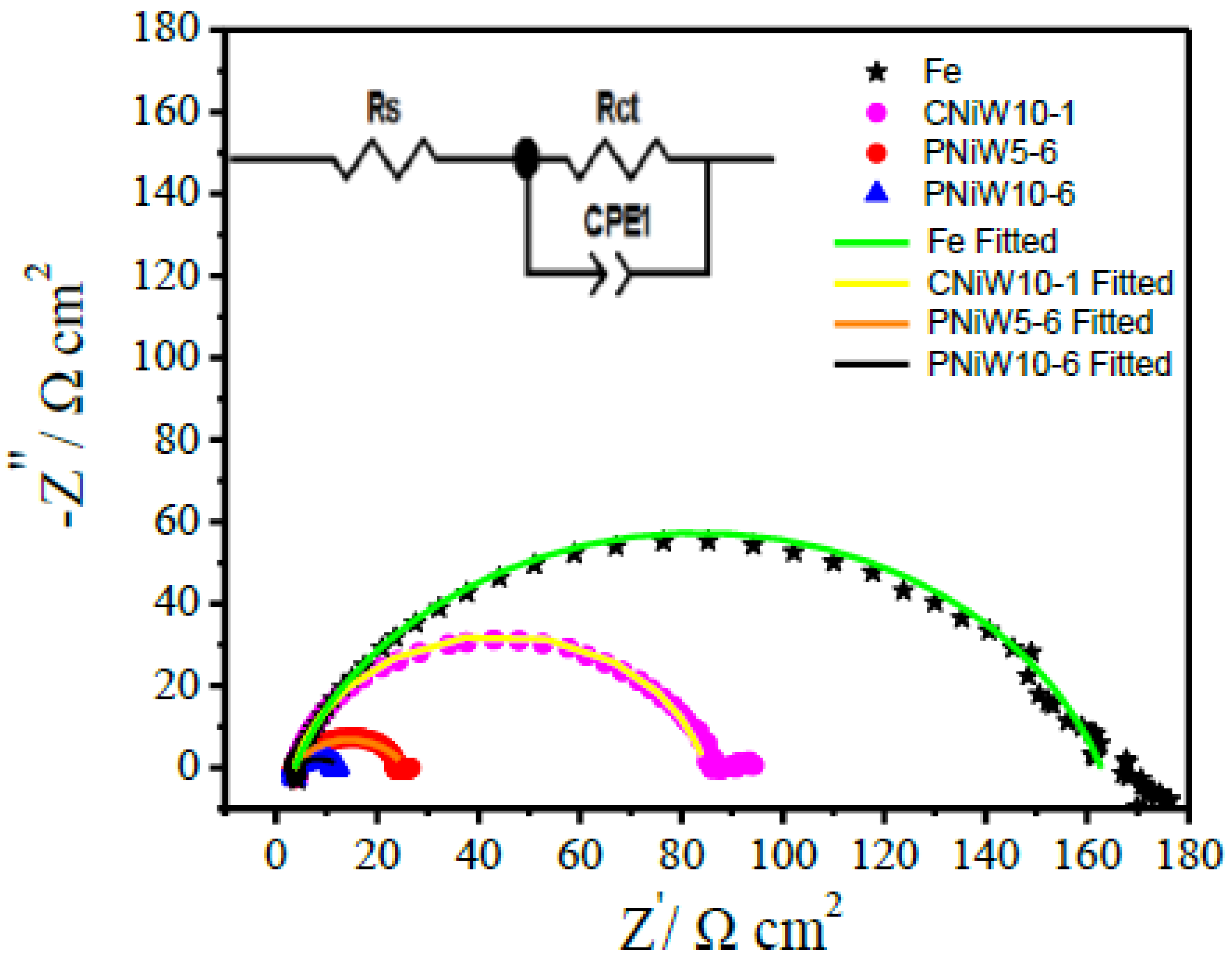
| Samples | Rs/Ω cm2 | Rct/Ω cm2 | CPE-T/mF cm−2 | n (0 < n < 1) |
|---|---|---|---|---|
| CNiW10-1 | 3.83 | 80.38 | 0.182 | 0.86 |
| PNiW5-6 | 3.68 | 22.07 | 2.625 | 0.71 |
| PNiW10-6 | 3.47 | 9.30 | 15.324 | 0.52 |
| Fe | 4.84 | 124.84 | 0.551 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, L.; Li, W.; Lu, L.; Tian, L.; Liu, Y.; Su, C.; Tian, W. Preparation of Porous Ni-W Alloys Electrodeposited by Dynamic Hydrogen Bubble Template and Their Alkaline HER Properties. Coatings 2024, 14, 957. https://doi.org/10.3390/coatings14080957
Li Y, Li L, Li W, Lu L, Tian L, Liu Y, Su C, Tian W. Preparation of Porous Ni-W Alloys Electrodeposited by Dynamic Hydrogen Bubble Template and Their Alkaline HER Properties. Coatings. 2024; 14(8):957. https://doi.org/10.3390/coatings14080957
Chicago/Turabian StyleLi, Yufei, Linghao Li, Wenzhe Li, Linfeng Lu, Lu Tian, Yangyang Liu, Changwei Su, and Weidong Tian. 2024. "Preparation of Porous Ni-W Alloys Electrodeposited by Dynamic Hydrogen Bubble Template and Their Alkaline HER Properties" Coatings 14, no. 8: 957. https://doi.org/10.3390/coatings14080957
APA StyleLi, Y., Li, L., Li, W., Lu, L., Tian, L., Liu, Y., Su, C., & Tian, W. (2024). Preparation of Porous Ni-W Alloys Electrodeposited by Dynamic Hydrogen Bubble Template and Their Alkaline HER Properties. Coatings, 14(8), 957. https://doi.org/10.3390/coatings14080957





