An Advanced Surface Treatment Technique for Coating Three-Dimensional-Printed Polyamide 12 by Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens Preparation
2.2. Specimens Grouping
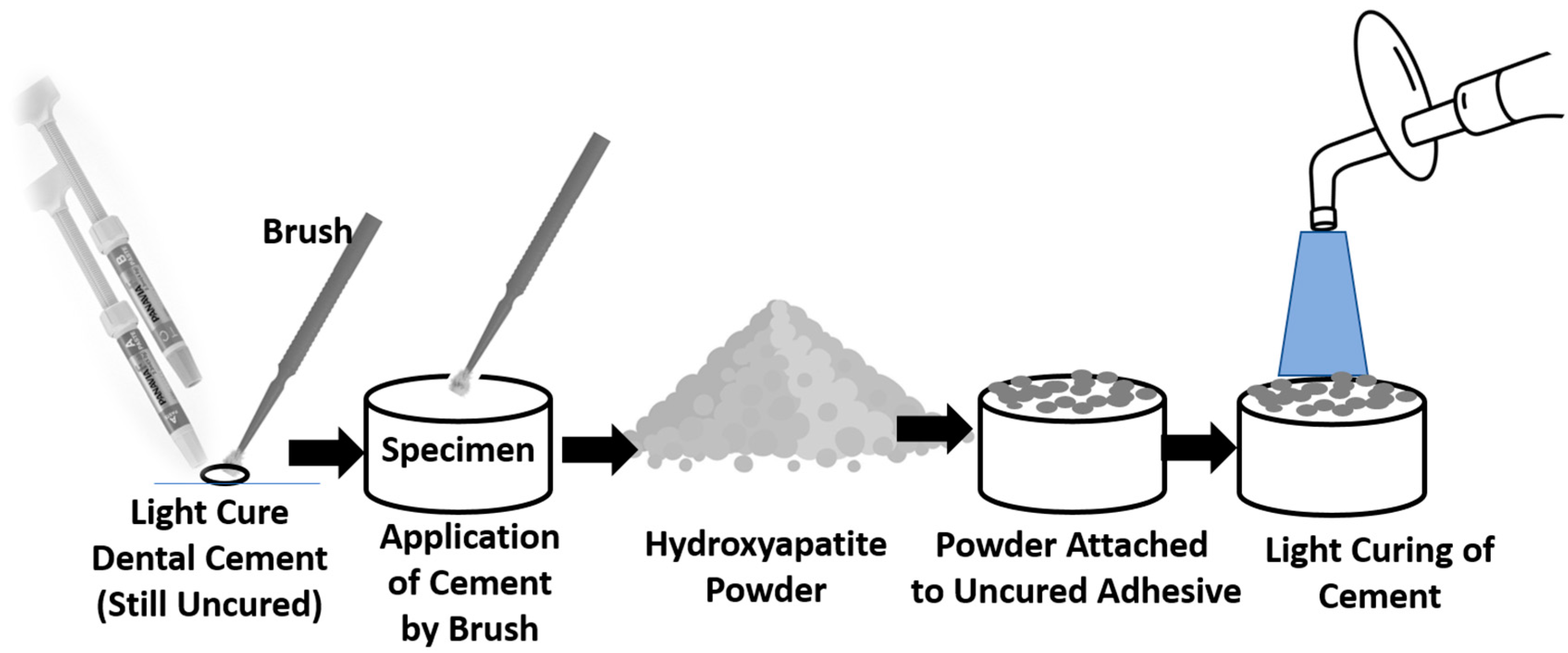
2.3. Surface Treatment
2.4. Characterisation by Fourier-Transform Infrared and Transmission Electron Microscopy
2.5. Storage in Phosphate-Buffered Saline
2.6. Examination of Surface Microstructure and Elemental Analysis
2.7. Coat Adhesion Test
3. Results
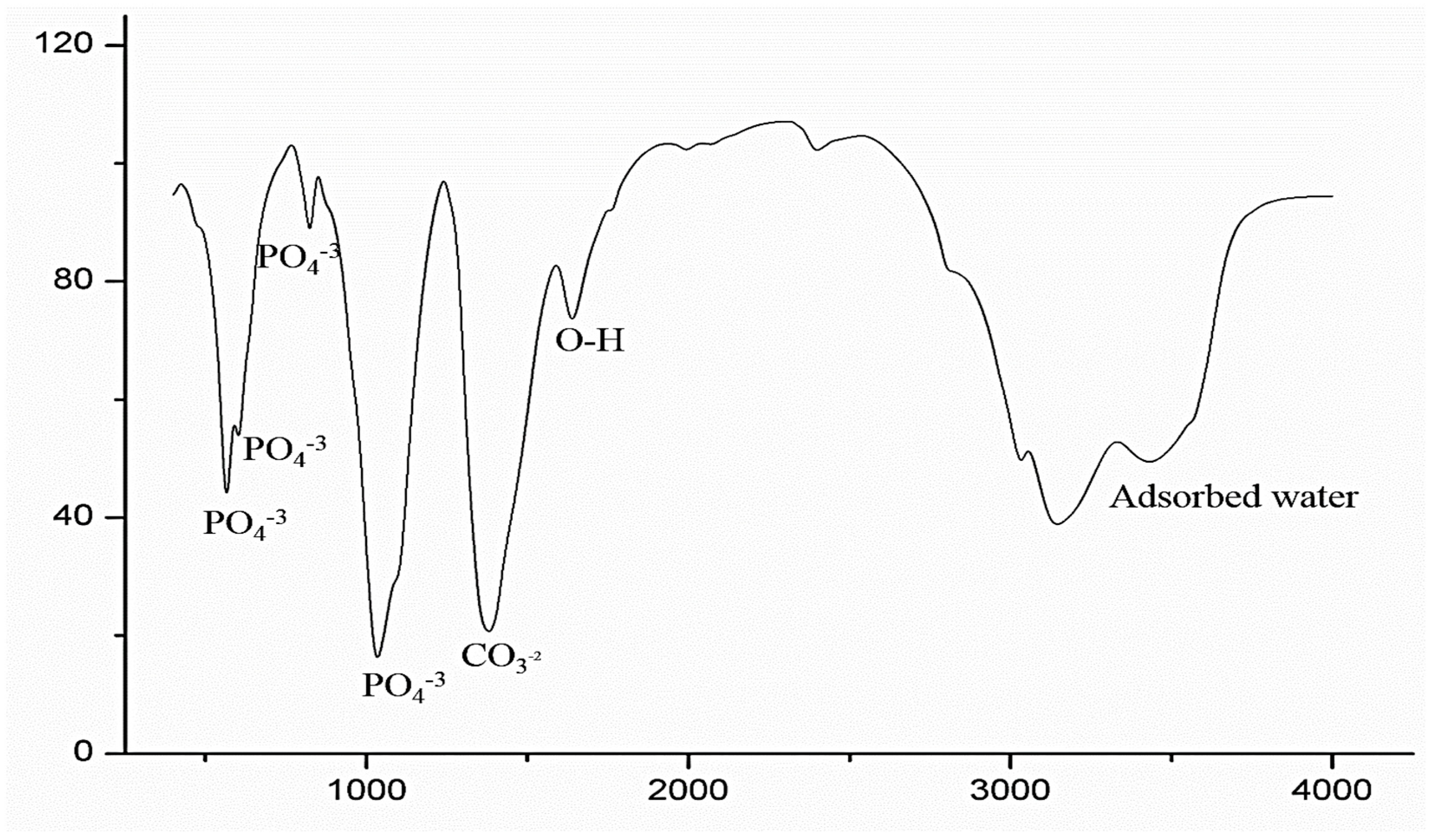
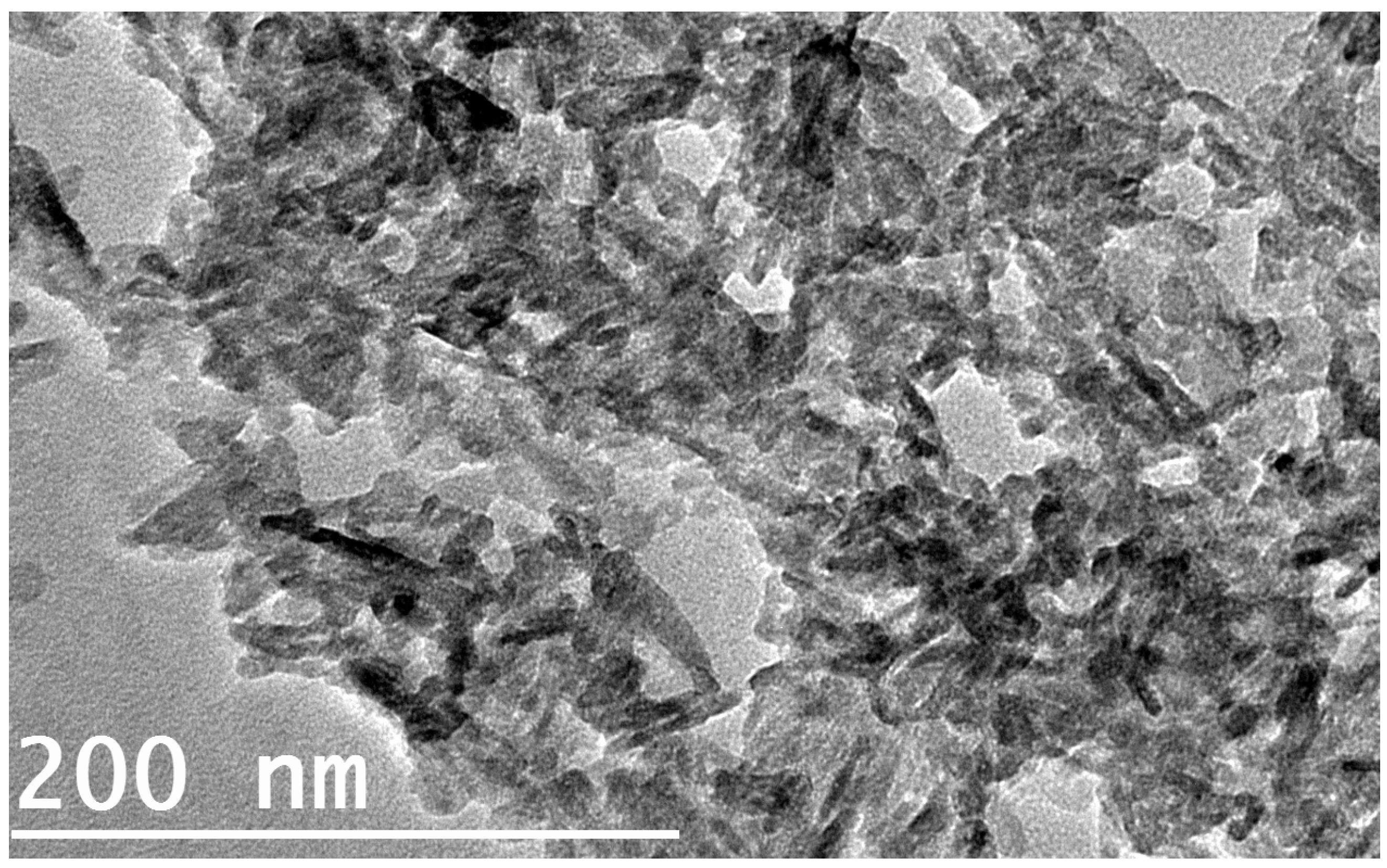
3.1. FTIR and TEM
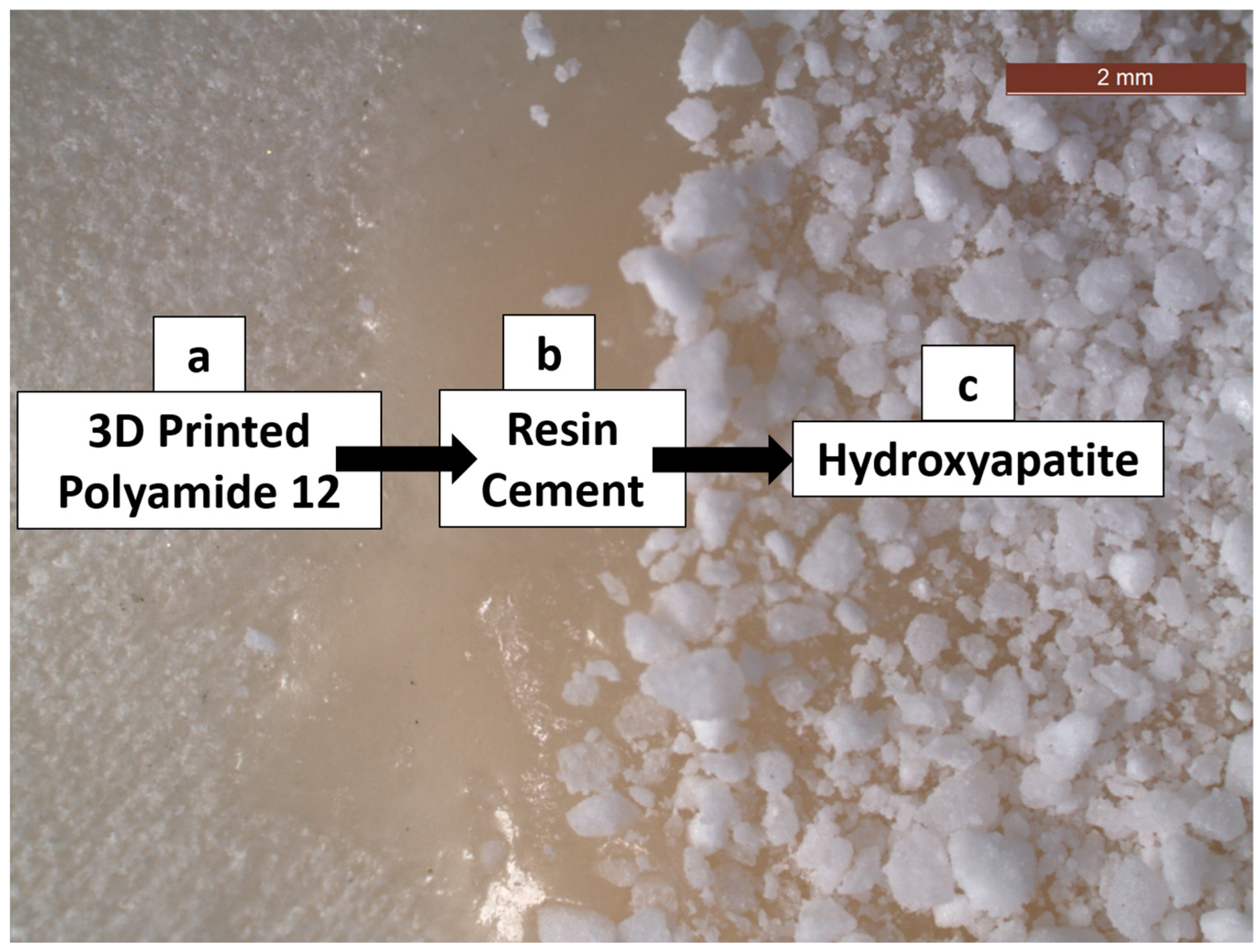
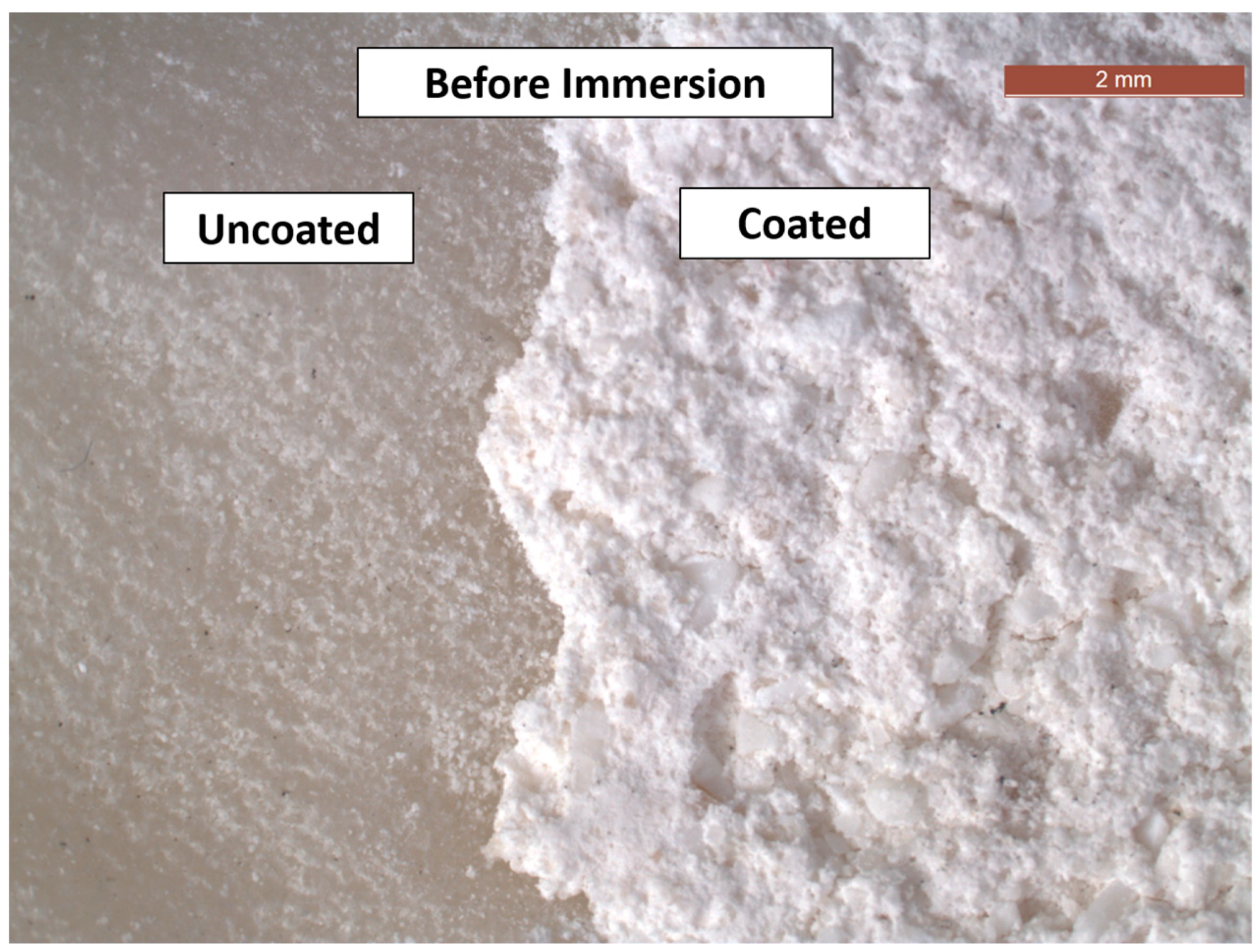
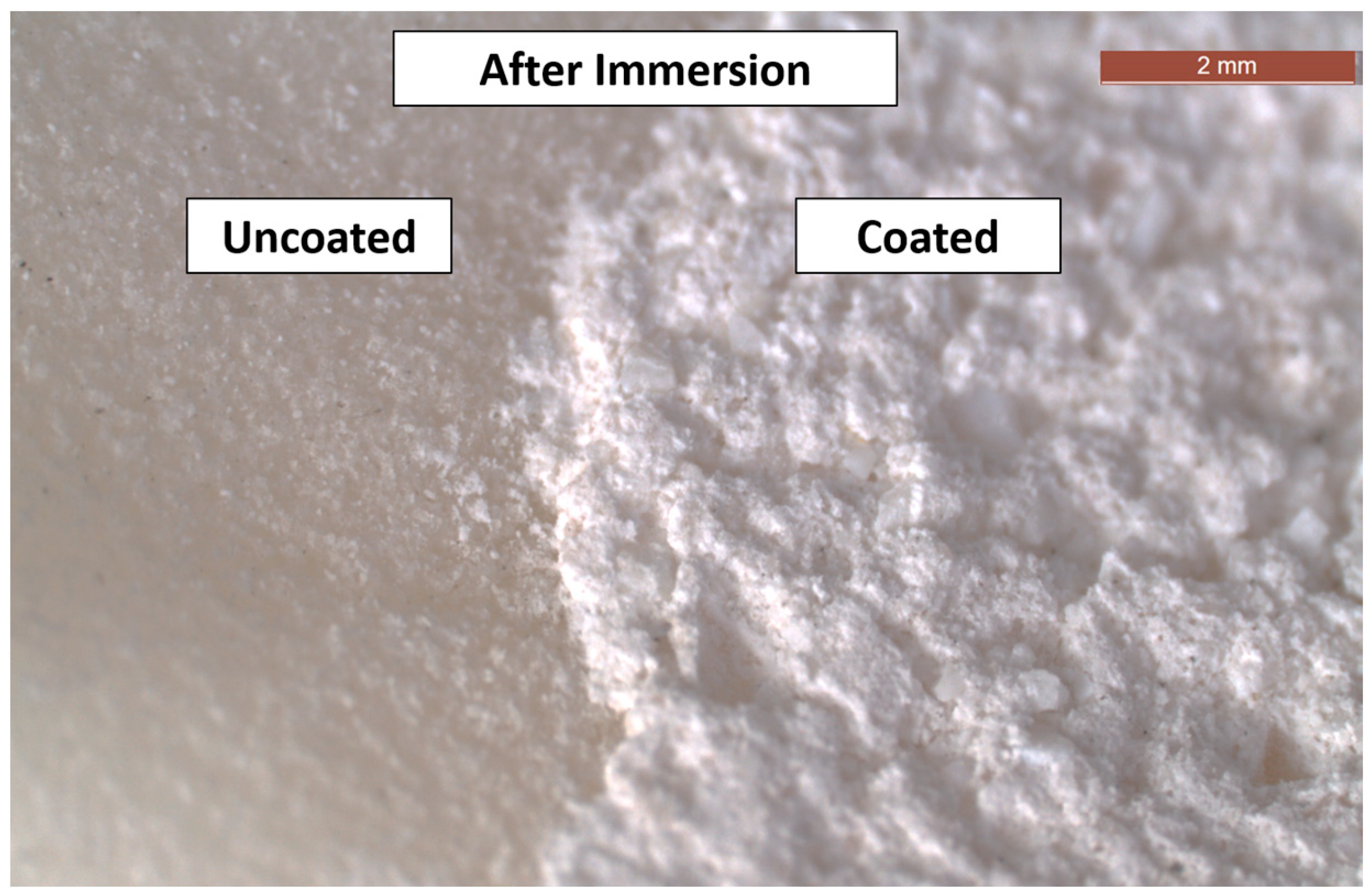
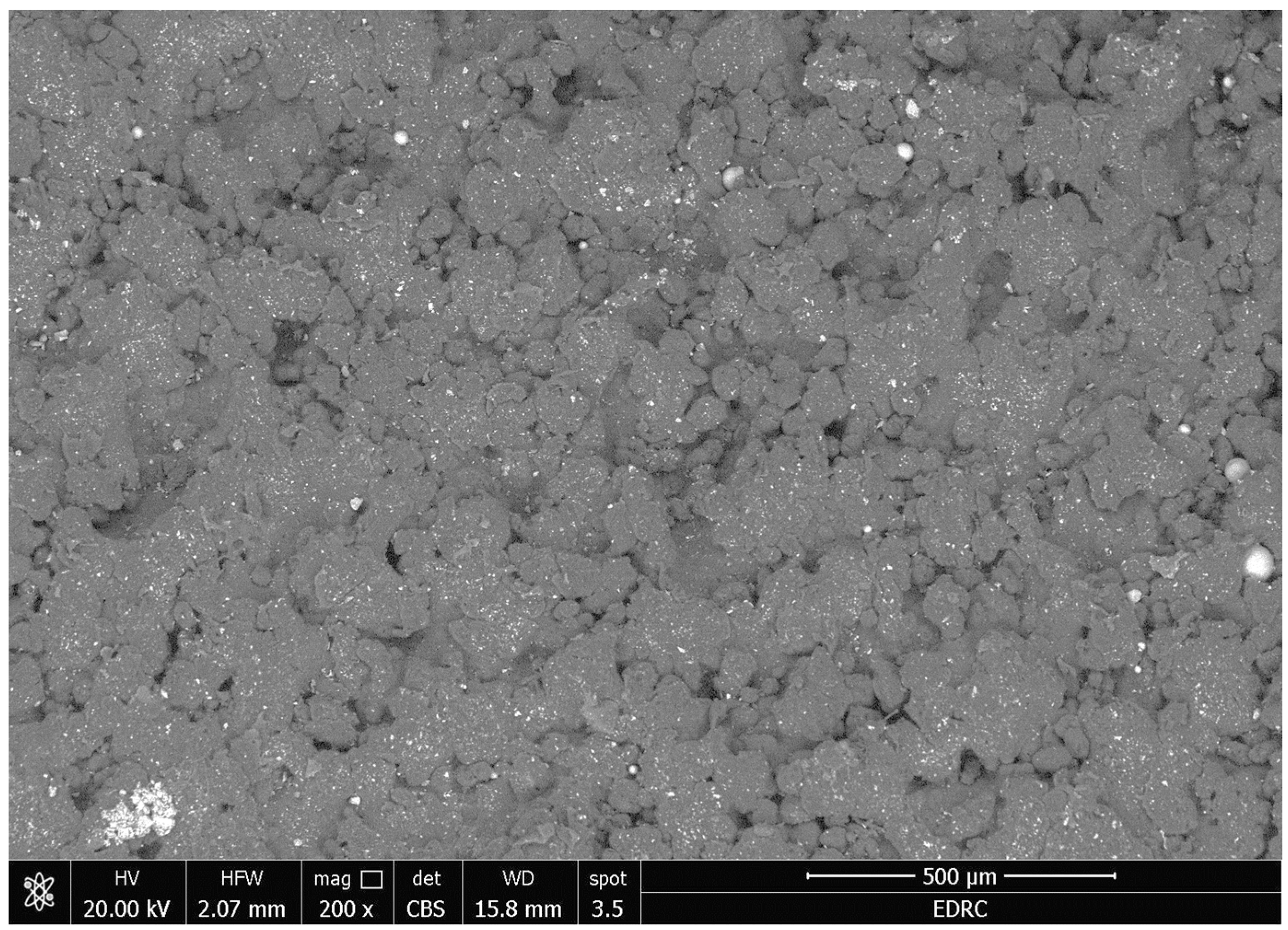
3.2. SEM and EDXA
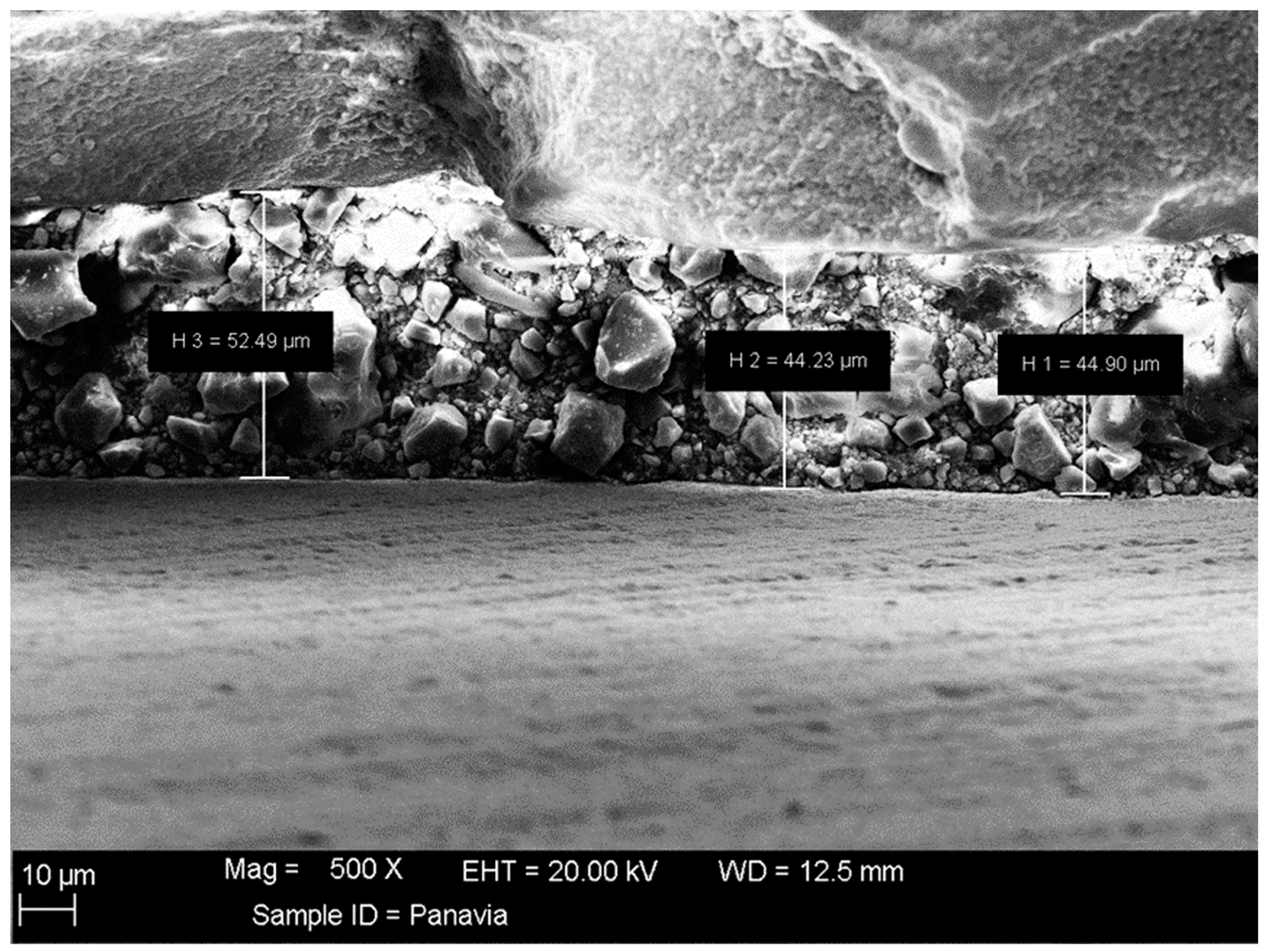
3.3. Coat Adhesion Test
4. Discussion
5. Conclusions
- The results of the FTIR spectroscopy of the coated specimens confirmed the HA bands. The TEM micrographs revealed HA-agglomerated particles in the coat.
- The coat was stable after immersion in PBS, as observed by SEM and EDXA as well as the coat adhesion test, which demonstrated a stable coat with just a few loose coating flakes (area removed <5%) on the surface of the HA-coated specimens.
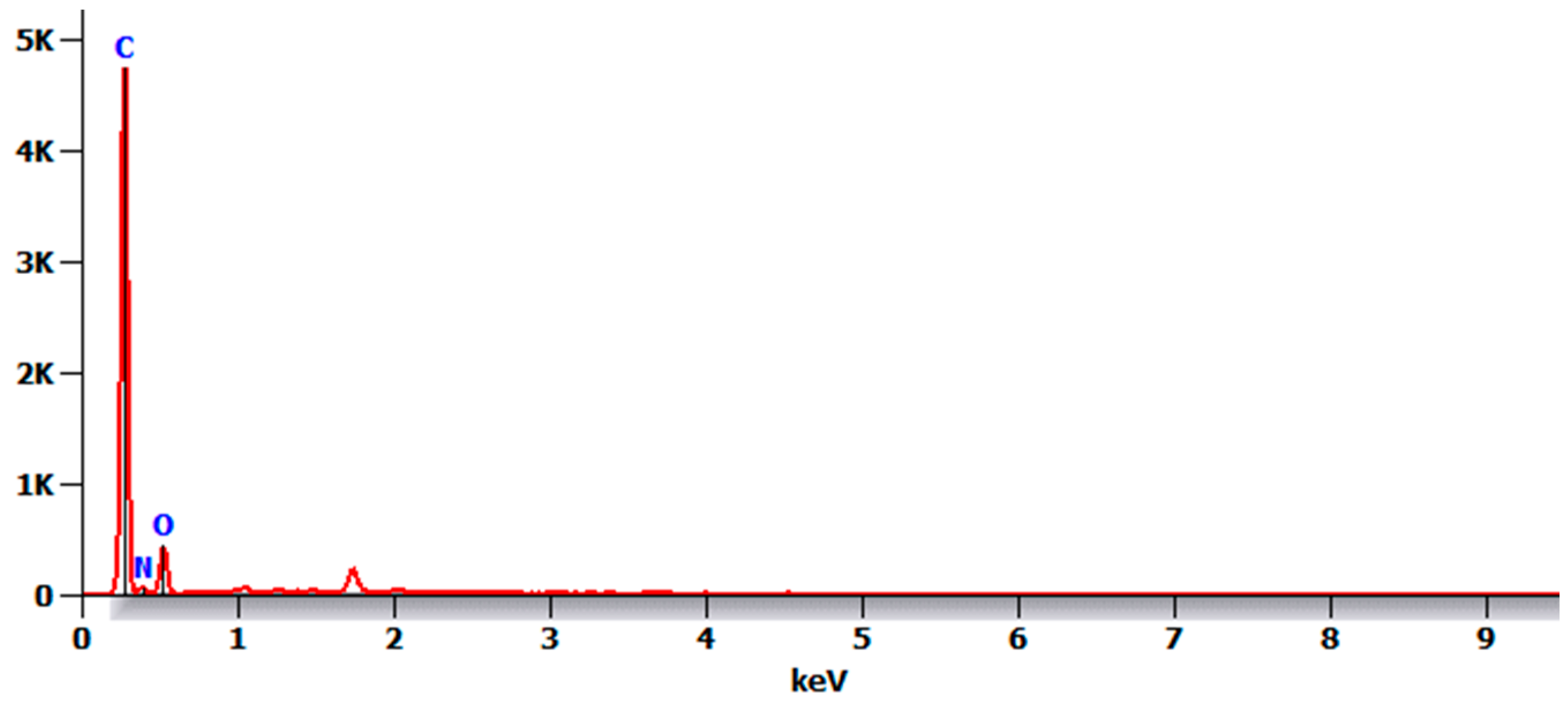
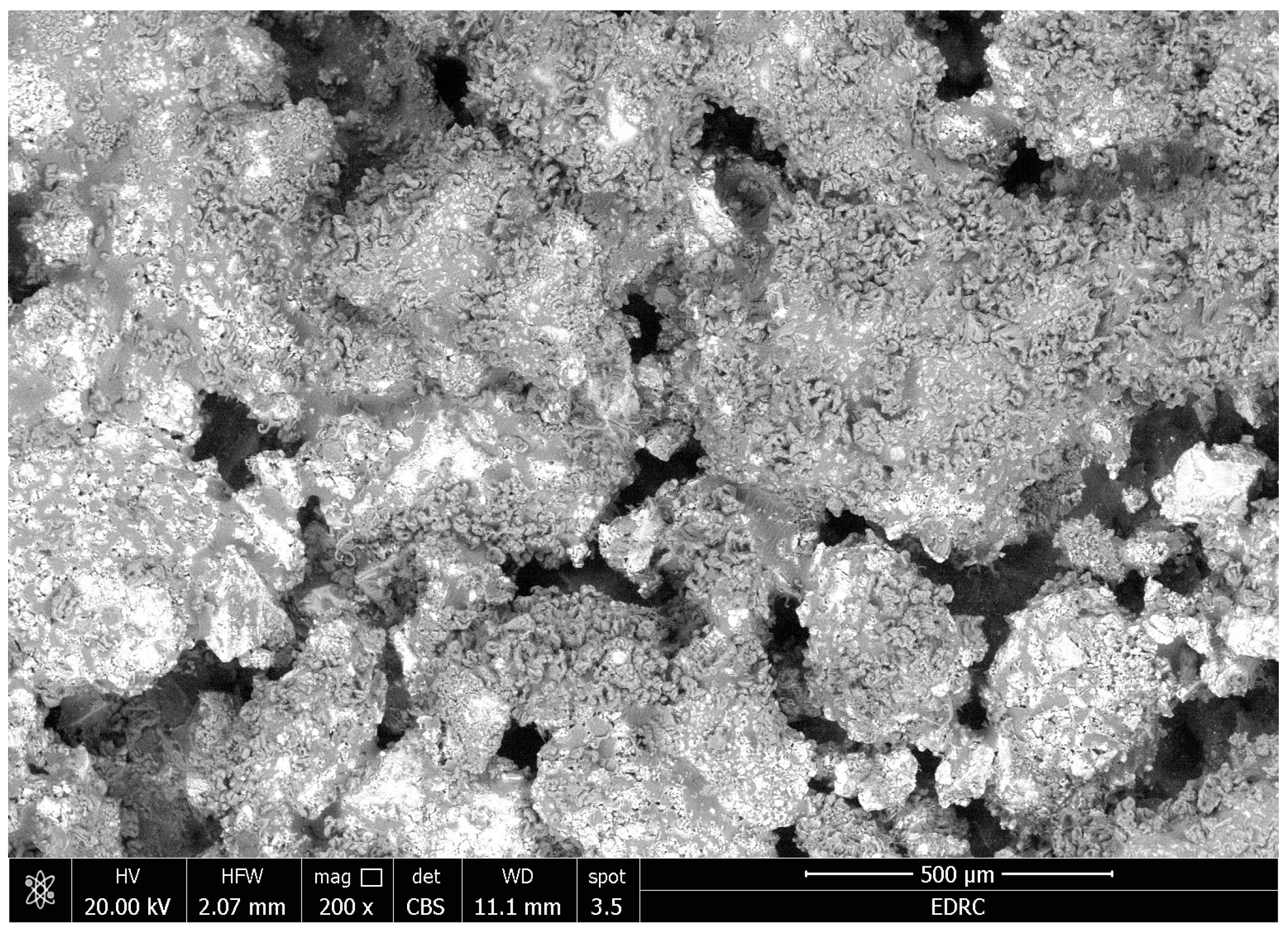
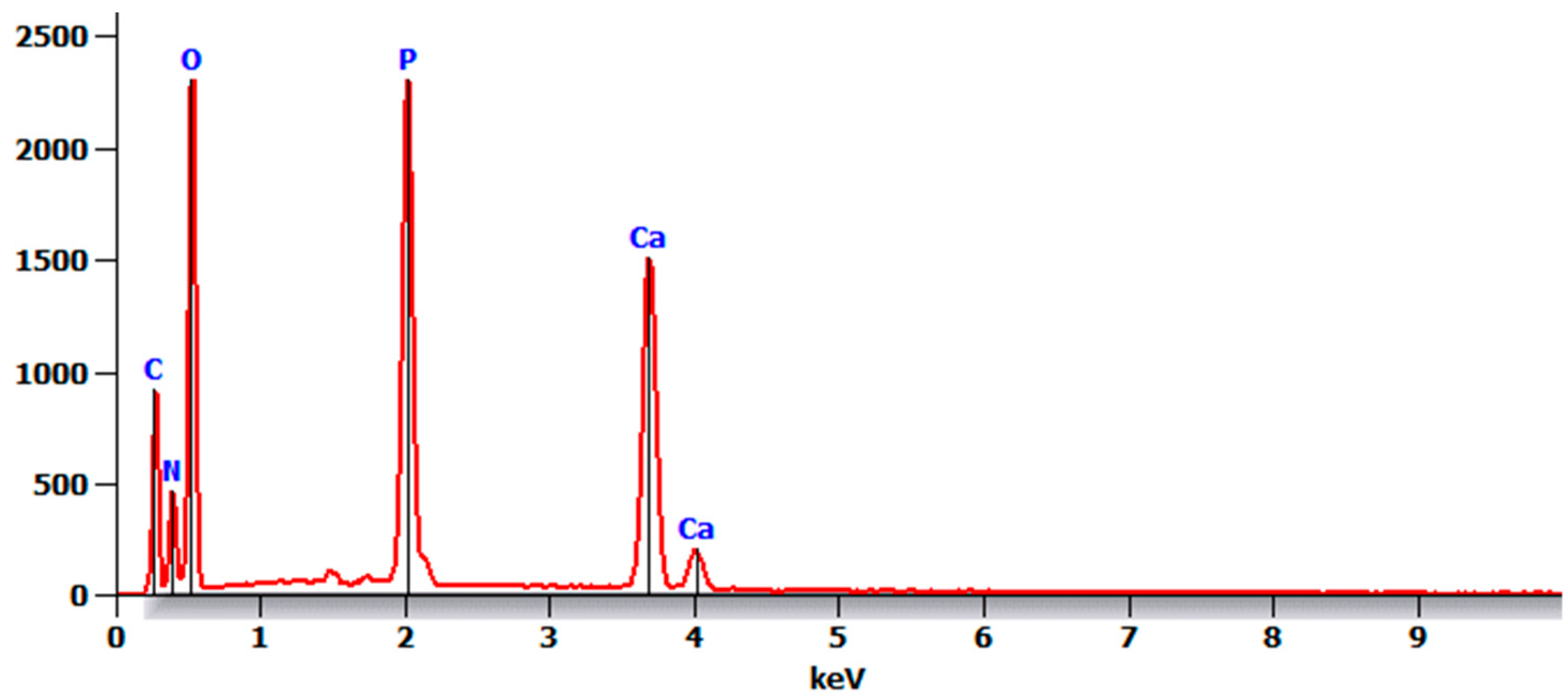
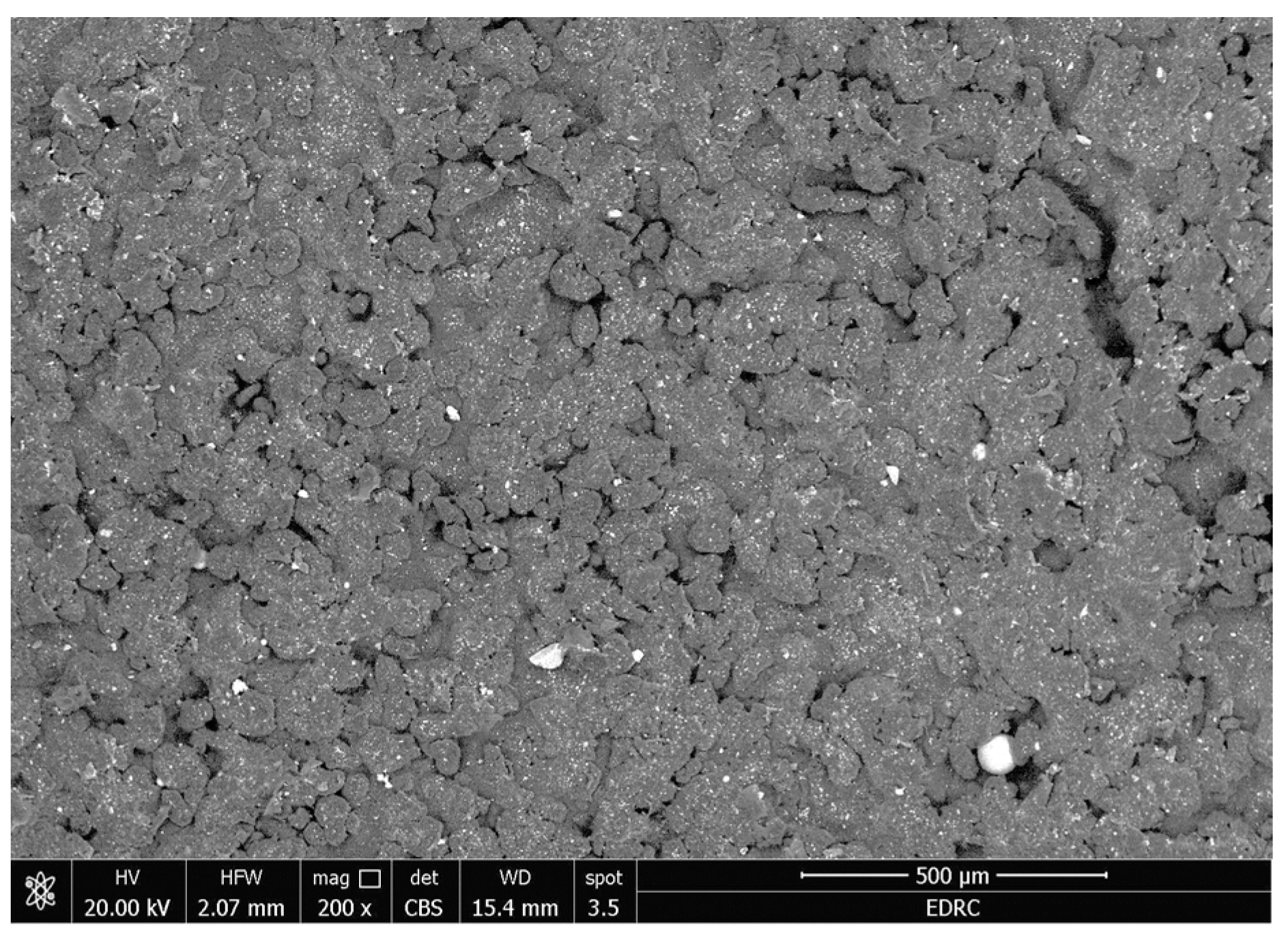
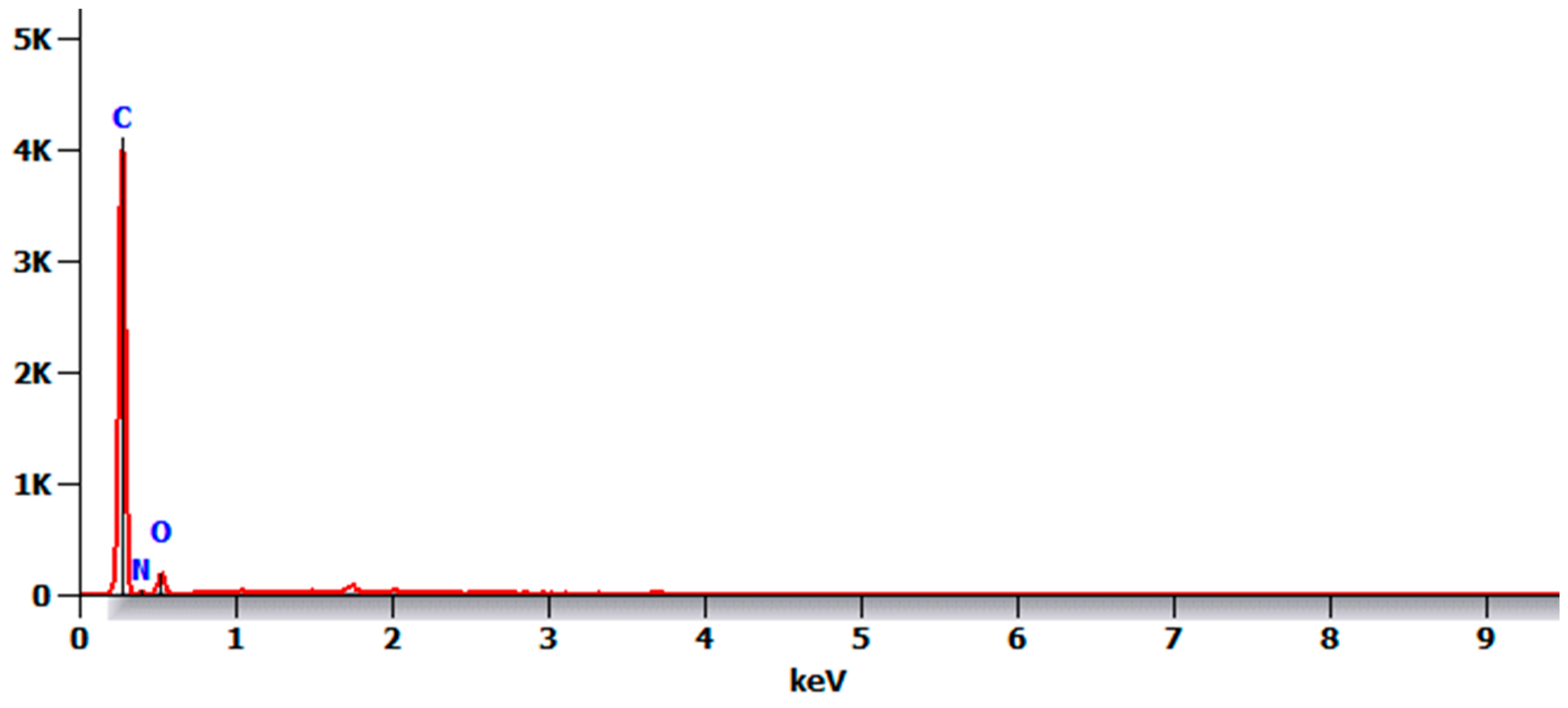
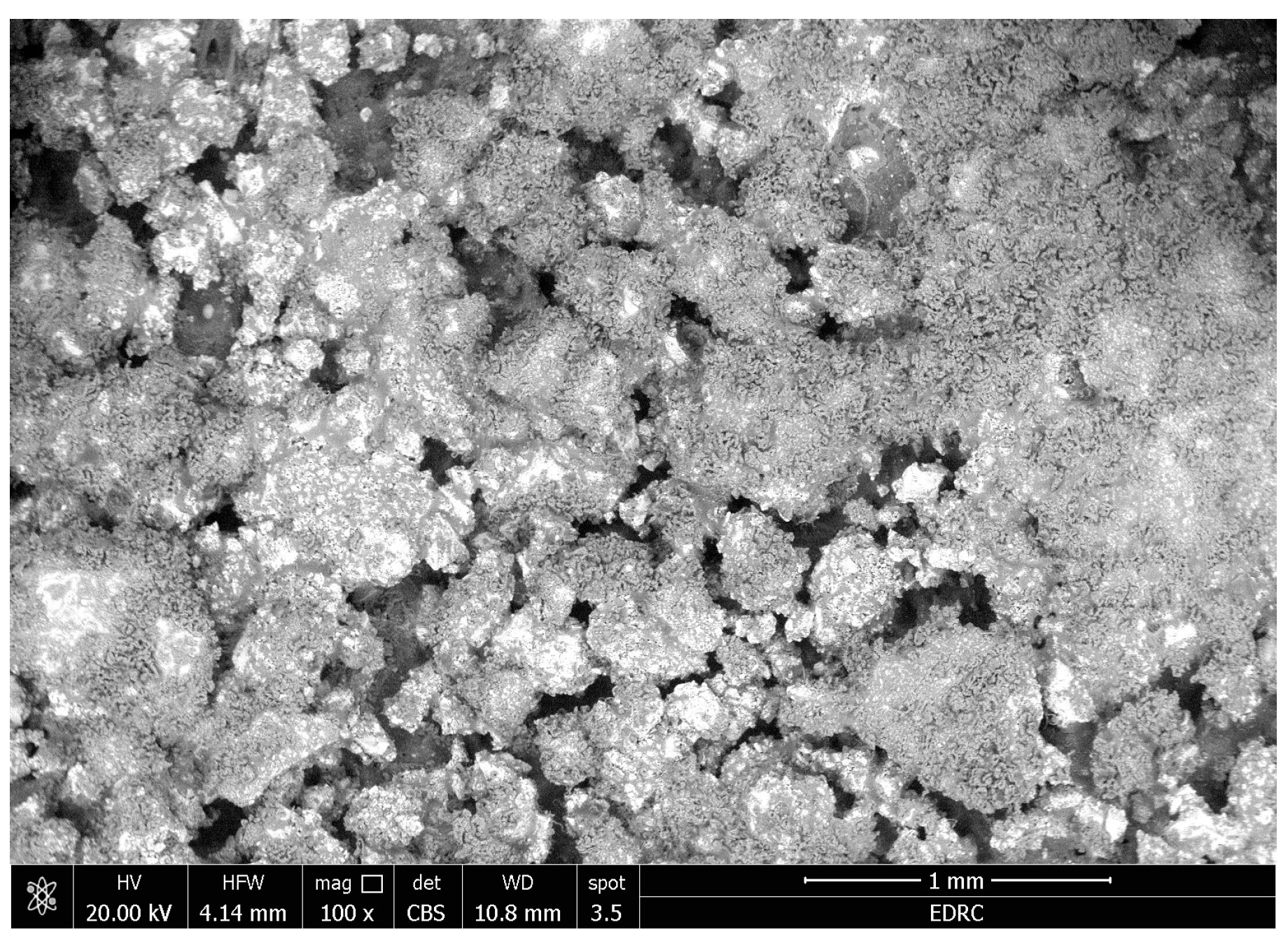
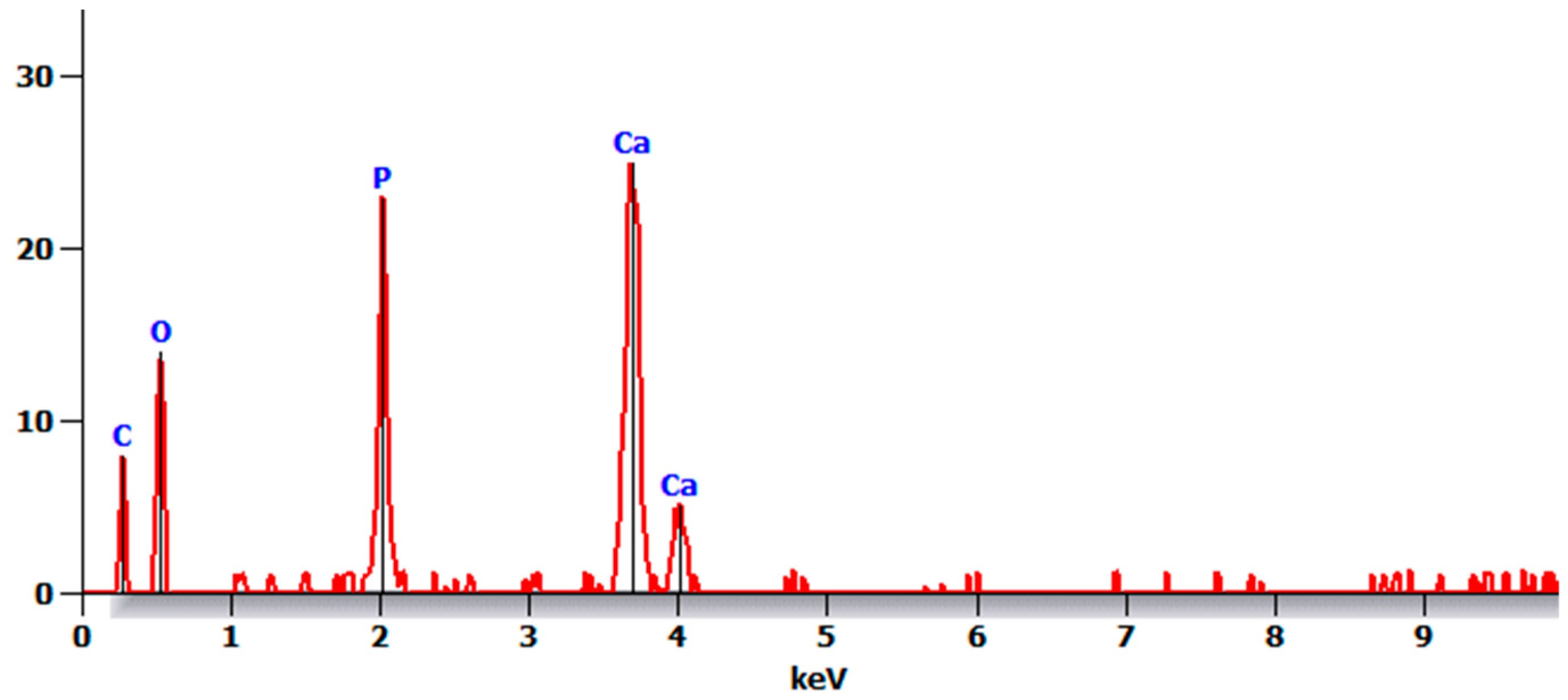
- There were no major changes in both the coated and uncoated (control) specimens before and after immersion in PBS. The SEM micrographs of the control 3D-printed polyamide 12 specimens illustrated the sintered 3D-printed particles with minimal porosity. Their EDXA revealed the presence of carbon, nitrogen, and oxygen. The microstructure of the coated specimens showed deposited clusters of calcium and phosphorus on the surface in addition to carbon, nitrogen, and oxygen.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Earar, K.; Iliescu, A.A.; Popa, G.; Iliescu, A.; Rudnic, I.; Feier, R.; Voinea-Georgescu, R.N. Additive vs. Subtractive CAD/CAM Procedures in Manufacturing of the PMMA Interim Dental Crowns a Comparative in Vitro Study of Internal Fit. Rev. Chim. 2020, 71, 405–410. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Abdelnabi, A.; Othman, M.S.; Bayoumi, R.E. Alterations in Surface Gloss and Hardness of Direct Dental Resin Composites and Indirect CAD/CAM Composite Block after Single Application of Bifluorid 10 Varnish: An In Vitro Study. J. Compos. Sci. 2024, 8, 58. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Bhardwaj, A.; Verma, S. Additive Manufacturing: A 3-Dimensional Approach in Periodontics. J. Adv. Med. Med. Res. 2020, 32, 105–117. [Google Scholar] [CrossRef]
- Moon, J.-M.; Jeong, C.-S.; Lee, H.-J.; Bae, J.-M.; Choi, E.-J.; Kim, S.-T.; Park, Y.-B.; Oh, S.-H. A Comparative Study of Additive and Subtractive Manufacturing Techniques for a Zirconia Dental Product: An Analysis of the Manufacturing Accuracy and the Bond Strength of Porcelain to Zirconia. Materials 2022, 15, 5398. [Google Scholar] [CrossRef]
- Jeong, M.; Radomski, K.; Lopez, D.; Liu, J.T.; Lee, J.D.; Lee, S.J. Materials and Applications of 3D Printing Technology in Dentistry: An Overview. Dent. J. 2023, 12, 1. [Google Scholar] [CrossRef]
- Cai, H.; Xu, X.; Lu, X.; Zhao, M.; Jia, Q.; Jiang, H.-B.; Kwon, J.-S. Dental Materials Applied to 3D and 4D Printing Technologies: A Review. Polymers 2023, 15, 2405. [Google Scholar] [CrossRef]
- Revilla-León, M.; Sadeghpour, M.; Özcan, M. An Update on Applications of 3D Printing Technologies Used for Processing Polymers Used in Implant Dentistry. Odontology 2020, 108, 331–338. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric Materials and Films in Dentistry: An Overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Biopolymer Coatings for Biomedical Applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Maniadi, A.; Velidakis, E.; Mountakis, N.; Kechagias, J.D. Sustainable Additive Manufacturing: Mechanical Response of Polyamide 12 over Multiple Recycling Processes. Materials 2021, 14, 466. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Kok, W.K.; Dikshit, V.; Feng, S.; Li, K.H.H.; Zhang, Y. 3D Printing Biocompatible Materials with Multi Jet Fusion for Bioreactor Applications. Int. J. Bioprint. 2023, 9, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Arafat, S.W.; Ibrahim, W.H.; Shaker, S.; Aldainy, D.G.; Salama, D.; Shaheen, H.A. Reconstruction of Cranial Bone Defects Using Polyamide 12 Patient-Specific Implant: Long Term Follow Up. J. Craniofac. Surg. 2022, 33, 1825–1828. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, H.A.; Shaker, S.; Ibrahim, W.H.; AlDainy, D.G.; Salama, D.; Emara, A.S. Delayed Complex Fronto-Zygomatico-Orbital Reconstruction Using Patient Specific 3D Printed Implants; A Report of Three Complex Cases—A Short Communication. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 3877–3903. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.C.; Piñeiro, O.G.; Alves, A.C.; Carneiro, O.S. On the Reuse of SLS Polyamide 12 Powder. Materials 2022, 15, 5486. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, T.M.; Saniour, S.H.; Sherief, M.A.; Zaki, D.Y. Effect of Incorporation of 20 Wt% Amorphous Nano-Hydroxyapatite Fillers in Poly Methyl Methacrylate Composite on the Compressive Strength. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1136–1141. [Google Scholar] [CrossRef]
- Abdelnabi, A.; Hamza, M.K.; El-Borady, O.M.; Hamdy, T.M. Effect of Different Formulations and Application Methods of Coral Calcium on Its Remineralization Ability on Carious Enamel. Open Access Maced. J. Med. Sci. 2020, 8, 94–99. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Mousa, S.M.A.; Sherief, M.A. Effect of Incorporation of Lanthanum and Cerium-Doped Hydroxyapatite on Acrylic Bone Cement Produced from Phosphogypsum Waste. Egypt. J. Chem. 2020, 63, 1823–1832. [Google Scholar] [CrossRef]
- Dutta, S.R.; Passi, D.; Singh, P.; Bhuibhar, A. Ceramic and Non-Ceramic Hydroxyapatite as a Bone Graft Material: A Brief Review. Ir. J. Med. Sci. 2015, 184, 101–106. [Google Scholar] [CrossRef]
- Hamdy, T.M. Dental Biomaterial Scaffolds in Tooth Tissue Engineering: A Review. Curr. Oral Health Reports 2023, 10, 14–21. [Google Scholar] [CrossRef]
- Chae, M.H.; Lee, Y.K.; Kim, K.N.; Lee, J.H.; Choi, B.J.; Choi, H.J.; Park, K.T. The Effect of Hydroxyapatite on Bonding Strength in Light Curing Glass Ionomer Dental Cement. Key Eng. Mater. 2006, 309, 881–884. [Google Scholar] [CrossRef]
- Razali, R.A.C.; Rahim, N.A.; Zainol, I.; Sharif, A.M. Preparation of Dental Composite Using Hydroxyapatite from Natural Sources and Silica. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Domingo, C.; Arcs, R.W.; Lpez-Macipe, A.; Osorio, R.; Rodrguez-Clemente, R.; Murtra, J.; Fanovich, M.A.; Toledano, M. Dental Composites Reinforced with Hydroxyapatite: Mechanical Behavior and Absorption/Elution Characteristics. J. Biomed. Mater. Res. 2001, 56, 297–305. [Google Scholar] [CrossRef]
- Ong, J.L.; Chan, D.C.N. Hydroxyapatite and Their Use as Coatings in Dental Implants: A Review. Crit. Rev. Biomed. Eng. 2000, 28, 667–707. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Naghib, S.M. 3D and 4D Printing Hydroxyapatite-Based Scaffolds for Bone Tissue Engineering and Regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of Hydroxyapatite for Biomedical Applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic Coatings in Implant Dentistry: A Quick Update. J. Funct. Biomater. 2023, 15, 15. [Google Scholar] [CrossRef]
- Carvalho, P.C.K.; Almeida, C.C.M.S.; Souza, R.O.A.; Tango, R.N. The Effect of a 10-MDP-Based Dentin Adhesive as Alternative for Bonding to Implant Abutment Materials. Materials 2022, 15, 5449. [Google Scholar] [CrossRef]
- Al-Shehri, E.Z.; Al-Zain, A.O.; Sabrah, A.H.; Al-Angari, S.S.; Al Dehailan, L.; Eckert, G.J.; Özcan, M.; Platt, J.A.; Bottino, M.C. Effects of Air-Abrasion Pressure on the Resin Bond Strength to Zirconia: A Combined Cyclic Loading and Thermocycling Aging Study. Restor. Dent. Endod. 2017, 42, 206–215. [Google Scholar] [CrossRef]
- Pekkan, G. Radiopacity of Dental Materials: An Overview. Avicenna J. Dent. Res. 2016, 8, 8. [Google Scholar] [CrossRef]
- ASTM D3359/D3359M-17; Standard Test Methods for Measuring Adhesion by Tape Test. American Society for Testing and Materials, ASTM International: West Conshohocken, PA, USA, 2017.
- Torabinejad, B.; Mohammadi-Rovshandeh, J.; Davachi, S.M.; Zamanian, A. Synthesis and Characterization of Nanocomposite Scaffolds Based on Triblock Copolymer of L-Lactide, ε-Caprolactone and Nano-Hydroxyapatite for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 199–210. [Google Scholar] [CrossRef]
- Zakręcki, A.; Cieślik, J.; Bazan, A.; Turek, P. Innovative Approaches to 3D Printing of PA12 Forearm Orthoses: A Comprehensive Analysis of Mechanical Properties and Production Efficiency. Materials 2024, 17, 663. [Google Scholar] [CrossRef]
- Pimentel de Oliveira, R.; de Paula, B.L.; Ribeiro, M.E.; Alves, E.; Costi, H.T.; Silva, C. Evaluation of the Bond Strength of Self-Etching Adhesive Systems Containing HEMA and 10-MDP Monomers: Bond Strength of Adhesives Containing HEMA and 10-MDP. Int. J. Dent. 2022, 2022, 5756649. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, T.M. Polymerization Shrinkage in Contemporary Resin-Based Dental Composites: A Review Article. Egypt. J. Chem. 2021, 64, 3087–3092. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A Novel Hydroxyapatite -Hardystonite Nanocomposite Ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and Characterization of Nano-Hydroxyapatite (n-HAP) Using the Wet Chemical Technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Bouropoulos, N.; Stampolakis, A.; Mouzakis, D.E. Dynamic Mechanical Properties of Calcium Alginate-Hydroxyapatite Nanocomposite Hydrogels. Sci. Adv. Mater. 2010, 2, 239–242. [Google Scholar] [CrossRef]
- Bandeira, L.C.; Ciuffi, K.J.; Calefi, P.S.; Nassar, E.J.; Silva, J.V.L.; Oliveira, M.; Maia, I.A.; Salvado, I.M.; Fernandes, M.H.V. Effect of Calcium Phosphate Coating on Polyamide Substrate for Biomaterial Applications. J. Braz. Chem. Soc. 2012, 23, 810–817. [Google Scholar] [CrossRef][Green Version]
- Alhotan, A.; Abdelraouf, R.M.; El-Korashy, S.A.; Labban, N.; Alotaibi, H.; Matinlinna, J.P.; Hamdy, T.M. Effect of Adding Silver-Doped Carbon Nanotube Fillers to Heat-Cured Acrylic Denture Base on Impact Strength, Microhardness, and Antimicrobial Activity: A Preliminary Study. Polymers 2023, 15, 2976. [Google Scholar] [CrossRef]
- Chen, C.-W.; Ranganathan, P.; Mutharani, B.; Shiu, J.-W.; Rwei, S.-P.; Chang, Y.-H.; Chiu, F.-C. Synthesis of High-Value Bio-Based Polyamide 12,36 Microcellular Foams with Excellent Dimensional Stability and Shape Recovery Properties. Polymers 2024, 16, 159. [Google Scholar] [CrossRef]
- Guo, B.; Xu, Z.; Luo, X.; Bai, J. A Detailed Evaluation of Surface, Thermal, and Flammable Properties of Polyamide 12/Glass Beads Composites Fabricated by Multi Jet Fusion. Virtual Phys. Prototyp. 2021, 16, S39–S52. [Google Scholar] [CrossRef]
- Lewandowski, G.; Rytwińska, E.; Milchert, E. Physical Properties and Application of Polyamide 12. Polimery/Polymers 2006. [Google Scholar] [CrossRef]
- Morano, C.; Alfano, M.; Pagnotta, L. Effect of Strain Rates and Heat Exposure on Polyamide (PA12) Processed via Selective Laser Sintering. Materials 2023, 16, 4654. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Vokhidova, N.R.; Ergashev, K.H.; Rashidova, S.S. Hydroxyapatite-Chitosan Bombyx Mori: Synthesis and Physicochemical Properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3357–3368. [Google Scholar] [CrossRef]
- Beaufils, S.; Rouillon, T.; Millet, P.; Le Bideau, J.; Weiss, P.; Chopart, J.-P.; Daltin, A.-L. Synthesis of Calcium-Deficient Hydroxyapatite Nanowires and Nanotubes Performed by Template-Assisted Electrodeposition. Mater. Sci. Eng. C 2019, 98, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical Development of Simulated Body Fluids Used in Biomedical Applications: A Review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Qian, M.; Zhang, H.; Chen, C.; Xie, H.; Tay, F.R. Chemical Affinity of 10-Methacryloyloxydecyl Dihydrogen Phosphate to Dental Zirconia: Effects of Molecular Structure and Solvents. Dent. Mater. 2017, 33, e415–e427. [Google Scholar] [CrossRef]
- Chen, C.; Lee, I.S.; Zhang, S.M.; Yang, H.C. Biomimetic Apatite Formation on Calcium Phosphate-Coated Titanium in Dulbecco’s Phosphate-Buffered Saline Solution Containing CaCl2 with and without Fibronectin. Acta Biomater. 2010, 6, 2274–2281. [Google Scholar] [CrossRef]
- Cieplik, F.; Rupp, C.M.; Hirsch, S.; Muehler, D.; Enax, J.; Meyer, F.; Hiller, K.-A.; Buchalla, W. Ca2+ Release and Buffering Effects of Synthetic Hydroxyapatite Following Bacterial Acid Challenge. BMC Oral Health 2020, 20, 85. [Google Scholar] [CrossRef]
- Shokry, M.; Al-Zordk, W.E.G.; Ghazy, M.H. Influence of Different Primer/Resin Cement Systems on Retention of Monolithic Zirconia Crowns. Mansoura J. Dent. 2021, 8, 53–57. [Google Scholar] [CrossRef]
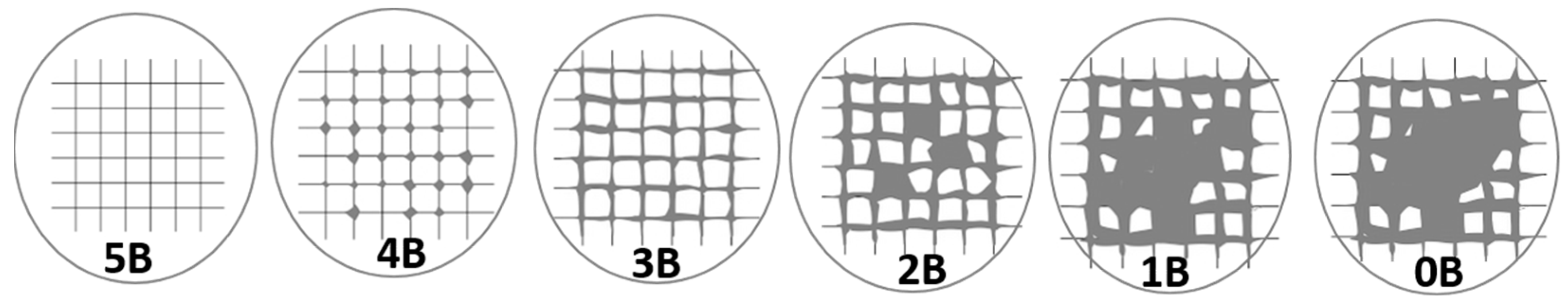
| Score | Description and Area% removed |
| 5B | The whole coat is attached, and the margins of the incisions are perfectly smooth (Area removed is zero). |
| 4B | Minor coating flakes detached at lines of intersections (Area removed <5%). |
| 3B | Little coating flakes come off at cut intersections and around edges, (5 to 15% of the lattice). |
| 2B | Parts of the squares and their edges have chipped off from the coat (15–35%). |
| 1B | Whole squares have come away from the coat, and enormous ribbons with cut edges have flaked (35–65%). |
| 0B | Detachment and flaking are worse than in Grade 1 (>65%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhotan, A.; Alhijji, S.; Abdalbary, S.A.; Bayoumi, R.E.; Matinlinna, J.P.; Hamdy, T.M.; Abdelraouf, R.M. An Advanced Surface Treatment Technique for Coating Three-Dimensional-Printed Polyamide 12 by Hydroxyapatite. Coatings 2024, 14, 1181. https://doi.org/10.3390/coatings14091181
Alhotan A, Alhijji S, Abdalbary SA, Bayoumi RE, Matinlinna JP, Hamdy TM, Abdelraouf RM. An Advanced Surface Treatment Technique for Coating Three-Dimensional-Printed Polyamide 12 by Hydroxyapatite. Coatings. 2024; 14(9):1181. https://doi.org/10.3390/coatings14091181
Chicago/Turabian StyleAlhotan, Abdulaziz, Saleh Alhijji, Sahar Ahmed Abdalbary, Rania E. Bayoumi, Jukka P. Matinlinna, Tamer M. Hamdy, and Rasha M. Abdelraouf. 2024. "An Advanced Surface Treatment Technique for Coating Three-Dimensional-Printed Polyamide 12 by Hydroxyapatite" Coatings 14, no. 9: 1181. https://doi.org/10.3390/coatings14091181
APA StyleAlhotan, A., Alhijji, S., Abdalbary, S. A., Bayoumi, R. E., Matinlinna, J. P., Hamdy, T. M., & Abdelraouf, R. M. (2024). An Advanced Surface Treatment Technique for Coating Three-Dimensional-Printed Polyamide 12 by Hydroxyapatite. Coatings, 14(9), 1181. https://doi.org/10.3390/coatings14091181







