Changes in 25-(OH) Vitamin D Levels during the SARS-CoV-2 Outbreak: Lockdown-Related Effects and First-to-Second Wave Difference—An Observational Study from Northern Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. UV and Ozone Data
2.2. Clinical Data
2.3. Statistical Analysis
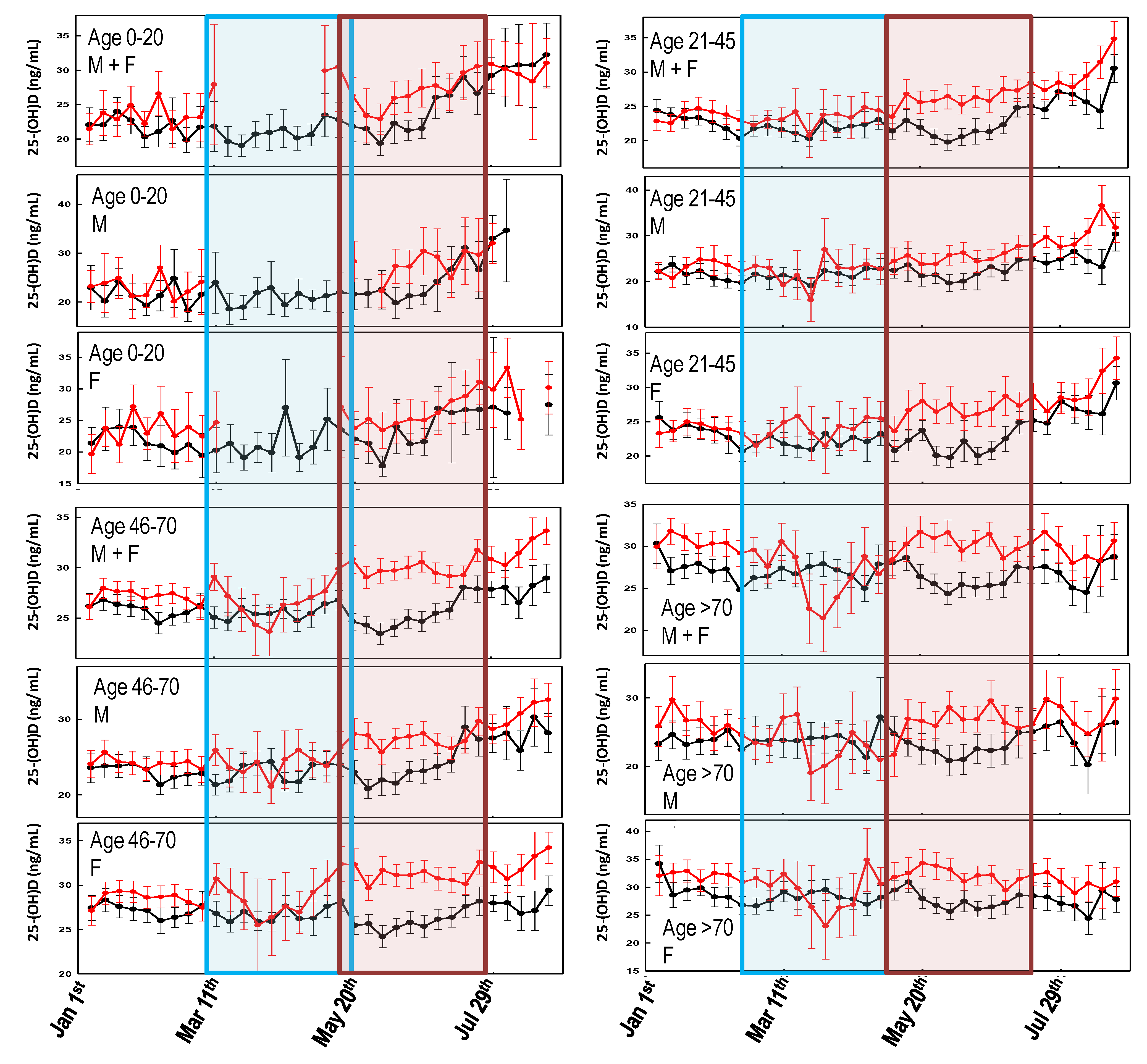
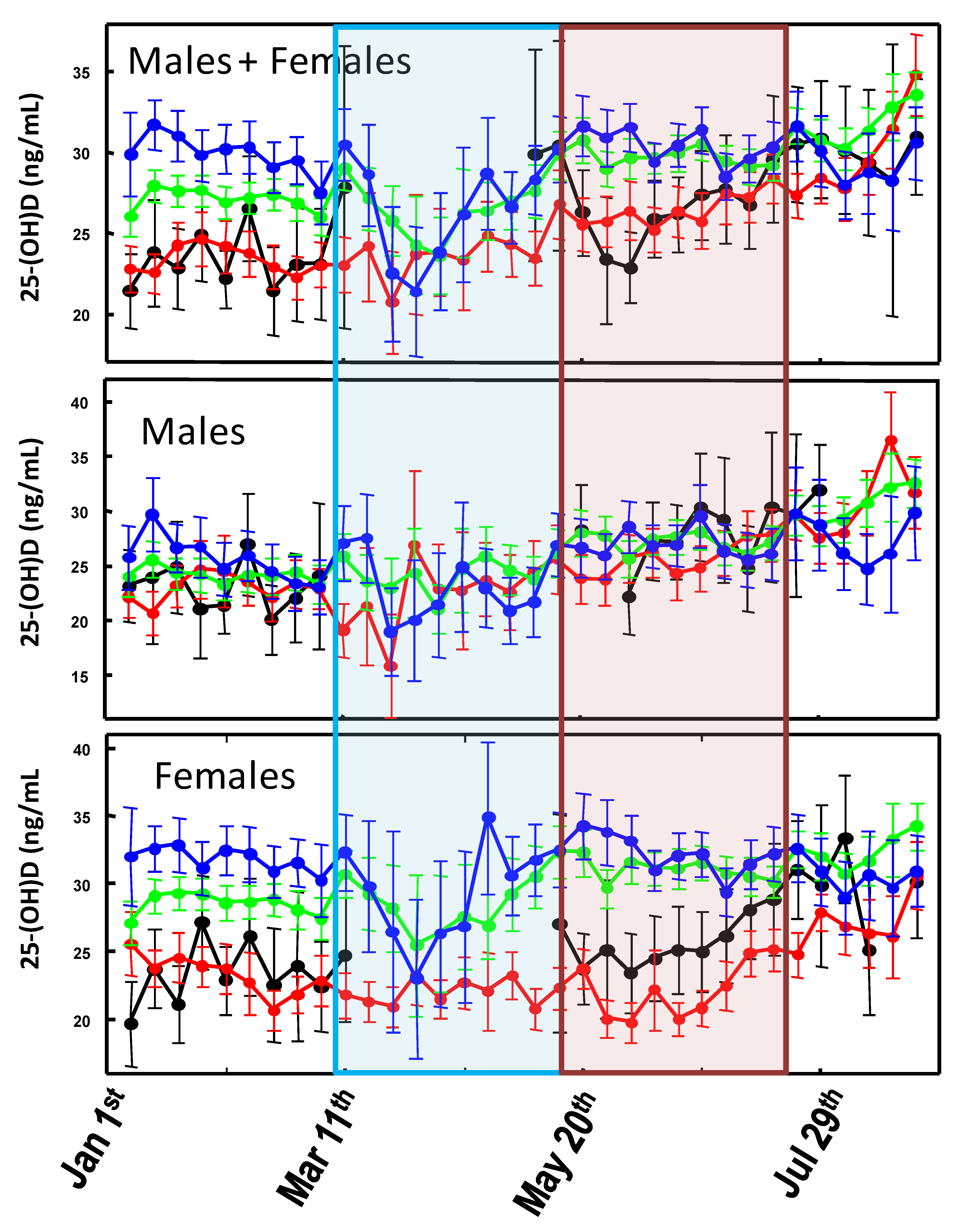
3. Results
3.1. Lockdown Effect
3.2. First Wave vs. Second Wave
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.J.; et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Locatelli, M.; Briguglio, M.; Lombardi, G. Is there a link between vitamin D status, SARS-CoV-2 infection risk and COVID-19 severity? Cell Biochem. Funct. 2021, 39, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Gregori, S.; Casorati, M.; Amuchastegui, S.; Smiroldo, S.; Davalli, A.M.; Adorini, L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001, 167, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am. J. Physicol. Gastrointest. Lliver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef]
- Colombini, A.; Perego, S.; Ardoino, I.; Marasco, E.; Lombardi, G.; Fiorilli, A.; Biganzoli, E.; Tettamanti, G.; Ferraretto, A. Evaluation of a possible direct effect by casein phosphopeptides on paracellular and vitamin D controlled transcellular calcium transport mechanisms in intestinal human HT-29 and Caco2 cell lines. Food Funct. 2013, 4, 1195–1203. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Liu, T.J.; Fu, J.H.; Xu, W.; Wu, L.L.; Hou, A.N.; Xue, X.D. Vitamin D/VDR signaling attenuates lipopolysaccharideinduced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef]
- Subramanian, K.; Bergman, P.; Henriques-Normark, B. Vitamin D Promotes Pneumococcal Killing and Modulates Inflammatory Responses in Primary Human Neutrophils. J. Innate Immun. 2017, 9, 375–386. [Google Scholar] [CrossRef]
- Gough, M.E.; Graviss, E.A.; May, E.E. The dynamic immunomodulatory effects of vitamin D3 during Mycobacterium infection. Innate Immun. 2017, 23, 506–523. [Google Scholar] [CrossRef]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Yang, C.S.; Jin, H.S.; Kim, K.K.; Lee, Z.W.; Lee, S.H.; Kim, J.M.; Jo, E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bouillon, R.; Ebeling, P.R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C.T.; Bilezikian, J.P. Controversies in Vitamin D: Summary Statement From an International Conference. J. Clin. Endocrinol. Metab. 2019, 104, 234–240. [Google Scholar] [CrossRef]
- Ferrari, D.; Locatelli, M. No significant association between vitamin D and COVID-19. A retrospective study from a northern Italian hospital. Int. J. Vitamin Nutr. Res. 2020. [Google Scholar] [CrossRef]
- Jones, G. Interpreting vitamin D assay results: Proceed with caution. Clin. J. Am. Soc. Nephrol. 2015, 10, 331–334. [Google Scholar] [CrossRef]
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Med. 2017, 27, 030501. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Nowson, C.A.; McGrath, J.J.; Ebeling, P.R.; Haikerwal, A.; Daly, R.M.; Sanders, K.M.; Seibel, M.J.; Mason, R.S. Vitamin D and health in adults in Australia and New Zealand: A position statement. Med. J. Aust. 2012, 196, 686–687. [Google Scholar] [CrossRef]
- Ferrari, D.; Lombardi, G.; Strollo, M.; Pontillo, M.; Motta, A.; Locatelli, M. Association between solar ultraviolet doses and vitamin D clinical routine data in European mid-latitude population between 2006 and 2018. Photochem. Photobiol. Sci. 2019, 18, 2696–2706. [Google Scholar] [CrossRef]
- Valks, P.J.M.; Veefkind, J.P.; Eskes, H.J.; Brinksma, E.J.; de Haan, J.F. Retrieval and validation of ozone columns derived from measurements of SCIAMACHY on Envisat. Atmos. Chem. Phys. Discuss. 2010, 5, 4429–4475. [Google Scholar] [CrossRef]
- Ferrari, D.; Manca, M.; Premaschi, S.; Banfi, G.; Locatelli, M. Toxicological investigation in blood samples from suspected impaired driving cases in the Milan area: Possible loss of evidence due to late blood sampling. Forensic Sci. Int. 2018, 288, 211–217. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Whittemore, P.B. COVID-19 fatalities, latitude, sunlight, and vitamin D. Am. J. Infect. Control 2020, 48, 1042–1044. [Google Scholar] [CrossRef]
- Rhodes, J.; Dunstan, F.; Laird, E.; Subramanian, S.; Kenny, R.A. COVID-19 mortality increases with northerly latitude after adjustment for age suggesting a link with ultraviolet and vitamin D. BMJ Nutr. Prev. Health 2020, 3, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Avoidance of vitamin D deficiency to slow the COVID-19 pandemic. BMJ Nutr. Prev. Health 2020, 3, 67–73. [Google Scholar] [CrossRef]
- Moozhipurath, R.K.; Kraft, L.; Skiera, B. Evidence of protective role of Ultraviolet-B (UVB) radiation in reducing COVID-19 deaths. Sci. Rep. 2020, 10, 17705. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liu, M.; Ren, B.; Wu, Z.; Yu, X.; Peng, C.; Tian, J. Sunlight ultraviolet radiation dose is negatively correlated with the percent positive of SARS-CoV-2 and four other common human coronaviruses in the U.S. Sci. Total Environ. 2021, 751, 141816. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.U.; Stiboy, E.; Hassan, M.Z.; Chan, M.; Islam, M.S.; Haider, N.; Jaffe, A.; Homaira, N. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine 2021, 39, 667–677. [Google Scholar] [CrossRef]
- Yin, H.; Sun, T.; Yao, L.; Jiao, Y.; Ma, L.; Lin, L.; Graff, J.C.; Aleya, L.; Postlethwaite, A.; Gu, W.; et al. Association between population density and infection rate suggests the importance of social distancing and travel restriction in reducing the COVID-19 pandemic. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef]

| SARS-CoV-2 + | SARS-CoV-2 − | ||||||
|---|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Total | p-Value | |
| N | 83 (64.8%) | 45 (35.2%) | 128 (100%) | 107 (48.9%) | 112 (51.1%) | 219 (100%) | <0.05 * |
| Age (years) | 62.7 ± 14.2 | 69.3 ± 15.6 | 65.0 ± 15.0 | 62.8 ± 19.5 | 54.3 ± 20.1 | 58.7 ± 20.2 | <0.05 |
| 25-(OH)D (ng/mL) | 19.7 ± 13.5 | 25.8 ± 19.6 | 21.8 ± 16.1 | 23.1 ± 15.0 | 22.6 ± 13.1 | 22.8 ± 14.0 | 0.39 |
| SARS-CoV-2 + | |||
| Males | Females | Total | |
| N | 101 (53.7%) | 87 (46.3%) | 188 (100%) |
| Age (years) | 58.9 ± 21.6 | 61.4 ± 18.8 | 60.0 ± 20.3 |
| 25-(OH)D (ng/mL) | 23.7 ± 11.5 ### | 26.7 ± 14.7 | 25.1 ± 13.2 |
| SARS-CoV-2 − | |||
| Males | Females | Total | |
| N | 547 (46.1%) | 638 (53.9%) | 1185 (100%) |
| Age (years) | 55.5 ± 23.9 | 54.0 ± 22.3 | 54.7 ± 23.0 |
| 25-(OH)D (ng/mL) | 24.9 ± 12.9 ### | 28.2 ± 13.5 | 26.7 ± 13.3 |
| Whole population | |||
| Males | Females | Total | |
| N | 9164 (33.1%) | 18,520 (66.9%) | 27,684 (100%) |
| Age (years) | 56.6 ± 19.6 | 59.0 ± 18.3 | 58.2 ± 18.8 |
| 25-(OH)D (ng/mL) | 29.0 ± 12.0 | 31.7 ± 13.5 | 30.8 ± 13.1 |
| <46 | 46–65 | >65 | ||
|---|---|---|---|---|
| Females | ||||
| 25-(OH)D (ng/mL) | SARS-CoV-2 + | * (13) | 30.4 ± 14.8 (32) | 22.2 ± 13.5 (42) § ### |
| SARS-CoV-2 – | 27.7 ± 11.3 (200) | 29.5 ± 14.2 (201) ## | 27.6 ± 14.4 (237) ### | |
| Total | 29.2 ± 11.9 (3728) | 32.2 ± 13.3 (7490) | 32.7 ± 15.0 (7300) | |
| Males | ||||
| 25-(OH)D (ng/mL) | SARS-CoV-2 + | 28.5 ± 10.6 (21) | 23.1 ± 12.8 (34) ## | 22.0 ± 10.5 (46) ## |
| SARS-CoV-2 − | 26.5 ± 10.3 (161) ## | 26.2 ± 13.0 (147) ### | 23.1 ± 14.2 (239) ### | |
| Total | 29.5 ± 11.6 (2335) | 29.4 ± 11.4 (3572) | 28.3 ± 13.8 (3257) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, D.; Locatelli, M.; Faraldi, M.; Lombardi, G. Changes in 25-(OH) Vitamin D Levels during the SARS-CoV-2 Outbreak: Lockdown-Related Effects and First-to-Second Wave Difference—An Observational Study from Northern Italy. Biology 2021, 10, 237. https://doi.org/10.3390/biology10030237
Ferrari D, Locatelli M, Faraldi M, Lombardi G. Changes in 25-(OH) Vitamin D Levels during the SARS-CoV-2 Outbreak: Lockdown-Related Effects and First-to-Second Wave Difference—An Observational Study from Northern Italy. Biology. 2021; 10(3):237. https://doi.org/10.3390/biology10030237
Chicago/Turabian StyleFerrari, Davide, Massimo Locatelli, Martina Faraldi, and Giovanni Lombardi. 2021. "Changes in 25-(OH) Vitamin D Levels during the SARS-CoV-2 Outbreak: Lockdown-Related Effects and First-to-Second Wave Difference—An Observational Study from Northern Italy" Biology 10, no. 3: 237. https://doi.org/10.3390/biology10030237
APA StyleFerrari, D., Locatelli, M., Faraldi, M., & Lombardi, G. (2021). Changes in 25-(OH) Vitamin D Levels during the SARS-CoV-2 Outbreak: Lockdown-Related Effects and First-to-Second Wave Difference—An Observational Study from Northern Italy. Biology, 10(3), 237. https://doi.org/10.3390/biology10030237







