Myco-Synthesis of Silver Nanoparticles and Their Bioactive Role against Pathogenic Microbes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of AgNPs from F. oxysporum
2.2. Characterization of Nanoparticles
2.2.1. Ultraviolet–Visible Spectroscopy (UV–Vis Spectroscopy)
2.2.2. Differential Light Scattering (DLS)
2.2.3. Transmission Electron Microscopy (TEM) Analysis
2.2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3. Antibacterial Activity
2.4. Antifungal Activity
2.5. Preparation of Cells for SEM Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Fungal-Mediated Nanoparticles
3.2. Spectrophotometric and Differential Light Scattering Analyses of Silver Nanoparticles
3.3. FTIR and TEM Analyses
3.4. Antibacterial Property
3.5. Antifungal Activity
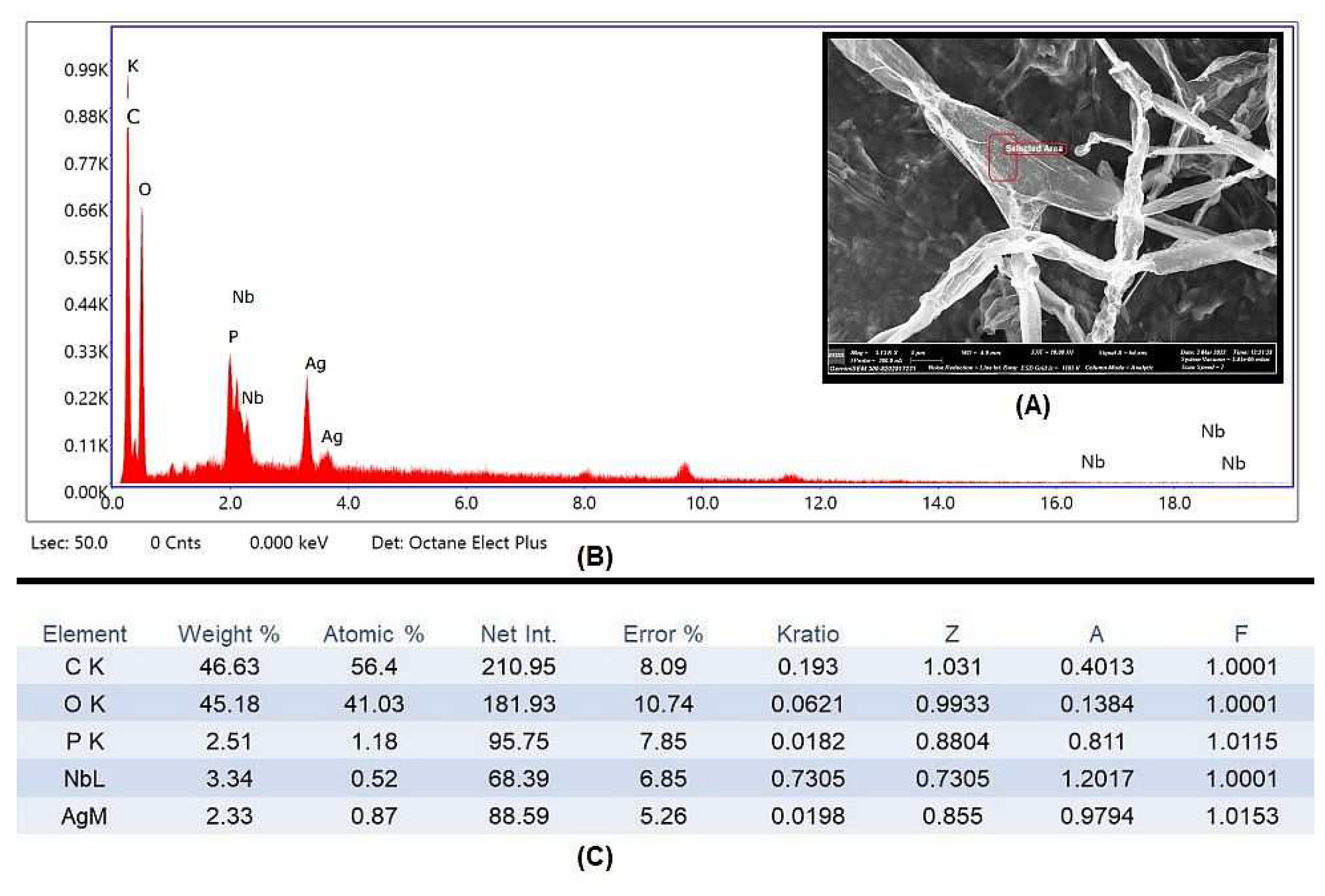
3.6. Scanning Electron Microscopy (SEM) and Energy-Dispersive Spectra (EDX)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljeldah, M.M. Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Dugassa, J.; Shukuri, N. Review on Antibiotic Resistance and Its Mechanism of Development. J. Health Med. Nurs. 2017, 1, 1–17. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Fatima, F. In Vitro Antimicrobicidal and Cytotoxicity Efficacy of Gold Nanoparticles Synthesized from Alternaria Brassicae (KF934409). SOJPPS 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal Nanoparticles against Multi-Drug-Resistance Bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Ercan, U.K.; Bakay, E.; Topaloğlu, N.; Rosenholm, J.M. Evolving Technologies and Strategies for Combating Antibacterial Resistance in the Advent of the Postantibiotic Era. Adv. Funct. Mater. 2020, 30, 1908783. [Google Scholar] [CrossRef]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Bano, N.; Iqbal, D.; Al Othaim, A.; Kamal, M.; Albadrani, H.M.; Algehainy, N.A.; Alyenbaawi, H.; Alghofaili, F.; Amir, M.; Roohi. Antibacterial Efficacy of Synthesized Silver Nanoparticles of Microbacterium Proteolyticum LA2(R) and Streptomyces Rochei LA2(O) against Biofilm Forming Meningitis Causing Microbes. Sci. Rep. 2023, 13, 4150. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Fatima, F.; Pathak, N.; Verma, S.R.; Bajpai, P. Toxicity and Immunomodulatory Efficacy of Biosynthesized Silver Myconanosomes on Pathogenic Microbes and Macrophage Cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1637–1645. [Google Scholar] [CrossRef]
- Fatima, F.; Siddiqui, S.; Khan, W.A. Nanoparticles as Novel Emerging Therapeutic Antibacterial Agents in the Antibiotics Resistant Era. Biol. Trace Elem. Res. 2021, 199, 2552–2564. [Google Scholar] [CrossRef]
- Ahmad, N.; Bhatnagar, S.; Saxena, R.; Iqbal, D.; Ghosh, A.K.; Dutta, R. Biosynthesis and Characterization of Gold Nanoparticles: Kinetics, In Vitro and In Vivo Study. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 553–564. [Google Scholar] [CrossRef]
- Bala, M.; Kumar Bansal, S.; Fatima, F. Nanotechnology: A Boon for Agriculture. Mater. Today Proc. 2023, 73, 267–270. [Google Scholar] [CrossRef]
- Khatoon, A.; Khan, F.; Ahmad, N.; Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Al-Qahtani, M.H.; Abuzenadah, A.M.; Tabrez, S.; Ahmed, A.B.F.; et al. Silver Nanoparticles from Leaf Extract of Mentha Piperita: Eco-Friendly Synthesis and Effect on Acetylcholinesterase Activity. Life Sci. 2018, 209, 430–434. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological Agents for Synthesis of Nanoparticles and Their Applications. J. King Saud Univ.—Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Ahmad, J. Evaluation of Antioxidant and Antimicrobial Activity of Ficus Carica Leaves: An In Vitro Approach. J. Plant Pathol. Microb. 2012, 4, 1. [Google Scholar] [CrossRef]
- Akhter, F.; Hashim, A.; Khan, M.S.; Ahmad, S.; Iqbal, D.; Srivastava, A.K.; Siddiqui, M.H. Antioxidant, α-Amylase Inhibitory and Oxidative DNA Damage Protective Property of Boerhaavia Diffusa (Linn.) Root. S. Afr. J. Bot. 2013, 88, 265–272. [Google Scholar] [CrossRef]
- Akhter, F.; Alvi, S.S.; Ahmad, P.; Iqbal, D.; Alshehri, B.M.; Khan, M.S. Therapeutic Efficacy of Boerhaavia Diffusa (Linn.) Root Methanolic Extract in Attenuating Streptozotocin-Induced Diabetes, Diabetes-Linked Hyperlipidemia and Oxidative-Stress in Rats. Biomed. Res. Ther. 2019, 6, 3293–3306. [Google Scholar] [CrossRef]
- Alsagaby, S.A.; Iqbal, D.; Ahmad, I.; Patel, H.; Mir, S.A.; Madkhali, Y.A.; Oyouni, A.A.A.; Hawsawi, Y.M.; Alhumaydhi, F.A.; Alshehri, B.; et al. In Silico Investigations Identified Butyl Xanalterate to Competently Target CK2α (CSNK2A1) for Therapy of Chronic Lymphocytic Leukemia. Sci. Rep. 2022, 12, 17648. [Google Scholar] [CrossRef]
- Alvi, S.; Ahmad, P.; Ishrat, M.; Iqbal, D.; Khan, S. Secondary Metabolites from Rosemary (Rosmarinus officinalis L.): Structure, Biochemistry and Therapeutic Implications Against Neurodegenerative Diseases. In Natural Bio-Active Compounds; Springer: Singapore, 2019; pp. 1–24. ISBN 9789811372049. [Google Scholar]
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Das, D.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; et al. Mechanistic Role of HPV-Associated Early Proteins in Cervical Cancer: Molecular Pathways and Targeted Therapeutic Strategies. Crit. Rev. Oncol./Hematol. 2022, 174, 103675. [Google Scholar] [CrossRef]
- Bijani, S.; Iqbal, D.; Mirza, S.; Jain, V.; Jahan, S.; Alsaweed, M.; Madkhali, Y.; Alsagaby, S.A.; Banawas, S.; Algarni, A.; et al. Green Synthesis and Anticancer Potential of 1,4-Dihydropyridines-Based Triazole Derivatives: In Silico and In Vitro Study. Life 2022, 12, 519. [Google Scholar] [CrossRef]
- Iqbal, D.; Pawar, R.K.; Sharma, R.K. Physico-Chemical Standardization of Butea Monosperma (Lam). Int. J. Pharm. Qual. Assur. 2010, 2, 49–51. [Google Scholar]
- Iqbal, D.; Khan, M.S.; Khan, M.S.; Ahmad, S.; Srivastava, A.K. An in Vitro and Molecular Informatics Study to Evaluate the Antioxidative and β-Hydroxy-β-Methylglutaryl-CoA Reductase Inhibitory Property of Ficus Virens Ait. Phytother. Res. 2014, 28, 899–908. [Google Scholar] [CrossRef]
- Iqbal, D.; Khan, M.S.; Khan, M.S.; Ahmad, S.; Hussain, M.S.; Ali, M. Bioactivity Guided Fractionation and Hypolipidemic Property of a Novel HMG-CoA Reductase Inhibitor from Ficus Virens Ait. Lipids Health Dis. 2015, 14, 15. [Google Scholar] [CrossRef]
- Iqbal, D.; Khan, M.S.; Khan, A.; Ahmad, S. Extenuating the Role of Ficus Virens Ait and Its Novel Bioactive Compound on Antioxidant Defense System and Oxidative Damage in Cigarette Smoke Exposed Rats. Biomed. Res. Ther. 2016, 3, 723–732. [Google Scholar] [CrossRef]
- Iqbal, D.; Khan, A.; Ansari, I.A.; Khan, M.S. Investigating The Role of Novel Bioactive Compound from Ficus Virens Ait on Cigarette Smoke Induced Oxidative Stress and Hyperlipidemia in Rats. Iran. J. Pharm. Res. 2017, 16, 1089–1103. [Google Scholar]
- Jahan, S.; Ansari, U.A.; Siddiqui, A.J.; Iqbal, D.; Khan, J.; Banawas, S.; Alshehri, B.; Alshahrani, M.M.; Alsagaby, S.A.; Redhu, N.S.; et al. Nobiletin Ameliorates Cellular Damage and Stress Response and Restores Neuronal Identity Altered by Sodium Arsenate Exposure in Human IPSCs-Derived HNPCs. Pharmaceuticals 2022, 15, 593. [Google Scholar] [CrossRef]
- Jana, A.; Bhattacharjee, A.; Das, S.S.; Srivastava, A.; Choudhury, A.; Bhattacharjee, R.; De, S.; Perveen, A.; Iqbal, D.; Gupta, P.K.; et al. Molecular Insights into Therapeutic Potentials of Hybrid Compounds Targeting Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 3512–3528. [Google Scholar] [CrossRef]
- Khushtar, M.; Siddiqui, H.H.; Dixit, R.K.; Khan, M.S.; Iqbal, D.; Rahman, M.A. Amelioration of Gastric Ulcers Using a Hydro-Alcoholic Extract of Triphala in Indomethacin-Induced Wistar Rats. Eur. J. Integr. Med. 2016, 8, 546–551. [Google Scholar] [CrossRef]
- Alhoqail, W.A.; Alothaim, A.S.; Suhail, M.; Iqbal, D.; Kamal, M.; Asmari, M.M.; Jamal, A. Husk-like Zinc Oxide Nanoparticles Induce Apoptosis through ROS Generation in Epidermoid Carcinoma Cells: Effect of Incubation Period on Sol-Gel Synthesis and Anti-Cancerous Properties. Biomedicines 2023, 11, 320. [Google Scholar] [CrossRef]
- Fatima, F.; Bajpai, P.; Pathak, N.; Singh, S.; Priya, S.; Verma, S.R. Antimicrobial and Immunomodulatory Efficacy of Extracellularly Synthesized Silver and Gold Nanoparticles by a Novel Phosphate Solubilizing Fungus Bipolaris Tetramera. BMC Microbiol. 2015, 15, 52. [Google Scholar] [CrossRef]
- Iqbal, D.; Dukhyil, A.B.; Khan, M.S. Geno-Protective, Free Radical Scavenging and Antimicrobial Potential of Hyptis Suaveolens Methanolic Fraction: An In-Vitro Study. J. Pharm. Res. Int. 2021, 33, 46–57. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Fatima, F.; Verma, S.R.; Pathak, N.; Bajpai, P. Extracellular Mycosynthesis of Silver Nanoparticles and Their Microbicidal Activity. J Glob. Antimicrob. Resist. 2016, 7, 88–92. [Google Scholar] [CrossRef]
- Fatima, F.; Wahid, I. Eco-Friendly Synthesis of Silver and Copper Nanoparticles by Shizophyllum Commune Fungus and Its Biomedical Applications. Int. J. Environ. Sci. Technol. 2022, 19, 7915–7926. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Fatima, F.; Pathak, N.; Srivastava, D.; Verma, S.R. Molecular Detection and Exploration of Diversity Among Fungal Consortium Involved in Phosphate Solubilization. Geomicrobiol. J. 2021, 38, 29–35. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of Silver Nanoparticles by Green Synthesis Method Using Pedalium Murex Leaf Extract and Their Antibacterial Activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Metuku, R.P.; Pabba, S.; Burra, S.; Hima Bindu N, S.V.S.S.S.L.; Gudikandula, K.; Singara Charya, M.A. Biosynthesis of Silver Nanoparticles from Schizophyllum Radiatum HE 863742.1: Their Characterization and Antimicrobial Activity. 3 Biotech 2014, 4, 227–234. [Google Scholar] [CrossRef]
- Cohen Hyams, T.; Mam, K.; Killingsworth, M.C. Scanning Electron Microscopy as a New Tool for Diagnostic Pathology and Cell Biology. Micron 2020, 130, 102797. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A Comprehensive Review on Green Nanomaterials Using Biological Systems: Recent Perception and Their Future Applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid Synthesis of Silver Nanoparticles from Fusarium Oxysporum by Optimizing Physicocultural Conditions. Sci. World J. 2013, 2013, e796018. [Google Scholar] [CrossRef]
- Daniel, S.C.G.K.; Banu, B.N.; Harshiny, M.; Nehru, K.; Ganesh, P.S.; Kumaran, S.; Sivakumar, M. Ipomea Carnea-Based Silver Nanoparticle Synthesis for Antibacterial Activity against Selected Human Pathogens. J. Exp. Nanosci. 2014, 9, 197–209. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of Size Controlled Silver Nanoparticles by Fusarium Oxysporum, Their Antibacterial and Antitumor Activities. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Abdel-Galil, E.; Aziz, O.; Zhran, M.; Amin, M. Characterization and Sorption Behavior of Some Toxic Metal Ions on Fusarium Oxysporum as Biomass Adsorbent. Desalination Water Treat. 2018, 133, 134–145. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Hashem, K.A.; Authman, S.H.; Hameed, L. In Vivo Antibacterial Activity of Alkaline Phosphatase Isolates from Escherichia Coli Isolated from Diarrhea Patients against Pseudomonas Aeruginosa. Pharma Innov. J. 2016, 5, 32–36. [Google Scholar]
- Fatima, F.; Ahmad, M.M.; Verma, S.; Pathak, N. Relevance of Phosphate Solubilizing Microbes in Sustainable Crop Production: A Review. Int. J. Environ. Sci. Technol. 2021, 19, 9283–9296. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Oliveira Junior, A.G.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal Activity of Silver Nanoparticles and Simvastatin against Toxigenic Species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of Silver Nanoparticles on Candida Albicans Biofilms: An Ultrastructural Study. J. Nanobiotechnol. 2015, 13, 91. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Fu, P. Fungus-(Alternaria Sp.) Mediated Silver Nanoparticles Synthesis, Characterization, and Screening of Antifungal Activity against Some Phytopathogens. J. Nanotechnol. 2020, 2020, e8828878. [Google Scholar] [CrossRef]
- Kasithevar, M.; Saravanan, M.; Prakash, P.; Kumar, H.; Ovais, M.; Barabadi, H.; Shinwari, Z.K. Green Synthesis of Silver Nanoparticles Using Alysicarpus Monilifer Leaf Extract and Its Antibacterial Activity against MRSA and CoNS Isolates in HIV Patients. J. Interdiscip. Nanomed. 2017, 2, 131–141. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Hadi, A.; Iqbal, D.; Alharbi, R.; Jahan, S.; Darwish, O.; Alshehri, B.; Banawas, S.; Palanisamy, M.; Ismail, A.; Aldosari, S.; et al. Myco-Synthesis of Silver Nanoparticles and Their Bioactive Role against Pathogenic Microbes. Biology 2023, 12, 661. https://doi.org/10.3390/biology12050661
Abdel-Hadi A, Iqbal D, Alharbi R, Jahan S, Darwish O, Alshehri B, Banawas S, Palanisamy M, Ismail A, Aldosari S, et al. Myco-Synthesis of Silver Nanoparticles and Their Bioactive Role against Pathogenic Microbes. Biology. 2023; 12(5):661. https://doi.org/10.3390/biology12050661
Chicago/Turabian StyleAbdel-Hadi, Ahmed, Danish Iqbal, Raed Alharbi, Sadaf Jahan, Omar Darwish, Bader Alshehri, Saeed Banawas, Manikanadan Palanisamy, Ahmed Ismail, Sahar Aldosari, and et al. 2023. "Myco-Synthesis of Silver Nanoparticles and Their Bioactive Role against Pathogenic Microbes" Biology 12, no. 5: 661. https://doi.org/10.3390/biology12050661
APA StyleAbdel-Hadi, A., Iqbal, D., Alharbi, R., Jahan, S., Darwish, O., Alshehri, B., Banawas, S., Palanisamy, M., Ismail, A., Aldosari, S., Alsaweed, M., Madkhali, Y., Kamal, M., & Fatima, F. (2023). Myco-Synthesis of Silver Nanoparticles and Their Bioactive Role against Pathogenic Microbes. Biology, 12(5), 661. https://doi.org/10.3390/biology12050661









