Unpredictable Repeated Stress in Rainbow Trout (Oncorhynchus mykiss) Shifted the Immune Response against a Fish Parasite
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Method
2.1. Fish
2.2. Parasite
2.3. Unpredictable Repeated Stress, Challenge, and Sampling
2.4. Water Environmental DNA (eDNA) I. multifiliis Standard Curve
2.5. Water eDNA Sampling and Purification
2.6. Fish Samples RNA Purification
2.7. Fish Samples cDNA Synthesis
2.8. qPCR
2.8.1. Fish Samples
2.8.2. Water Sample
2.9. Statistics
2.9.1. Fish Mortality
2.9.2. Parasite Burden and Infection Severity
2.9.3. Fish Gene Expression
2.9.4. Association between Parasite Burden, Infection Severity and Gene Expression
2.10. Ethical Statement
3. Results
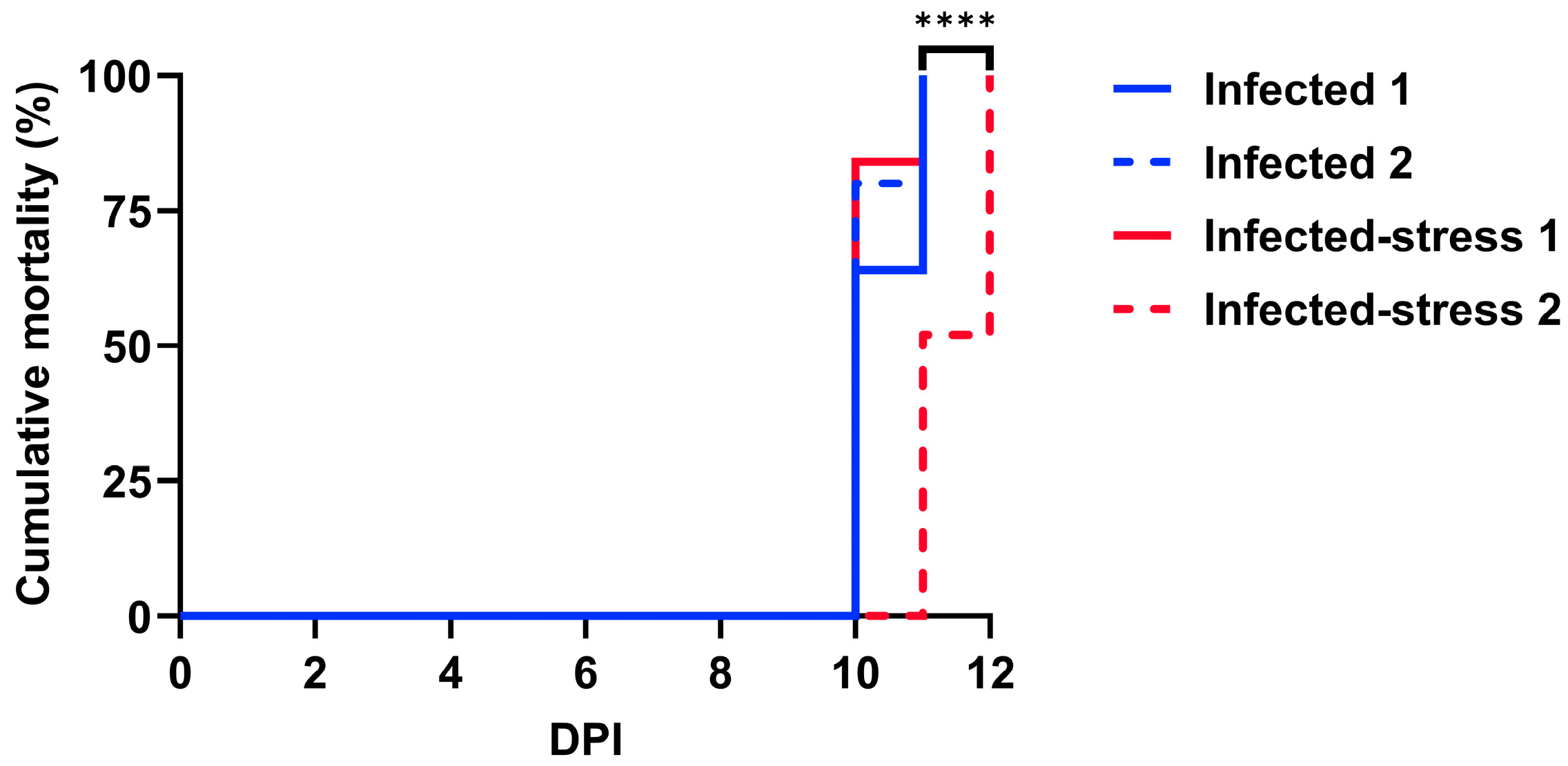
3.1. Fish Cumulative Mortality
3.2. Parasite Count, Burden and eDNA Levels
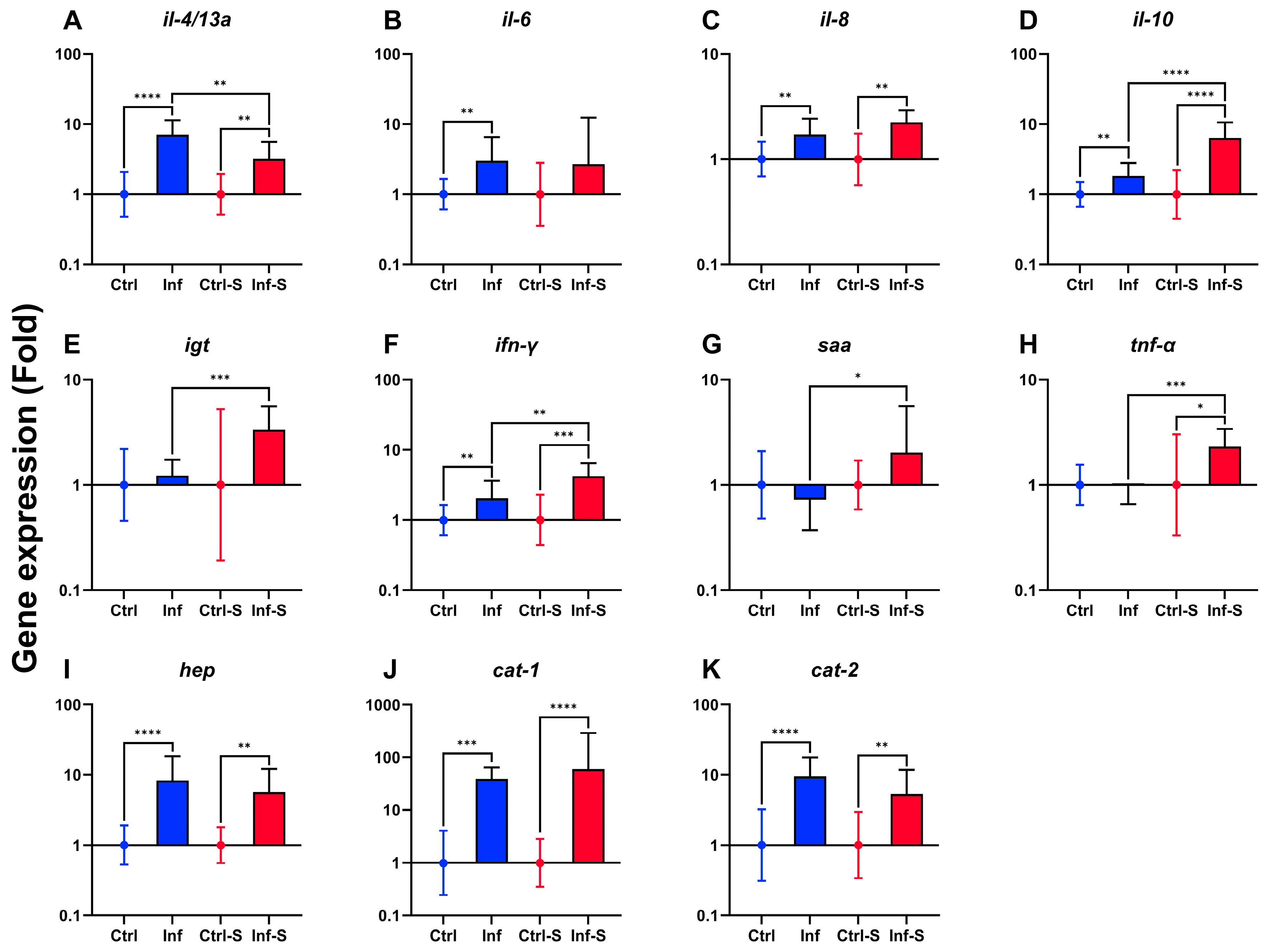
3.3. Immune Gene Expression in Gill
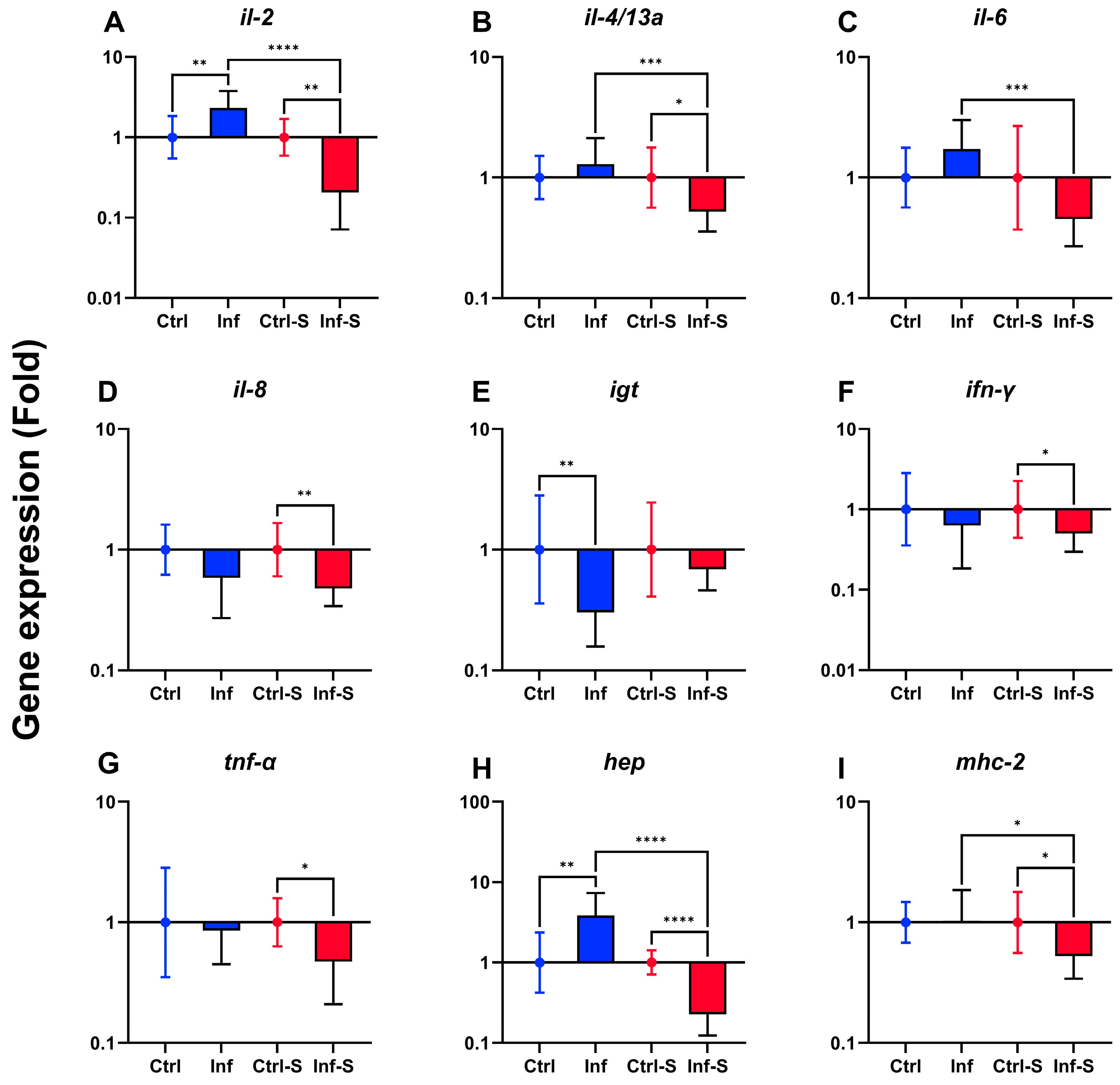
3.4. Immune Gene Expression in Spleen
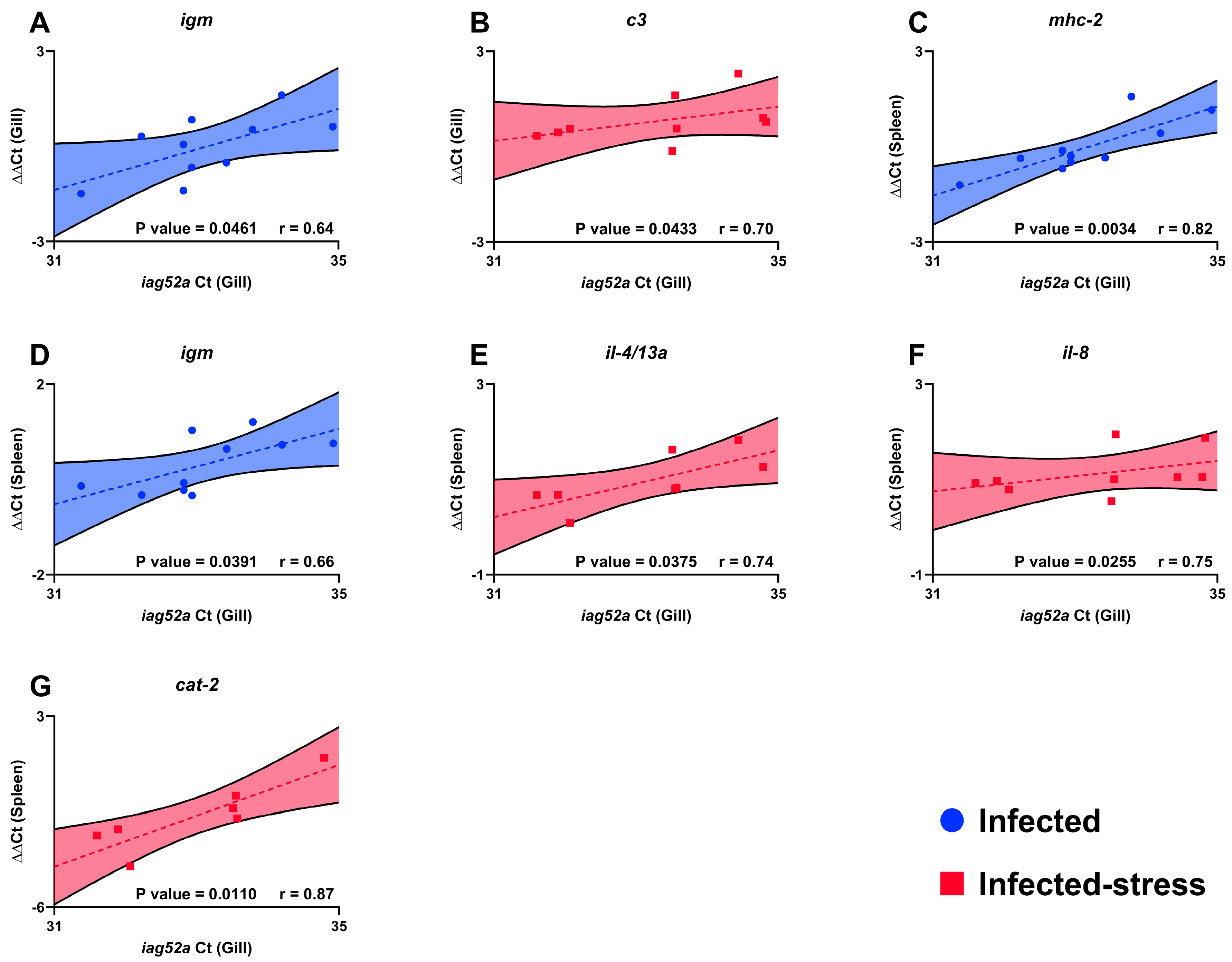
3.5. Association between Trophont Count, Immune Gene Expression and Infection Severity
4. Discussion
4.1. Rainbow Trout Typical Immune Gene Expression against Ichthyophthirius multifiliis
4.2. Rainbow Trout Impaired Immune Gene Expression against Ichthyophthirius multifiliis Induced by Unpredictable Repeated Stress
4.3. Rainbow Trout Mortality and Parasite Burden in Relation with Ich Water eDNA Levels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, R.A. Ichthyophthirius multifiliis fouquet and ichthyophthiriosis in freshwater teleosts. Adv. Parasitol. 2005, 59, 159–241. [Google Scholar] [CrossRef] [PubMed]
- MacColl, E.; Therkelsen, M.D.; Sherpa, T.; Ellerbrock, H.; Johnston, L.A.; Jariwala, R.H.; Chang, W.; Gurtowski, J.; Schatz, M.C.; Hossain, M.M.; et al. Molecular genetic diversity and characterization of conjugation genes in the fish parasite Ichthyophthirius multifiliis. Mol. Phylogenet. Evol. 2015, 86, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.Z.; Li, H.; Yu, H. Ichthyophthiriasis: Emphases on the epizootiology. Lett. Appl. Microbiol. 2013, 57, 91–101. [Google Scholar] [CrossRef]
- Buchmann, K.; Nielsen, M.E. Chemoattraction of Ichthyophthirius multifliis (Ciliophora) theronts to host molecules. Int. J. Parasitol. 1999, 29, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Maceda-Veiga, A.; Salvadó, H.; Vinyoles, D.; de Sostoa, A. Outbreaks of Ichthyophthirius multifiliis in redtail barbs Barbus haasi in a mediterranean stream during drought. J. Aquat. Anim. Health 2009, 21, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.R.; Larsen, T.B.; Buchmann, K. Parasite infections in recirculated rainbow trout (Oncorhynchus mykiss) farms. Aquaculture 2009, 289, 91–94. [Google Scholar] [CrossRef]
- Buchmann, K.; Bresciani, J. Parasitic infection in pond-reared rainbow trout Oncorhynchus mykiss in Denmark. Dis. Aquat. Org. 1997, 28, 125–138. [Google Scholar] [CrossRef]
- Barton, B.A.; Morgan, J.D.; Vijayan, M.M. Physiological and Condition-Related Indicators of Environmental Stress in Fish. Am. Fish. Soc. 2002, 111–148. [Google Scholar]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish. In Fish Physiology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, pp. 1–34. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef]
- Fischer, U.; Dijkstra, J.M.; Köllner, B.; Kiryu, I.; Koppang, E.O.; Hordvik, I.; Sawamoto, Y.; Ototake, M. The ontogeny of MHC class I expression in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2005, 18, 49–60. [Google Scholar] [CrossRef]
- Piato, Â.L.; Capiotti, K.M.; Tamborski, A.R.; Oses, J.P.; Barcellos, L.J.; Bogo, M.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Marana, M.H.; Chettri, J.K.; Salten, M.B.; Bach-Olesen, N.E.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Primary immunization using low antigen dosages and immunological tolerance in rainbow trout. Fish Shellfish Immunol. 2020, 105, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Raida, M.; Buchmann, K. Temperature-dependent expression of immune-relevant genes in rainbow trout following Yersinia ruckeri vaccination. Dis. Aquat. Org. 2007, 77, 41–52. [Google Scholar] [CrossRef]
- Purcell, M.K.; Kurath, G.; Garver, K.A.; Herwig, R.P.; Winton, J.R. Quantitative expression profiling of immune response genes in rainbow trout following infectious haematopoietic necrosis virus (IHNV) infection or DNA vaccination. Fish Shellfish Immunol. 2004, 17, 447–462. [Google Scholar] [CrossRef]
- Raida, M.; Buchmann, K. Development of adaptive immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) surviving an infection with Yersinia ruckeri. Fish Shellfish Immunol. 2008, 25, 533–541. [Google Scholar] [CrossRef]
- Bahlool, Q.Z.M.; Skovgaard, A.; Kania, P.W.; Buchmann, K. Effects of excretory/secretory products from Anisakis simplex (Nematoda) on immune gene expression in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 734–739. [Google Scholar] [CrossRef]
- Olsen, M.M.; Kania, P.W.; Heinecke, R.D.; Skjoedt, K.; Rasmussen, K.J.; Buchmann, K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: Molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011, 30, 859–869. [Google Scholar] [CrossRef]
- Raida, M.K.; Buchmann, K. Innate immune response in rainbow trout (Oncorhynchus mykiss) against primary and secondary infections with Yersinia ruckeri O1. Dev. Comp. Immunol. 2009, 33, 35–45. [Google Scholar] [CrossRef]
- Xueqin, J.; Kania, P.W.; Buchmann, K. Comparative effects of four feed types on white spot disease susceptibility and skin immune parameters in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2012, 35, 127–135. [Google Scholar] [CrossRef]
- Jaafar, R.; Ødegård, J.; Mathiessen, H.; Karami, A.M.; Marana, M.H.; von Gersdorff Jørgensen, L.; Zuo, S.; Nielsen, T.; Kania, P.W.; Buchmann, K. Quantitative trait loci (QTL) associated with resistance of rainbow trout Oncorhynchus mykiss against the parasitic ciliate Ichthyophthirius multifiliis. J. Fish Dis. 2020, 43, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.A.; Santana, P.A.; Salinas-Parra, N.; Beltrán, D.; Guzmán, F.; Vega, B.; Acosta, F.; Mercado, L. Immune Modulation Ability of Hepcidin from Teleost Fish. Animals 2022, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, R.D.; Buchmann, K. Inflammatory response of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) larvae against Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2013, 34, 521–528. [Google Scholar] [CrossRef]
- Abdel-Hafez, G.; Lahnsteiner, F.; Mansour, N.; Licek, E. Pathophysiology of Ichthyophthirius multifiliis infection in rainbow trout (Oncorhynchus mykiss) and chub (leuciscus cephalus). J. Comp. Pathol. 2014, 151, 394–399. [Google Scholar] [CrossRef]
- Kong, W.; Ding, G.; Cheng, G.; Yang, P.; Xu, Z. Mucosal immune responses to Ichthyophthirius multifiliis in the ocular mucosa of rainbow trout (Oncorhynchus mykiss, Walbaum), an ancient teleost fish. Mar. Life Sci. Technol. 2023, 6, 266–279. [Google Scholar] [CrossRef]
- Sigh, J.; Lindenstrøm, T.; Buchmann, K. Expression of pro-inflammatory cytokines in rainbow trout (Oncorhynchus mykiss) during an infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2004, 17, 75–86. [Google Scholar] [CrossRef]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Jafaar, R.M.; Dirks, R.P.; Buchmann, K. Transcriptomic analysis of immunity in rainbow trout (Oncorhynchus mykiss) gills infected by Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2019, 86, 486–496. [Google Scholar] [CrossRef]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Marnis, H.; Mathiessen, H.; Dirks, R.P.; Buchmann, K. Association between stress, metabolism, and growth in Ichthyophthirius multifiliis infected rainbow trout gills: Transcriptomic evidence. Aquaculture 2020, 526, 735384. [Google Scholar] [CrossRef]
- Shepherd, B.S.; Spear, A.R.; Philip, A.M.; Leaman, D.W.; Stepien, C.A.; Sepulveda-Villet, O.J.; Palmquist, D.E.; Vijayan, M.M. Effects of cortisol and lipopolysaccharide on expression of select growth-, stress- and immune-related genes in rainbow trout liver. Fish Shellfish Immunol. 2018, 74, 410–418. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.K.; Fayaz, S.; Caipang, C.M.A. Increased stocking density causes changes in expression of selected stress- and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 48, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.S.; Wen, H.S.; Li, J.F.; He, F.; Li, Y.; Qi, X. Effects of long-term crowding stress on neuro-endocrine-immune network of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 95, 180–189. [Google Scholar] [CrossRef]
- Khansari, A.R.; Balasch, J.C.; Vallejos-Vidal, E.; Teles, M.; Fierro-Castro, C.; Tort, L.; Reyes-López, F.E. Comparative study of stress and immune-related transcript outcomes triggered by Vibrio anguillarum bacterin and air exposure stress in liver and spleen of gilthead seabream (Sparus aurata), zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 86, 436–448. [Google Scholar] [CrossRef]
- Ren, Y.; Men, X.; Yu, Y.; Li, B.; Zhou, Y.; Zhao, C. Effects of transportation stress on antioxidation, immunity capacity and hypoxia tolerance of rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2022, 22, 100940. [Google Scholar] [CrossRef]
- Nielsen, C.V.; Buchmann, K. Prolonged in vitro cultivation of Ichthyophthirius multifiliis using an EPC cell line as substrate. Dis. Aquat. Org. 2000, 42, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hutson, K.S.; Cable, J.; Grutter, A.S.; Paziewska-Harris, A.; Barber, I. Aquatic Parasite Cultures and Their Applications. Trends Parasitol. 2018, 34, 1082–1096. [Google Scholar] [CrossRef]
- Dickerson, H.W.; Leff, A.A. Serotypic Variation Among Isolates of Ichthyophthirius multifiliis Based on Immobilization. J. Eukaryot. Microbiol. 1993, 40, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Swennes, A.G.; Noe, J.G.; Findly, R.C.; Dickerson, H.W. Differences in virulence between two serotypes of Ichthyophthirius multifiliis. Dis. Aquat. Org. 2006, 69, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K.; Nielsen, T.; Mathiessen, H.; Marana, M.H.; Duan, Y.; Jørgensen, L.V.; Zuo, S.; Karami, A.M.; Kania, P.W. Validation of two QTL associated with lower Ichthyophthirius multifiliis infection and delayed-time-to-death in rainbow trout. Aquac. Rep. 2022, 23, 101078. [Google Scholar] [CrossRef]
- Ewing, M.S.; Lynn, M.E.; Ewing, S.A. Critical Periods in Development of Ichthyuphthirius multifiliis (Ciliophora) Populations. J. Protozool. 1986, 33, 388–391. [Google Scholar] [CrossRef]
- Garcia, L.d.O.; Becker, A.G.; Cunha, M.A.; Baldisserotto, B.; Copatti, C.E.; Kochhann, D. Effects of Water pH and Hardness on Infection of Silver Catfish, Rhamdia quelen, Fingerlings by Ichthyophthirius multifiliis. J. World Aquac. Soc. 2011, 42, 399–405. [Google Scholar] [CrossRef]
- Ewing, M.S.; Hefler, C.; Kocan, K.M.; Stebler, E. Extracellular Calcium Concentration Affects Infectivity of Ichthyophthirius (Ciliophora) Theronts. J. Protozool. 1991, 38, 178–181. [Google Scholar] [CrossRef]
- Davis, K.B.; Griffin, B.R.; Gray, W.L. Effect of Handling Stress on Susceptibility of Channel Catfish Ictalurus punctatus to Ichthyophthirius multifiliis and Channel Catfish Virus Infection. Aquaculture. 2002, 214, 55–66. [Google Scholar] [CrossRef]
- Dickerson, H.W.; Findly, R.C. Immunity to Ichthyophthirius infections in fish: A synopsis. Dev. Comp. Immunol. 2014, 43, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.S.; Spira, D.T. Ichthyophthiriasis in the mirror carp Cyprinus carpio (L.) III. Pathology. J. Fish Biol. 1974, 6, 189–196. [Google Scholar] [CrossRef]
- Adams, M.B.; Ellard, K.; Nowak, B.F. Gross pathology and its relationship with histopathology of amoebic gill disease (AGD) in farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 2004, 27, 151–161. [Google Scholar] [CrossRef]

| Day Post Infection | Air Exposure | Chasing | Transfer | |
|---|---|---|---|---|
| −3 | 3:15 p.m. | |||
| −2 | 3:40 p.m. | |||
| −1 | 2:25 p.m. | |||
| 0 | Infection | |||
| 1 | 10:10 a.m. | 1:10 p.m. | ||
| 2 | 3:30 p.m. | 1:35 p.m. | ||
| 3 | 10:30 a.m. | 3:15 p.m. | ||
| 4 | 9:55 a.m. | 3:15 p.m. | ||
| 5 | 1:35 p.m. | 10:45 a.m. | ||
| 6 | 9:25 a.m. | 3:35 p.m. | ||
| 7 | Sampling | |||
| 8 | 10:22 a.m. | 1:55 p.m. | ||
| 9 | 1:40 p.m. | |||
| 10 | 2:40 p.m. | |||
| 11 | ||||
| 12 | Experiment end |
| Gene | Species | Sequence 5′-3′ | Product Size (bp) | Ref. |
|---|---|---|---|---|
| GenBank Acc. Num | ||||
| β-actin | O. mykiss | F: ACATCAAGGAGAAGCTGTGCTAC | 241 | [14] |
| AB196465 | R: TACGGATGTCCACGTCACAC | |||
| P: CCTCTCTGGAGAAGAGCTACGAGCTG | ||||
| elf-1α | O. mykiss | F: ACCCTCCTCTTGGTCGTTTC | 63 | [15] |
| AF498320 | R: TGATGACACCAACAGCAACA | |||
| P: GCTGTGCGTGACATGAGGCA | ||||
| arp | O. mykiss | F: GAAAATCATCCAATTGCTGGATG | 106 | [16] |
| AY505012 | R: CTTCCCACGCAAGGACAGA | |||
| P: CTATCCCAAATGTTTCATTGTCGGCGC | ||||
| il-1β | O. mykiss | F: ACATTGCCAACCTCATCATCG | 91 | [17] |
| AJ223954 | R: TTGAGCAGGTCCTTGTCCTTG | |||
| P: CATGGAGAGGTTAAAGGGTGGC | ||||
| il-2 | O. mykiss | F: ATGCAACACCACATCAGCAT | 110 | [14] |
| FJ571513 | R: TTTTCCAACCGCTTGTCTTC | |||
| P: TGCCACGGCCCTACAAAAGA | ||||
| il-4/13a | O. mykiss | F: ATCCTTCTCCTCTCTGTTGC | 139 | [18] |
| AB574337 | R: GAGTGTGTGTGTATTGTCCTG | |||
| P: CGCACCGGCAGCATAGAAGT | ||||
| il-6 | O. mykiss | F: ACTCCCCTCTGTCACACACC | 91 | [17] |
| DQ866150 | R: GGCAGACAGGTCCTCCACTA | |||
| P: CCACTGTGCTGATAGGGCTGG | ||||
| il-8 | O. mykiss | F: AGAATGTCAGCCAGCCTTGT | 69 | [17] |
| AJ279069 | R: TCTCAGACTCATCCCCTCAGT | |||
| P: TTGTGCTCCTGGCCCTCCTGA | ||||
| il-10 | O. mykiss | F: CGACTTTAAATCTCCCATCGAC | 70 | [15] |
| AB118099 | R: GCATTGGACGATCTCTTTCTTC | |||
| P: CATCGGAAACATCTTCCACGAGCT | ||||
| il-22 | O. mykiss | F: ATGACCACCACCACAGCATT | 64 | [19] |
| AM748537 | R: ATTCCTTTCCCCTCCTCCAT | |||
| P: CTTTCCGCAAGAAGTTGTCCGAG | ||||
| igm | O. mykiss | F: CTTGGCTTGTTGACGATGAG | 72 | [15] |
| AH014877—S63348 | R: GGCTAGTGGTGTTGAATTGG | |||
| P: TGGAGAGAACGAGCAGTTCAGCA | ||||
| igt | O. mykiss | F: AGCACCAGGGTGAAACCA | 73 | [15] |
| AY870265—AY870263 | R: GCGGTGGGTTCAGAGTCA | |||
| P: AGCAAGACGACCTCCAAAACAGAAC | ||||
| ifn-γ | O. mykiss | F: AAGGGCTGTGATGTGTTTCTG | 68 | [17] |
| FJ184374/FJ184375 | R: TGTACTGAGCGGCATTACTCC | |||
| P: TTGATGGGCTGGATGACTTTAGGA | ||||
| saa | O. mykiss | F: GGGAGATGATTCAGGGTTCCA | 79 | [20] |
| AM422446 | R: TTACGTCCCCAGTGGTTAGC | |||
| P: TCGAGGACACGAGGACTCAGCA | ||||
| tnf-α | O. mykiss | F: GGGGACAAACTGTGGACTGA | 75 | [17] |
| AJ277604/AJ401377 | R: GAAGTTCTTGCCCTGCTCTG | |||
| P: GACCAATCGACTGACCGACGTGGA | ||||
| c3 | O. mykiss | F: ATTGGCCTGTCCAAAACACA | 85 | [20] |
| AF271080 | R: AGCTTCAGATCAAGGAAGAAGTTC | |||
| P: TGGAATCTGTGTGTCTGAACCCC | ||||
| mhc-2 | O. mykiss | F: TGCCATGCTGATGTGCAG | 68 | [15] |
| AF115533 | R: GTCCCTCAGCCAGGTCACT | |||
| P: CGCCTATGACTTCTACCCCAAACAAAT | ||||
| hep | O. mykiss | F: GAGGAGGTTGGAAGCATTGA | 95 | [20] |
| AF281354 | R: TGACGCTTGAACCTGAAATG | |||
| P: AGTCCAGTTGGGGAACATCAACAG | ||||
| cox-2 | O. mykiss | F: GGGCTTTGACATCCTCAACA | 73 | [15] |
| NM_001124348.1 | R: CATCGGACAAGAACCCTTGA | |||
| P: CTTCATTGGAGAGGCTGGTGTGC | ||||
| cat-1 | O. mykiss | F: TCTCTCGTCCTGGGGTT | 189 | [14] |
| AY382478 | R: GTTGTAGCGTGCTGATCTATG | |||
| P: TAATTGGTCGTCCTGGGGGTGG | ||||
| cat-2 | O. mykiss | F: AAAGATTCCAAGGGGGGT | 135 | [21] |
| AY360356 | R: CAAAGGGTGTGTTGTGCTGT | |||
| P: GCTCTCGTCCTGGGTTTGGCTCC | ||||
| iag52a | I. mutifiliis | F: TTGGAACTGAAACTAACACAGCC | 240 | [22] |
| AF324424 | R: CTCCACCTGCAATTGCGGTA | |||
| P: TGCTGCTGCTTTCGTTCCTGGTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henard, C.; Li, H.; Nowak, B.F.; von Gersdorff Jørgensen, L. Unpredictable Repeated Stress in Rainbow Trout (Oncorhynchus mykiss) Shifted the Immune Response against a Fish Parasite. Biology 2024, 13, 769. https://doi.org/10.3390/biology13100769
Henard C, Li H, Nowak BF, von Gersdorff Jørgensen L. Unpredictable Repeated Stress in Rainbow Trout (Oncorhynchus mykiss) Shifted the Immune Response against a Fish Parasite. Biology. 2024; 13(10):769. https://doi.org/10.3390/biology13100769
Chicago/Turabian StyleHenard, Cyril, Hanxi Li, Barbara F. Nowak, and Louise von Gersdorff Jørgensen. 2024. "Unpredictable Repeated Stress in Rainbow Trout (Oncorhynchus mykiss) Shifted the Immune Response against a Fish Parasite" Biology 13, no. 10: 769. https://doi.org/10.3390/biology13100769
APA StyleHenard, C., Li, H., Nowak, B. F., & von Gersdorff Jørgensen, L. (2024). Unpredictable Repeated Stress in Rainbow Trout (Oncorhynchus mykiss) Shifted the Immune Response against a Fish Parasite. Biology, 13(10), 769. https://doi.org/10.3390/biology13100769






