Whole-Transcriptome Analysis Reveals Potential CeRNA Regulatory Mechanism in Takifugu rubripes against Cryptocaryon irritans Infection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals
2.3. Experimental Design
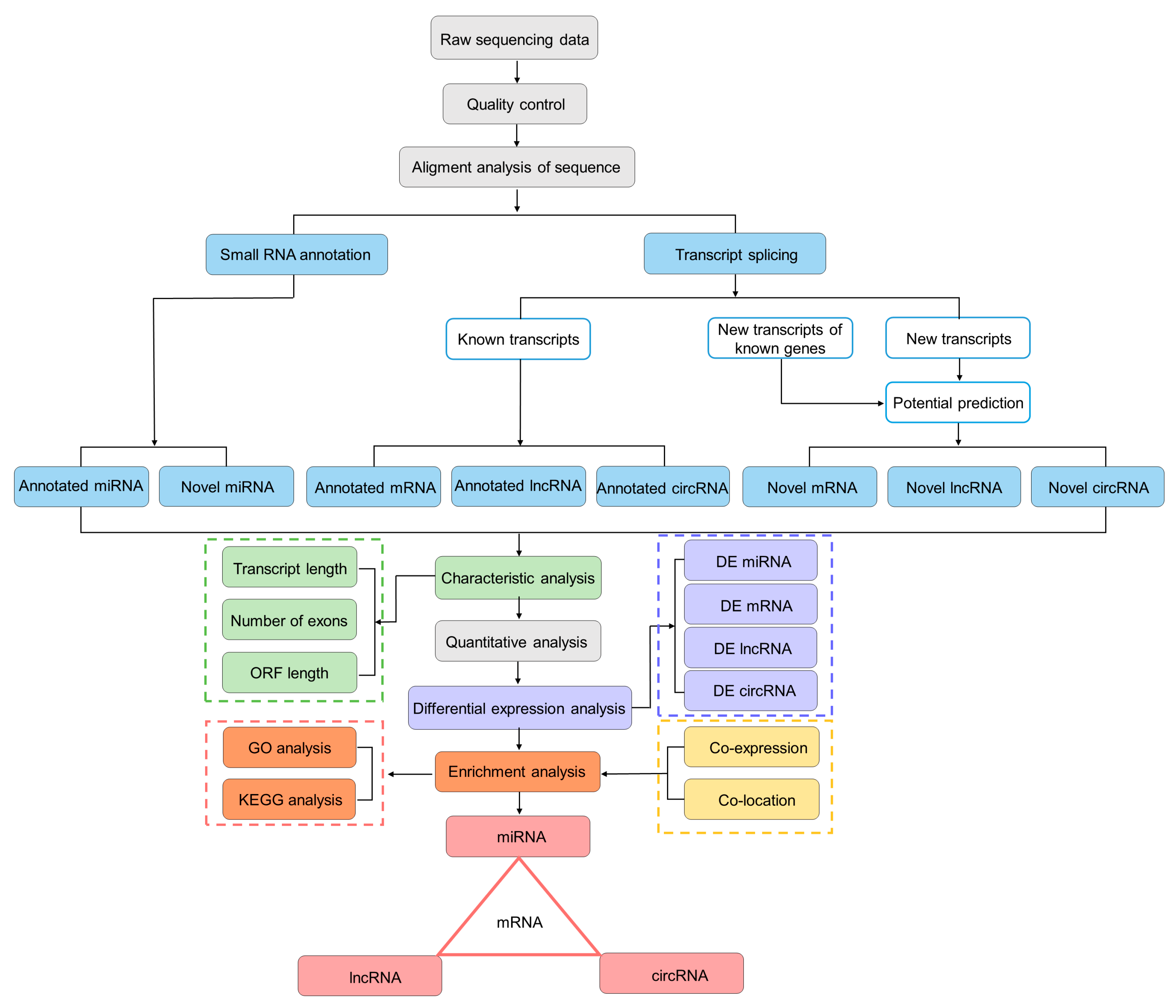
2.4. Library Construction and Illumina Sequencing
2.5. Construction of ceRNA Networks
2.6. Quantitative Real-Time PCR Verification
2.7. Statistical Analysis
3. Results
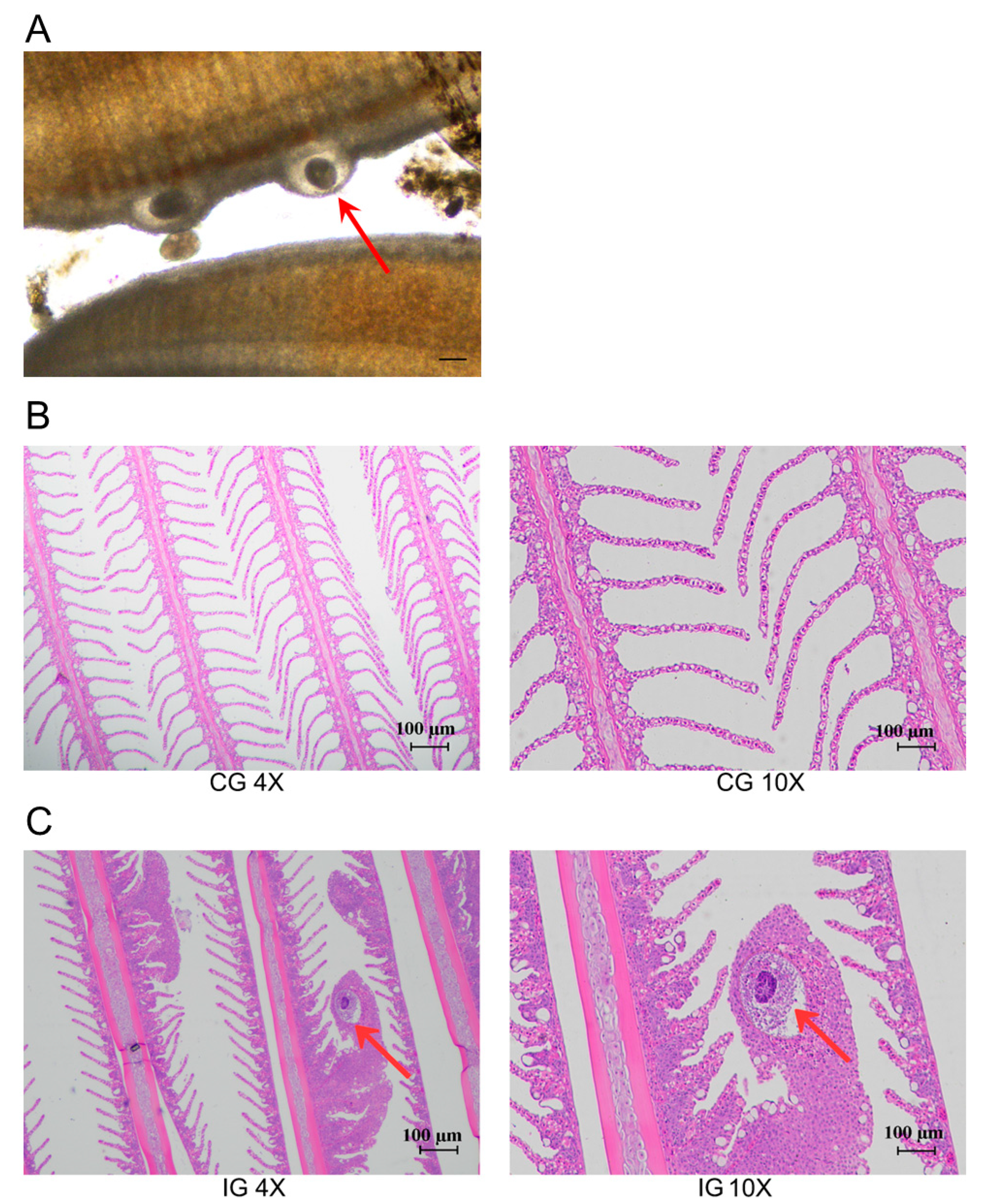
3.1. Pathological Tissue
3.2. RNA Sequencing and cDNA Library Construction
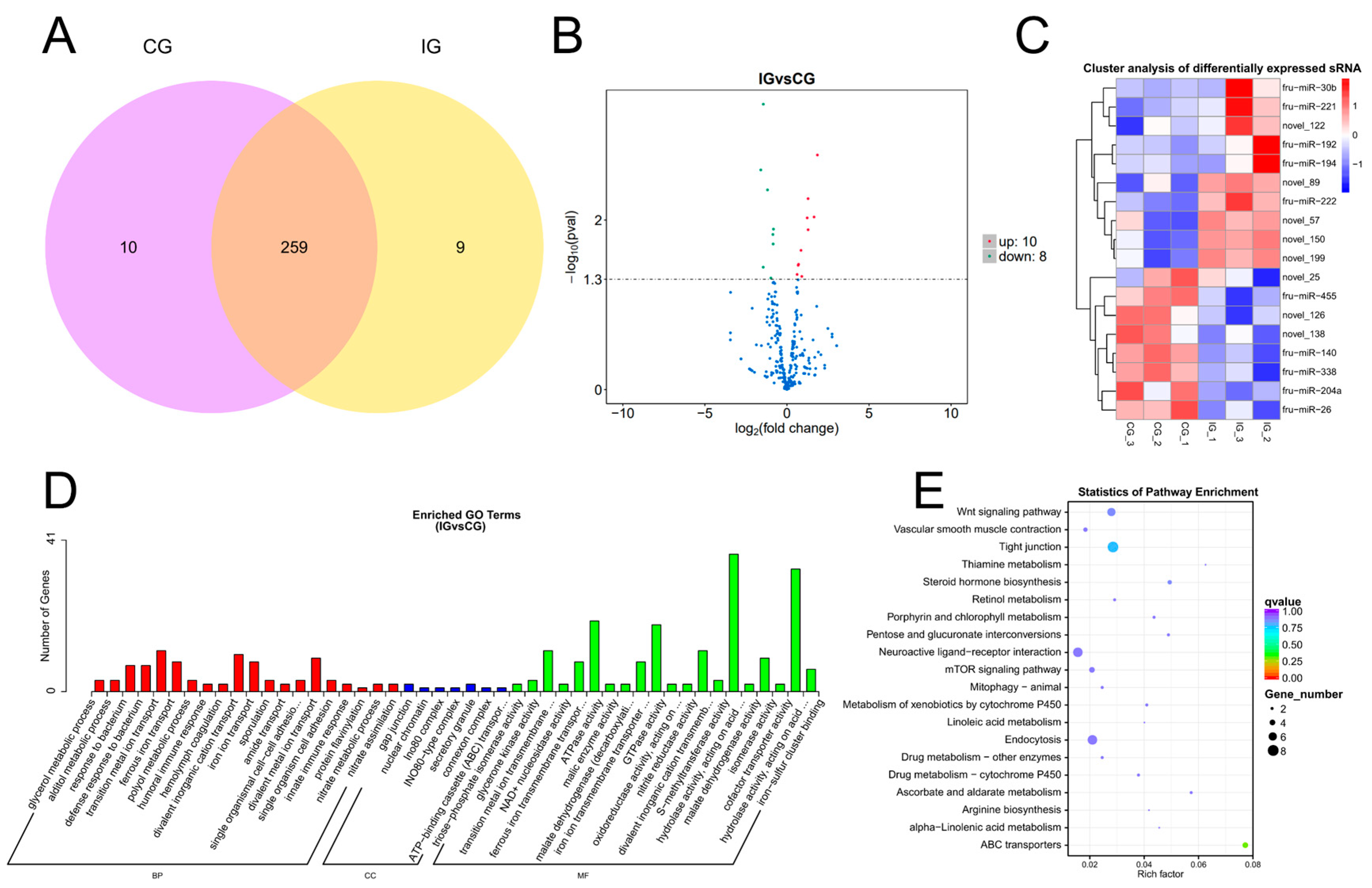
3.3. Expression Profiles and Enrichment Analysis of DEMs
3.4. Expression Profiles and Enrichment Analysis of DELs
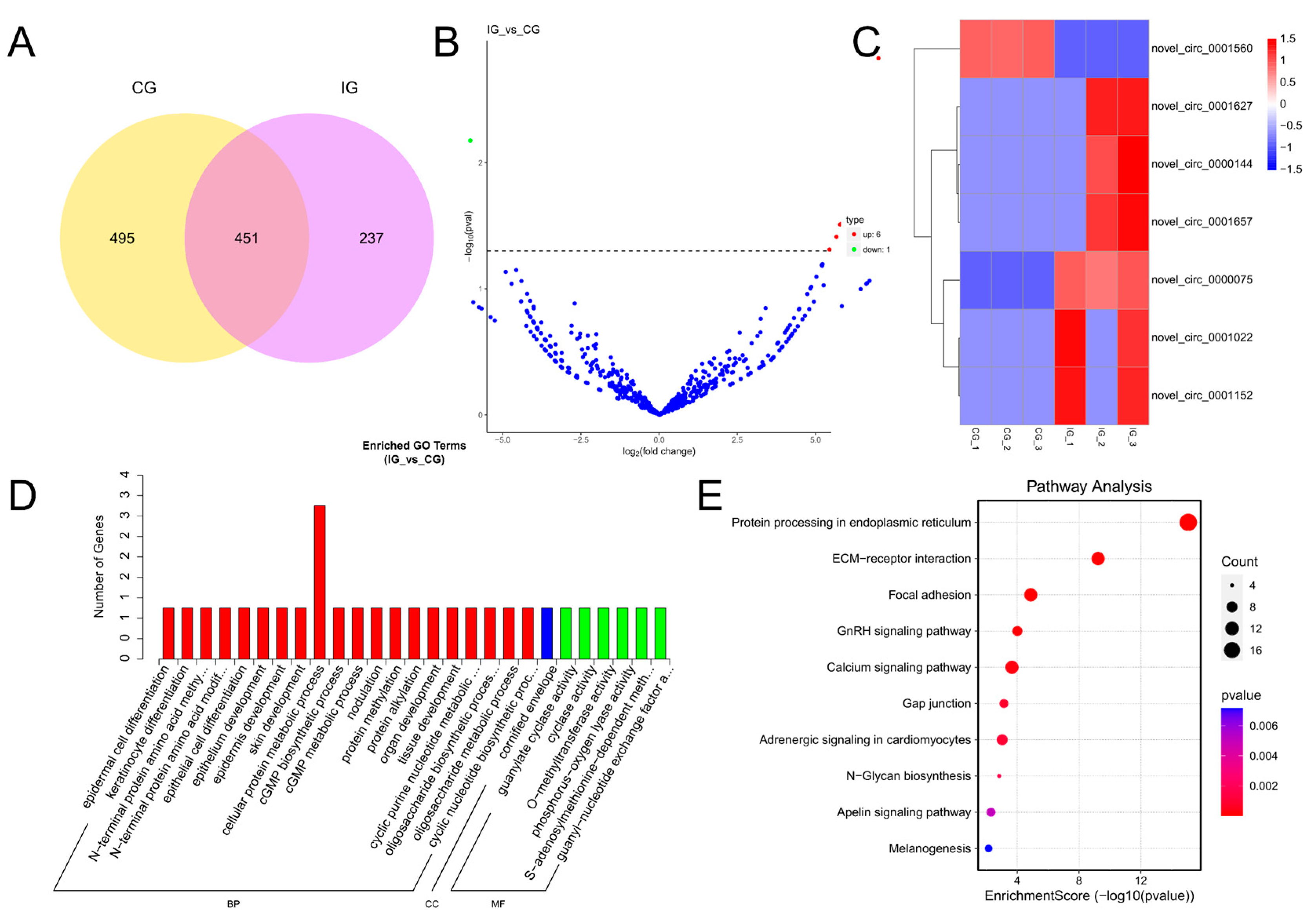
3.5. Expression Profiles and Enrichment Analysis of DECs
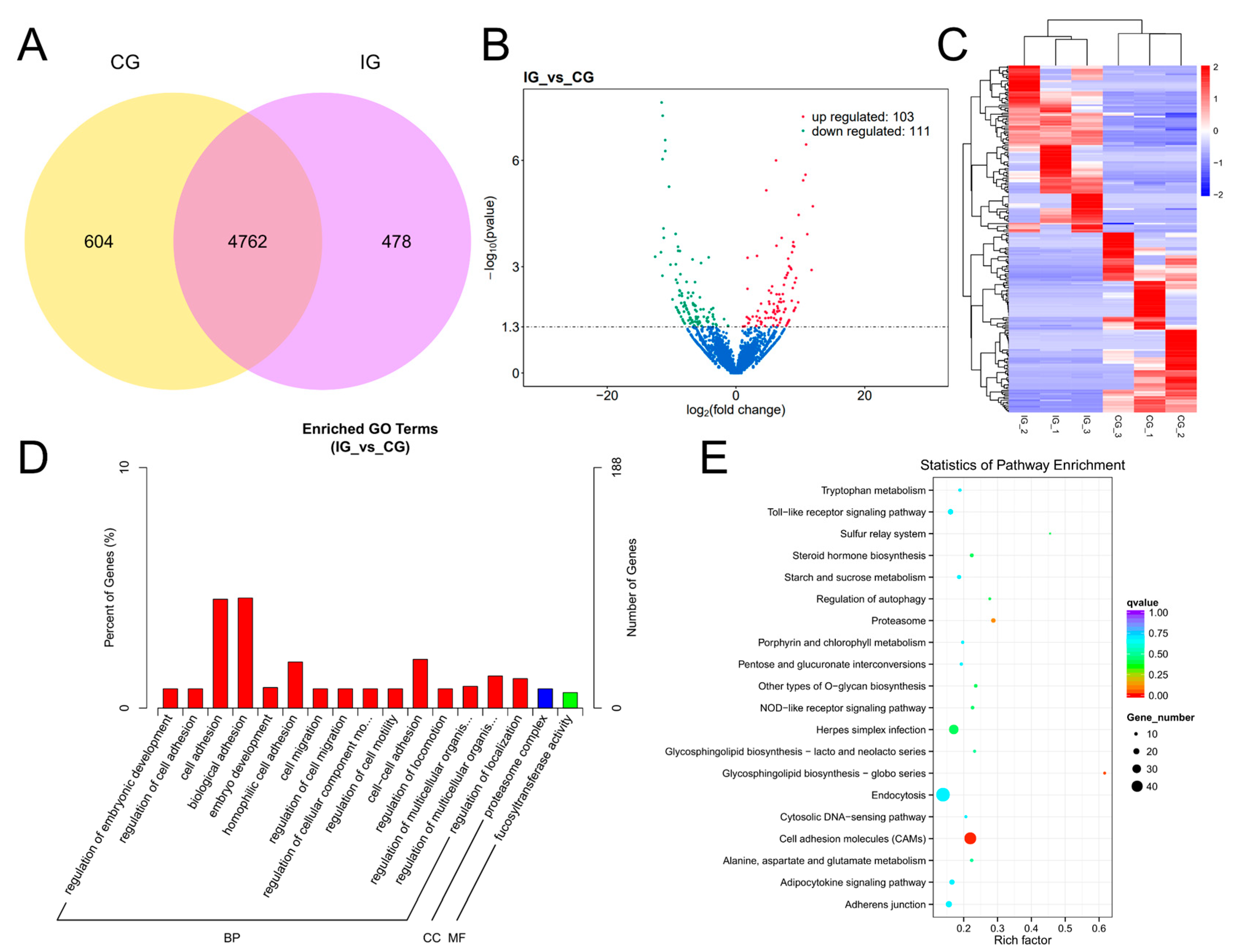
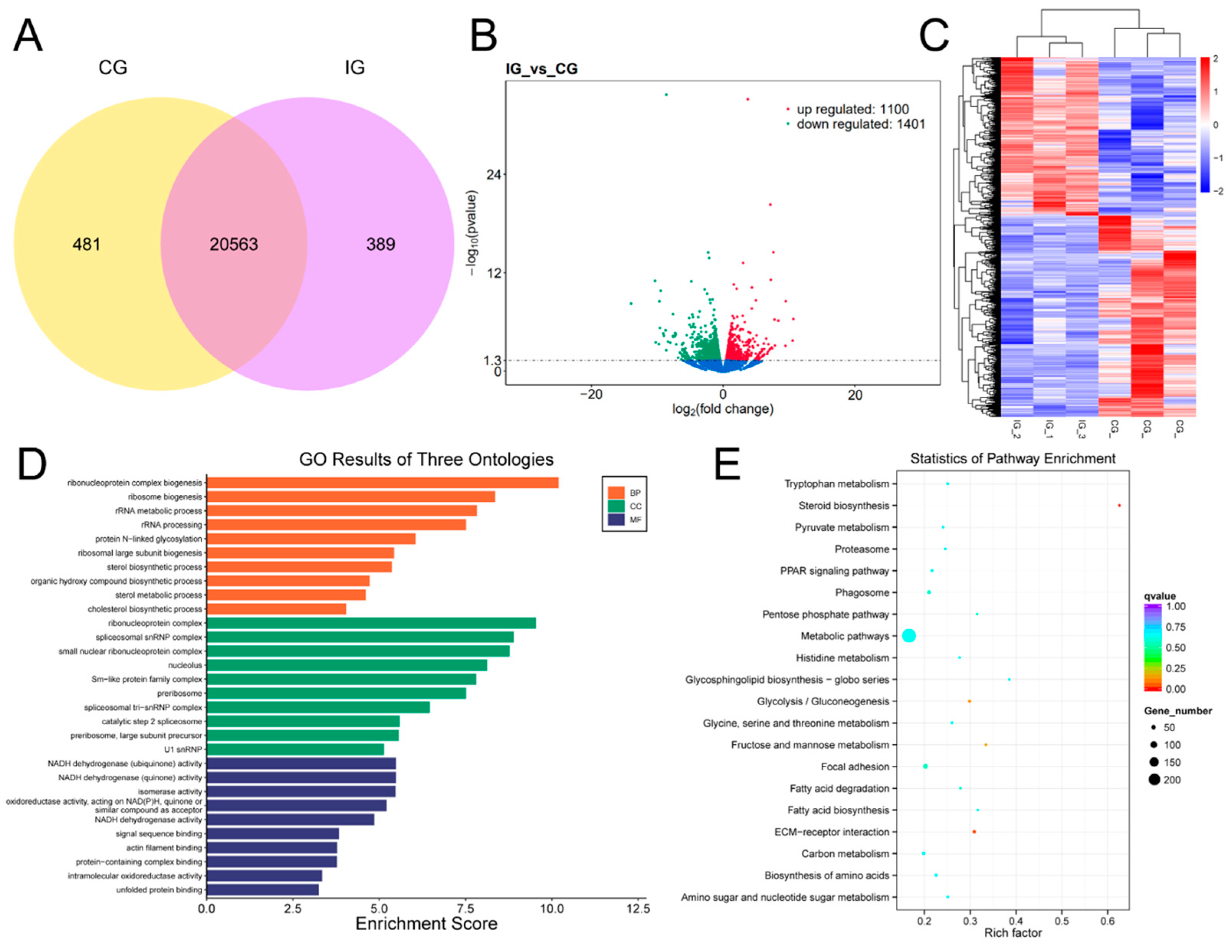
3.6. Expression Profiles and Enrichment Analysis of DEGs
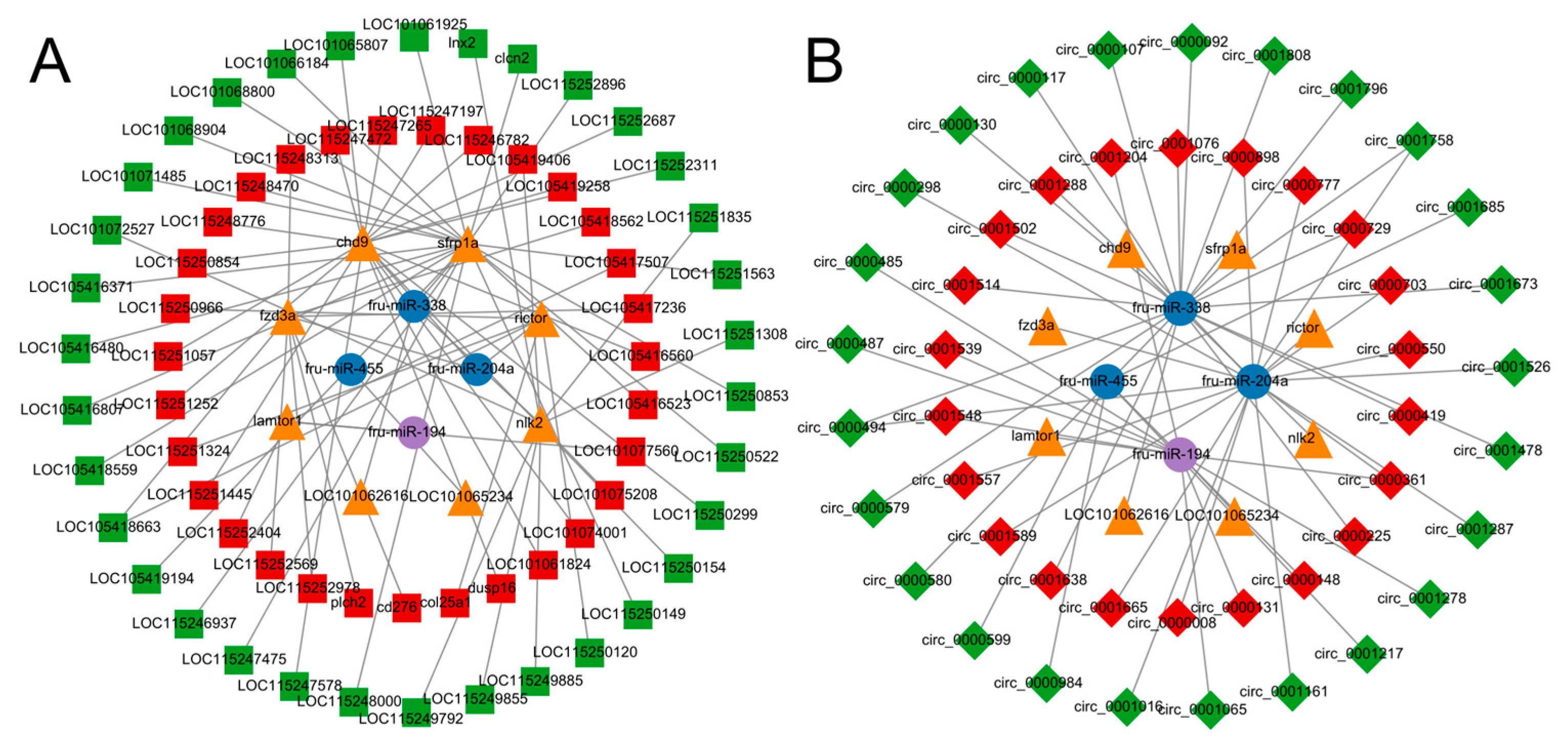
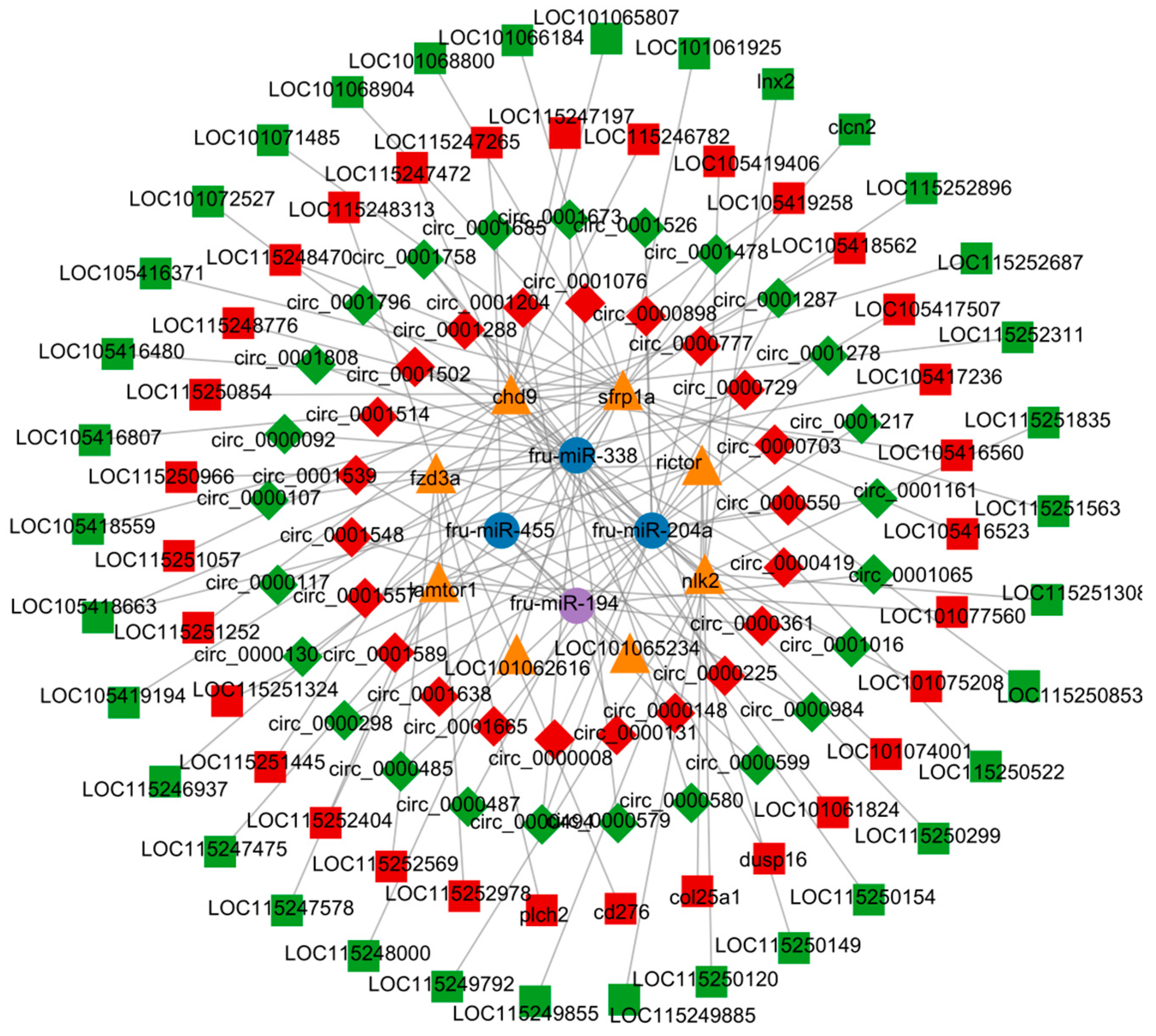
3.7. CeRNA Network Construction
3.8. lncRNA-circRNA-miRNA-mRNA Construction
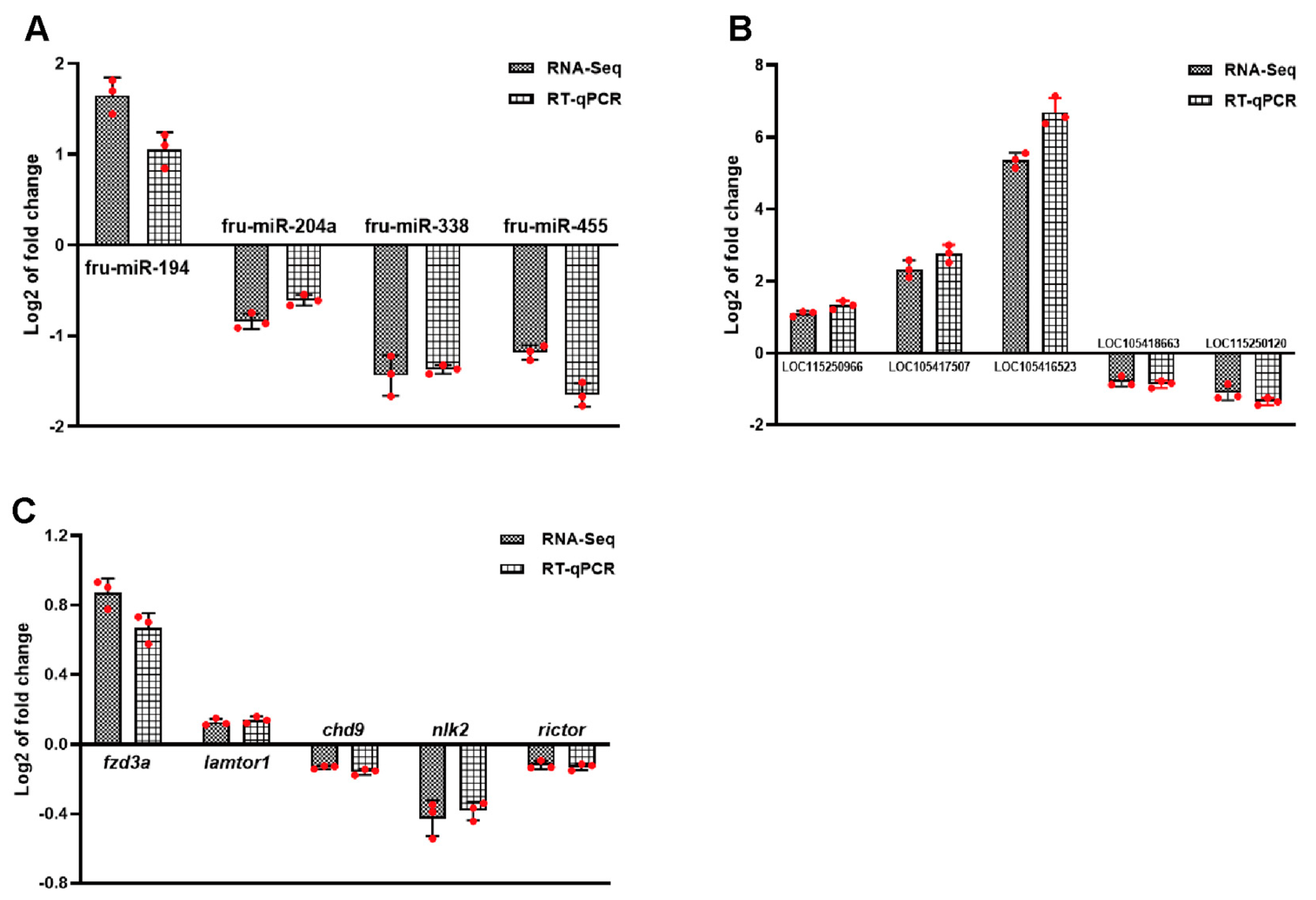
3.9. Validation of ncRNAs
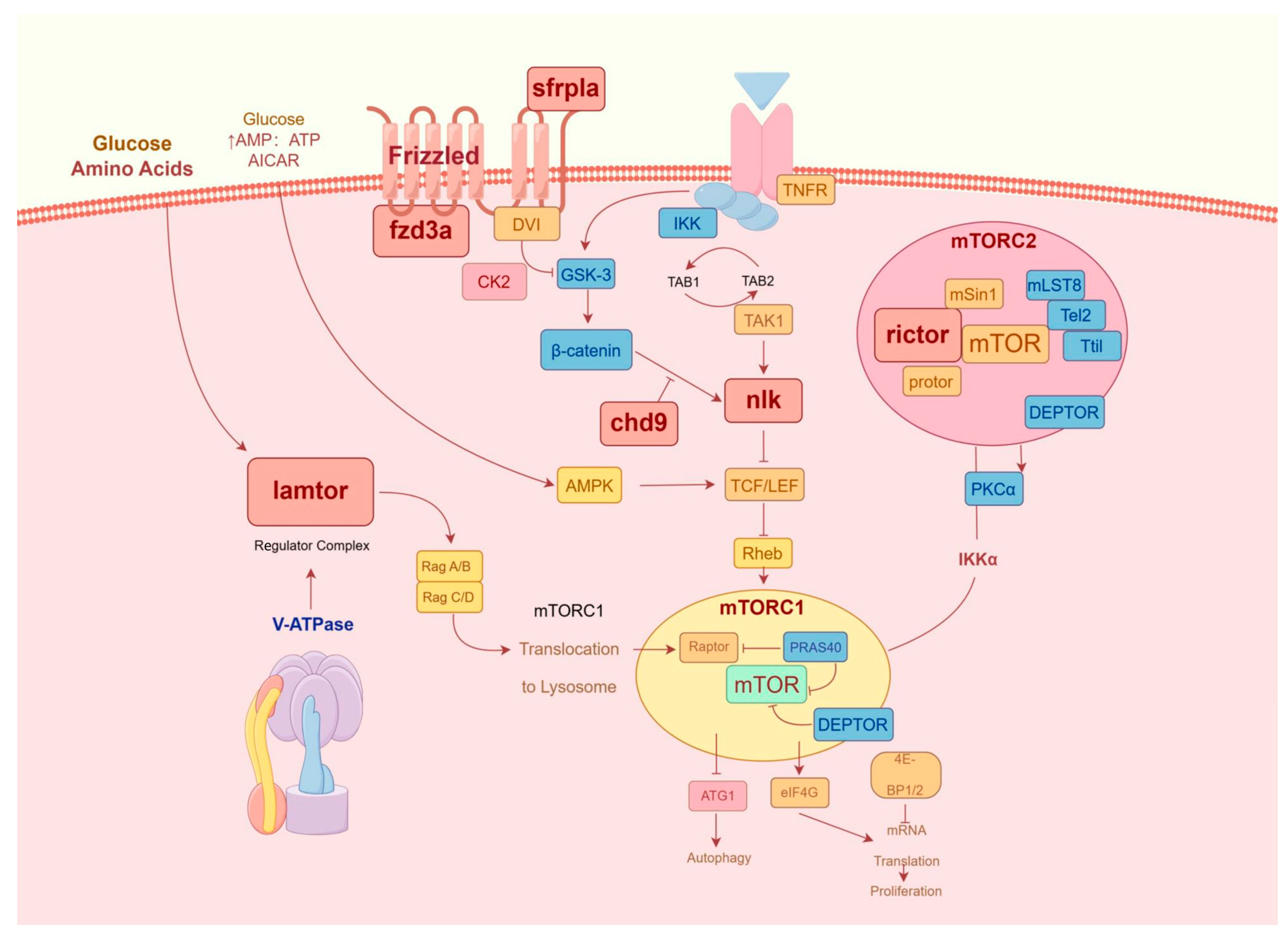
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Jiang, B.; Mo, Z.; Li, A.; Dan, X. Cryptocaryon irritans (Brown, 1951) is a serious threat to aquaculture of marine fish. Rev. Aquac. 2021, 14, 218–236. [Google Scholar] [CrossRef]
- Hirazawa, N.; Oshima, S.I.; Hara, T.; Mitsuboshi, T.; Hata, K. Antiparasitic effect of medium-chain fatty acids against the ciliate Cryptocaryon irritans infestation in the red sea bream Pagrus major. Aquaculture 2001, 198, 219–228. [Google Scholar] [CrossRef]
- Chang, L.C.; Karin, M. Mammalian MAP kinase signaling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Liao, K.; Lou, X.; Yang, Z. Comparative analysis of the economic feasibility of Tiger puffer (Takifugu rubripes) aquaculture in China. Aquacult. Int. 2023, 32, 939–961. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Hua, X.T.; Zhu, S.; Liu, Y.; Liu, P.F. Characterization and expression analysis of the complement component 8β from pufferfish (Takifugu rubripes). J. Aquac. Rep. 2021, 21, 100861. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-Lopez, F.; Toro-Ascuy, D.; Montero, R.; Maisey, K.; Acuna-Castillo, C.; Sunyer, J.O.; Parra, D.; Sandino, A.M.; Imarai, M. Induction of anti-inflammatory cytokine expression by IPNV in persistent infection. Fish Shellfish Immunol. 2014, 41, 172–182. [Google Scholar] [CrossRef]
- Rigos, G.; Padrós, F.; Golomazou, E.; Zarza, C. Antiparasitic approaches and strategies in European aquaculture, with emphasis on Mediterranean marine finfish farming: Present scenarios and future visions. Rev. Aquac. 2024, 16, 622–643. [Google Scholar] [CrossRef]
- Witzany, G.; Nowacki, M. Interactions Between Parasitic Ciliates and Their Hosts: Ichthyophthirius multifiliis and Cryptocaryon irritans as Examples. Biocommunication Ciliates 2016, 327–350. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef]
- Ma, R.; Yu, Y.; Liu, X.; Lei, Y.; Zhou, S.; Xie, X.; Jin, S.; Qian, D.; Yin, F. Transcriptomic analysis of Nibea albiflora skin in response to infection by Cryptocaryon irritans. Fish Shellfish Immunol. 2020, 98, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Josepriya, T.A.; Chien, K.H.; Lin, H.Y.; Huang, H.N.; Wu, C.J.; Song, Y.L. Immobilization antigen vaccine adjuvanted by parasitic heat shock protein 70C confers high protection in fish against cryptocaryonosis. Fish Shellfish Immunol. 2015, 45, 517–527. [Google Scholar] [CrossRef]
- Chu, Q.; Xu, T.; Zheng, W.; Chang, R.; Zhang, L. Long noncoding RNA MARL regulates antiviral responses through suppression miR-122-dependent MAVS downregulation in lower vertebrates. PLoS Pathog. 2020, 16, e1008670. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, Y.; Cao, Z.; Sun, Y.; Chen, Y.; Xiang, Y.; Wang, L.; Zhang, S.; Guo, W. Comparative analysis of the expression patterns of IL-1beta, IL-11, and IL-34 in golden pompano (Trachinotus ovatus) following different pathogens challenge. Fish Shellfish Immunol. 2019, 93, 863–870. [Google Scholar] [CrossRef]
- Wilhelm, B.T.; Marguerat, S.; Watt, S.; Schubert, F.; Wood, V.; Goodhead, I.; Penkett, C.J.; Rogers, J.; Bahler, J. Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature 2008, 453, 1239–1243. [Google Scholar] [CrossRef]
- Chen, R.; Mao, Y.; Wang, J.; Liu, M.; Qiao, Y.; Zheng, L.; Su, Y.; Ke, Q.; Zheng, W. Molecular mechanisms of an antimicrobial peptide piscidin (Lc-pis) in a parasitic protozoan, Cryptocaryon irritans. BMC Genom. 2018, 19, 192. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Karreth, F.A.; Reschke, M.; Ruocco, A.; Ng, C.; Chapuy, B.; Leopold, V.; Sjoberg, M.; Keane, T.M.; Verma, A.; Ala, U.; et al. The BRAF pseudogene functions as a competitive endogenous RNA and induces lymphoma in vivo. Cell 2015, 161, 319–332. [Google Scholar] [CrossRef]
- Tang, J.; Zhuo, H.; Zhang, X.; Jiang, R.; Ji, J.; Deng, L.; Qian, X.; Zhang, F.; Sun, B. A novel biomarker Linc00974 interacting with KRT19 promotes proliferation and metastasis in hepatocellular carcinoma. Cell Death Dis. 2014, 5, e1549. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xie, Q.; Xu, H.; Li, J.; Li, Y. Circular RNAs and cancer. Cancer Lett. 2017, 396, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.M.; Li, A.X.; Lin, X.T.; Teng, N.; Zhu, X.Q. A standardized method to propagate Cryptocaryon irritans on a susceptible host pompano Trachinotus ovatus. Aquaculture 2006, 258, 127–133. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Sudhagar, A.; Kumar, G.; El-Matbouli, M. Transcriptome Analysis Based on RNA-Seq in Understanding Pathogenic Mechanisms of Diseases and the Immune System of Fish: A Comprehensive Review. Int. J. Mol. Sci. 2018, 19, 245. [Google Scholar] [CrossRef]
- Faber, M.; Shaw, S.; Yoon, S.; de Paiva Alves, E.; Wang, B.; Qi, Z.; Okamura, B.; Hartikainen, H.; Secombes, C.J.; Holland, J.W. Comparative transcriptomics and host-specific parasite gene expression profiles inform on drivers of proliferative kidney disease. Sci. Rep. 2021, 11, 2149. [Google Scholar] [CrossRef]
- Bailey, C.; von Siebenthal, E.W.; Rehberger, K.; Segner, H. Transcriptomic analysis of the impacts of ethinylestradiol (EE2) and its consequences for proliferative kidney disease outcome in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 222, 31–48. [Google Scholar] [CrossRef]
- Sudhagar, A.; Ertl, R.; Kumar, G.; El-Matbouli, M. Transcriptome profiling of posterior kidney of brown trout, Salmo trutta, during proliferative kidney disease. Parasit. Vectors 2019, 12, 569. [Google Scholar] [CrossRef]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Jafaar, R.M.; Dirks, R.P.; Buchmann, K. Transcriptomic analysis of immunity in rainbow trout (Oncorhynchus mykiss) gills infected by Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2019, 86, 486–496. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Woldemariam, N.T.; Agafonov, O.; Sindre, H.; Hoyheim, B.; Houston, R.D.; Robledo, D.; Bron, J.E.; Andreassen, R. miRNAs Predicted to Regulate Host Anti-viral Gene Pathways in IPNV-Challenged Atlantic Salmon Fry Are Affected by Viral Load, and Associated with the Major IPN Resistance QTL Genotypes in Late Infection. Front. Immunol. 2020, 11, 2113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Li, Y.; Yin, J.; Ren, Y.; Shi, C.; Bergmann, S.M.; Zhu, X.; Zeng, W. Integrated analysis of mRNA-miRNA expression in Tilapia infected with Tilapia lake virus (TiLV) and identifies primarily immuneresponse genes. Fish Shellfish Immunol. 2020, 99, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Sun, L. Micro-Transcriptome Analysis Reveals Immune-Related MicroRNA Regulatory Networks of Paralichthys olivaceus Induced by Vibrio anguillarum Infection. Int. J. Mol. Sci. 2020, 21, 4252. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, Y.; Liu, H.; Yang, A.K.; Di, J.M.; Tan, G.M.; Wang, H.F.; Qiu, J.G.; Zhang, W.J.; Jiang, Q.W.; et al. MiR-194 functions as a tumor suppressor in laryngeal squamous cell carcinoma by targeting Wee1. J. Hematol. Oncol. 2017, 10, 32. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Wen, L.; Liu, Z.; Yan, F.; Gao, C. Identification and characterization of microRNAs in the spleen of common carp immune organ. J. Cell Biochem. 2014, 115, 1768–1778. [Google Scholar] [CrossRef]
- Wu, T.H.; Pan, C.Y.; Lin, M.C.; Hsieh, J.C.; Hui, C.F.; Chen, J.Y. In vivo screening of zebrafish microRNA responses to bacterial infection and their possible roles in regulating immune response genes after lipopolysaccharide stimulation. Fish. Physiol. Biochem. 2012, 38, 1299–1310. [Google Scholar] [CrossRef]
- Dinh, H.; Hong, Y.H.; Lillehoj, H.S. Modulation of microRNAs in two genetically disparate chicken lines showing different necrotic enteritis disease susceptibility. Vet. Immunol. Immunopathol. 2014, 159, 74–82. [Google Scholar] [CrossRef]
- Kong, L.; Sun, M.; Jiang, Z.; Li, L.; Lu, B. MicroRNA-194 Inhibits Lipopolysaccharide-Induced Inflammatory Response in Nucleus Pulposus Cells of the Intervertebral Disc by Targeting TNF Receptor-Associated Factor 6 (TRAF6). Med. Sci. Monit. 2018, 24, 3056–3067. [Google Scholar] [CrossRef]
- Wang, K.; Lai, C.; Gu, H.; Zhao, L.; Xia, M.; Yang, P.; Wang, X. miR-194 Inhibits Innate Antiviral Immunity by Targeting FGF2 in Influenza H1N1 Virus Infection. Front. Microbiol. 2017, 8, 2187. [Google Scholar] [CrossRef]
- Bao, C.; Li, Y.; Huan, L.; Zhang, Y.; Zhao, F.; Wang, Q.; Liang, L.; Ding, J.; Liu, L.; Chen, T.; et al. NF-kappaB signaling relieves negative regulation by miR-194 in hepatocellular carcinoma by suppressing the transcription factor HNF-1alpha. Sci. Signal 2015, 8, ra75. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Yan, Y.; Wang, H.; Bian, J.; Deng, Z.; Su, X.; Yang, Z.; Song, J. Hsa_circ_0001278 Facilitates Colorectal Cancer Progression via Sponging miR-338-5p and Regulating AMOTL1 Expression. Comb. Chem. High Throughput Screen. 2023, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Su, R.; Yao, F.; Peng, Y.; Luo, Q.; Li, J. Circulating circular RNAs hsa_circ_0001204 and hsa_circ_0001747 act as diagnostic biomarkers for active tuberculosis detection. Int. J. Clin. Exp. Pathol. 2018, 11, 586–594. [Google Scholar] [PubMed]
- Ma, X.; Wang, F.; Zhen, L.; Cai, Q. Hsa_circ_0001204 modulates inflammatory response of macrophages infected by Mycobacterium tuberculosis via TLR4/NF-kappaB signalling pathway. Clin. Exp. Pharmacol. Physiol. 2023, 50, 132–139. [Google Scholar] [CrossRef]
- Kimura, T.; Kumanogoh, A.; Okada, M. Roles of Lamtor1 in Macrophages, CD4+ T-cells, and Regulatory T-cells. Crit. Rev. Immunol. 2018, 38, 403–414. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, Z.; Sheng, Q.; Duan, W.; Zhao, H.; Zhou, J.; Deng, Q.; Pei, X. N-myristoyltransferase-1 deficiency blocks myristoylation of LAMTOR1 and inhibits bladder cancer progression. Cancer Lett. 2022, 529, 126–138. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Q.; Li, B.; Jiang, M. LAMTOR1 degrades MHC-II via the endocytic in hepatocellular carcinoma. Carcinogenesis 2022, 43, 1059–1070. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, H.; Zhang, Y.F.; Zhou, Z.; Wu, S. MicroRNA-29 enhances autophagy and cleanses exogenous mutant alphaB-crystallin in retinal pigment epithelial cells. Exp. Cell Res. 2019, 374, 231–248. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, N.; Peng, X.; Chen, L.; Meng, T.; Jiang, C.; Jin, J.; Zhang, J.; Duan, Q.; Tian, H.; et al. TRAF4-Mediated LAMTOR1 Ubiquitination Promotes mTORC1 Activation and Inhibits the Inflammation-Induced Colorectal Cancer Progression. Adv. Sci. 2024, 11, e2301164. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Y.; Zhu, S.; Li, F.; Xu, J.; Zhang, L.; Shu, H. circRNA circ_102049 Implicates in Pancreatic Ductal Adenocarcinoma Progression through Activating CD80 by Targeting miR-455-3p. Mediators Inflamm. 2021, 2021, 8819990. [Google Scholar] [CrossRef]
- Xu, X.W.; Zheng, B.A.; Hu, Z.M.; Qian, Z.Y.; Huang, C.J.; Liu, X.Q.; Wu, W.D. Circular RNA hsa_circ_000984 promotes colon cancer growth and metastasis by sponging miR-106b. Oncotarget 2017, 8, 91674–91683. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Li, M.; Hua, Q.; Shao, Y.; Zeng, H.; Liu, Y.; Diao, Q.; Zhang, H.; Qiu, M.; Zhu, J.; Li, X.; et al. Circular RNA circBbs9 promotes PM(2.5)-induced lung inflammation in mice via NLRP3 inflammasome activation. Environ. Int. 2020, 143, 105976. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Zeng, X.C.; Jiang, N.; Fu, B.S.; Guo, Y.; Yi, H.M.; Li, H.; Zhang, Q.; Chen, W.J.; et al. Down-regulation of microRNA-338-3p promoted angiogenesis in hepatocellular carcinoma. Biomed. Pharmacother. 2016, 84, 583–591. [Google Scholar] [CrossRef]
- Song, B.; Lin, H.X.; Dong, L.L.; Ma, J.J.; Jiang, Z.G. MicroRNA-338 inhibits proliferation, migration, and invasion of gastric cancer cells by the Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1290–1296. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Li, H.; Niu, X.; Liu, X.; Pei, D.; Guo, X.; Xu, X.; Li, Y. miR-338-3p inhibits the invasion of renal cell carcinoma by downregulation of ALK5. Oncotarget 2017, 8, 64106–64113. [Google Scholar] [CrossRef]
- Tong, Z.; Meng, X.; Wang, J.; Wang, L. MicroRNA-338-3p targets SOX4 and inhibits cell proliferation and invasion of renal cell carcinoma. Exp. Ther. Med. 2017, 14, 5200–5206. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Q.; Liao, P.; Zhang, P.; Sun, S.; Xu, Q. Mechanism of miR-338-3p in sepsis-induced acute lung injury via indirectly modulating ATF4. Transpl. Immunol. 2023, 76, 101681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, Y.; Liao, Q. Long noncoding RNA: A crosslink in biological regulatory network. Brief. Bioinform. 2018, 19, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; He, Y.; Lin, L.; Qi, Z.; Ma, L.; Li, L.; Su, Y. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour Biol. 2016, 37, 1683–1691. [Google Scholar] [CrossRef]
- Liu, G.; Wan, Q.; Li, J.; Hu, X.; Gu, X.; Xu, S. Circ_0038467 regulates lipopolysaccharide-induced inflammatory injury in human bronchial epithelial cells through sponging miR-338-3p. Thorac. Cancer 2020, 11, 1297–1308. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, C.; Wang, L. Knockdown of circ_0026579 ameliorates lipopolysaccharide (bacterial origin)-induced inflammatory injury in bronchial epithelium cells by targeting miR-338-3p/TBL1XR1 axis. Transpl. Immunol. 2022, 74, 101635. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Geng, Y.; Hu, C.; Wang, J.; Quan, Y.; Yu, T.; Zhou, X. Circ_0000419 acts as a miR-580 sponge to accelerate colorectal cancer progression via regulating DNMT3B. J. Biol. Regul. Homeost. Agents 2022, 36, 21–34. [Google Scholar]
- Li, S.; Sun, X.; Miao, S.; Lu, T.; Wang, Y.; Liu, J.; Jiao, W. hsa_circ_0000729, a potential prognostic biomarker in lung adenocarcinoma. Thorac. Cancer 2018, 9, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Wang, J.; Li, W.; Lu, Y.; Wu, X.; Long, X.; Luo, C.; Wei, H. hsa_circ_0000092 promotes hepatocellular carcinoma progression through up-regulating HN1 expression by binding to microRNA-338-3p. J. Cell Mol. Med. 2024, 28, e15010. [Google Scholar] [CrossRef]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef]
- Ishitani, T.; Ninomiya-Tsuji, J.; Nagai, S.; Nishita, M.; Meneghini, M.; Barker, N.; Waterman, M.; Bowerman, B.; Clevers, H.; Shibuya, H.; et al. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 1999, 399, 798–802. [Google Scholar] [CrossRef]
- Ishitani, T.; Hirao, T.; Suzuki, M.; Isoda, M.; Ishitani, S.; Harigaya, K.; Kitagawa, M.; Matsumoto, K.; Itoh, M. Nemo-like kinase suppresses Notch signalling by interfering with formation of the Notch active transcriptional complex. Nat. Cell Biol. 2010, 12, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kanei-Ishii, C.; Ninomiya-Tsuji, J.; Tanikawa, J.; Nomura, T.; Ishitani, T.; Kishida, S.; Kokura, K.; Kurahashi, T.; Ichikawa-Iwata, E.; Kim, Y.; et al. Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes. Dev. 2004, 18, 816–829. [Google Scholar] [CrossRef]
- Ohkawara, B.; Shirakabe, K.; Hyodo-Miura, J.; Matsuo, R.; Ueno, N.; Matsumoto, K.; Shibuya, H. Role of the TAK1-NLK-STAT3 pathway in TGF-beta-mediated mesoderm induction. Genes. Dev. 2004, 18, 381–386. [Google Scholar] [CrossRef]
- Kojima, H.; Sasaki, T.; Ishitani, T.; Iemura, S.; Zhao, H.; Kaneko, S.; Kunimoto, H.; Natsume, T.; Matsumoto, K.; Nakajima, K. STAT3 regulates Nemo-like kinase by mediating its interaction with IL-6-stimulated TGFbeta-activated kinase 1 for STAT3 Ser-727 phosphorylation. Proc. Natl. Acad. Sci. USA 2005, 102, 4524–4529. [Google Scholar] [CrossRef]
- Zeng, Y.A.; Rahnama, M.; Wang, S.; Sosu-Sedzorme, W.; Verheyen, E.M. Drosophila Nemo antagonizes BMP signaling by phosphorylation of Mad and inhibition of its nuclear accumulation. Development 2007, 134, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Ishitani, S.; Shimizu, N.; Matsumoto, K.; Itoh, M.; Ishitani, T. NLK positively regulates Wnt/beta-catenin signalling by phosphorylating LEF1 in neural progenitor cells. EMBO J. 2012, 31, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, C.; Wu, G.; Wang, Y.; Liu, R.; Yang, S.; He, S.; He, F.; Yuan, Q.; Huang, Y.; et al. Expression of NLK and its potential effect in ovarian cancer chemotherapy. Int. J. Gynecol. Cancer 2011, 21, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Emami, K.H.; Brown, L.G.; Pitts, T.E.; Sun, X.; Vessella, R.L.; Corey, E. Nemo-like kinase induces apoptosis and inhibits androgen receptor signaling in prostate cancer cells. Prostate 2009, 69, 1481–1492. [Google Scholar] [CrossRef]
- Li, S.Z.; Zhang, H.H.; Liang, J.B.; Song, Y.; Jin, B.X.; Xing, N.N.; Fan, G.C.; Du, R.L.; Zhang, X.D. Nemo-like kinase (NLK) negatively regulates NF-kappa B activity through disrupting the interaction of TAK1 with IKKbeta. Biochim. Biophys. Acta 2014, 1843, 1365–1372. [Google Scholar] [CrossRef][Green Version]
- Yu, T.; Zeng, Q.; Mao, H.; Liu, Y.; Zhang, H.; Wang, S.; Hu, C.; Xu, X. Grass carp (Ctenopharyngodon idella) NLK2 inhibits IFN I response through blocking MAVS-IRF3 axis. Fish Shellfish Immunol. 2022, 131, 206–217. [Google Scholar] [CrossRef]
- Kortenjann, M.; Wehrle, C.; Nehls, M.C.; Boehm, T. Only one nemo-like kinase gene homologue in invertebrate and mammalian genomes. Gene 2001, 278, 161–165. [Google Scholar] [CrossRef]
- Jones, S.E.; Jomary, C. Secreted Frizzled-related proteins: Searching for relationships and patterns. Bioessays 2002, 24, 811–820. [Google Scholar] [CrossRef]
- Esteve, P.; Lopez-Rios, J.; Bovolenta, P. SFRP1 is required for the proper establishment of the eye field in the medaka fish. Mech. Dev. 2004, 121, 687–701. [Google Scholar] [CrossRef]
- Pezeron, G.; Anselme, I.; Laplante, M.; Ellingsen, S.; Becker, T.S.; Rosa, F.M.; Charnay, P.; Schneider-Maunoury, S.; Mourrain, P.; Ghislain, J. Duplicate sfrp1 genes in zebrafish: sfrp1a is dynamically expressed in the developing central nervous system, gut and lateral line. Gene Expr. Patterns 2006, 6, 835–842. [Google Scholar] [CrossRef]
- Uren, A.; Reichsman, F.; Anest, V.; Taylor, W.G.; Muraiso, K.; Bottaro, D.P.; Cumberledge, S.; Rubin, J.S. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 2000, 275, 4374–4382. [Google Scholar] [CrossRef] [PubMed]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef]
- Thomson, J.M.; Newman, M.; Parker, J.S.; Morin-Kensicki, E.M.; Wright, T.; Hammond, S.M. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes. Dev. 2006, 20, 2202–2207. [Google Scholar] [CrossRef]
- Hu, B.; Sun, M.; Liu, J.; Hong, G.; Lin, Q. MicroRNA-204 suppressed proliferation and motility capacity of human hepatocellular carcinoma via directly targeting zinc finger E-box binding homeobox 2. Oncol. Lett. 2017, 13, 3823–3830. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Liu, X.; Yu, J.; Ban, T.; Zhang, Z.; Wang, P.; Huang, R.; Zheng, F.; Chang, Y.; Peng, W.; et al. Nuclear miR-204-3p mitigates metabolic dysfunction-associated steatotic liver disease in mice. J. Hepatol. 2024, 80, 834–845. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Chen, G.; Zhao, X. microRNA-204 modulates chemosensitivity and apoptosis of prostate cancer cells by targeting zinc-finger E-box-binding homeobox 1 (ZEB1). Am. J. Transl. Res. 2017, 9, 3599–3610. [Google Scholar]
- Canu, V.; Sacconi, A.; Lorenzon, L.; Biagioni, F.; Lo Sardo, F.; Diodoro, M.G.; Muti, P.; Garofalo, A.; Strano, S.; D’Errico, A.; et al. MiR-204 down-regulation elicited perturbation of a gene target signature common to human cholangiocarcinoma and gastric cancer. Oncotarget 2017, 8, 29540–29557. [Google Scholar] [CrossRef]
- Galasso, M.; Morrison, C.; Minotti, L.; Corra, F.; Zerbinati, C.; Agnoletto, C.; Baldassari, F.; Fassan, M.; Bartolazzi, A.; Vecchione, A.; et al. Loss of miR-204 expression is a key event in melanoma. Mol. Cancer 2018, 17, 71. [Google Scholar] [CrossRef]
- Ma, T.; Guo, J.; Han, J.; Li, L.; Ren, Y.; Huang, J.; Diao, G.; Zheng, X.; Zheng, Y. Circ_0001589/miR-1248/HMGB1 axis enhances EMT-mediated metastasis and cisplatin resistance in cervical cancer. Mol. Carcinog. 2023, 62, 1645–1658. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Liu, Q.; Wu, J.; Zheng, H.; Lin, B.; Huang, S. Circ_0001665 Contributes to the Occurrence of Vestibular Schwannoma via Targeting miR-302a-3p/Adam9/EGFR Signaling Pathway. Neuroscience 2022, 490, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Li, Y.; Feng, X.Z.; Li, D.B. Circular RNA circ_0001287 inhibits the proliferation, metastasis, and radiosensitivity of non-small cell lung cancer cells by sponging microRNA miR-21 and up-regulating phosphatase and tensin homolog expression. Bioengineered 2021, 12, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, P.; Yu, B.; Liu, J. The Circular RNA circXPO1 Promotes Tumor Growth via Sponging MicroRNA-23a in Prostate Carcinoma. Front. Oncol. 2021, 11, 712145. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, N.; Yu, H.; Zhang, X.; Cao, M.; Li, C. Construction and analysis of competing endogenous RNA ceRNA networks in the liver of black rockfish (Sebastes schlegelii) following Aeromonas salmonicida infection. Comp. Immunol. Rep. 2024, 6, 200124. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Fan, J.M.; Zhang, Z.M.; Ouyang, J.L.; Ni, T.T.; Wu, H.X.; Li, H. MicroRNA-204 targets signal transducer and activator of transcription 5 expression and inhibits proliferation of B-cell lymphoma cells. Mol. Med. Rep. 2015, 11, 4567–4572. [Google Scholar] [CrossRef]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef]
- Yanfeng, W.; Saint-Jeannet, J.P.; Klein, P.S. Wnt-frizzled signaling in the induction and differentiation of the neural crest. Bioessays 2003, 25, 317–325. [Google Scholar] [CrossRef]
- Slusarski, D.C.; Pelegri, F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev. Biol. 2007, 307, 1–13. [Google Scholar] [CrossRef]
- Kibardin, A.; Ossipova, O.; Sokol, S.Y. Metastasis-associated kinase modulates Wnt signaling to regulate brain patterning and morphogenesis. Development 2006, 133, 2845–2854. [Google Scholar] [CrossRef]
- Li, C.; Nguyen, V.; Clark, K.N.; Zahed, T.; Sharkas, S.; Filipp, F.V.; Boiko, A.D. Down-regulation of FZD3 receptor suppresses growth and metastasis of human melanoma independently of canonical WNT signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 4548–4557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, Y.; Wang, X.; Wang, J.; Xi, G. lncRNA SNHG10 Promotes the Proliferation and Invasion of Osteosarcoma via Wnt/beta-Catenin Signaling. Mol. Ther. Nucleic Acids 2020, 22, 957–970. [Google Scholar] [CrossRef]
- Kenneth, M.J.; Shishir, T.A.; Haque, F.K.M. In silico analysis reveals mir-98-5p as a potential inhibitor of tumor cell proliferation and metastasis in colorectal cancer by targeting the fzd3 receptor of the Wnt signaling pathway. J. Genet. Eng. Biotechnol. 2023, 21, 79. [Google Scholar] [CrossRef]
- Zhi, Y.; Du, P.; Li, Y.; Liu, H.; Jiang, T.; Zhao, X.; Li, X. SOX21-AS1 Augmented Cervical Cancer Growth by Triggering FZD3 to Activate the Wnt/beta-Catenin Signaling Pathway. Biochem. Genet. 2024. [Google Scholar] [CrossRef]
- Lamar, K.J.; Carvill, G.L. Chromatin Remodeling Proteins in Epilepsy: Lessons from CHD2-Associated Epilepsy. Front. Mol. Neurosci. 2018, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Ran, X.G.; Du, Y.Q.; Ren, F.; Tian, Y.; Wang, Y.; Chen, M.M. High CHD9 expression is associated with poor prognosis in clear cell renal cell carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 3697–3702. [Google Scholar] [PubMed]
- Xu, L.; Peng, H.; Huang, X.X.; Xia, Y.B.; Hu, K.F.; Zhang, Z.M. Decreased expression of chromodomain helicase DNA-binding protein 9 is a novel independent prognostic biomarker for colorectal cancer. Braz. J. Med. Biol. Res. 2018, 51, e7588. [Google Scholar] [CrossRef]
- Xia, T.; Pan, Z.; Zhang, J. CircPDZD8 promotes gastric cancer progression by regulating CHD9 via sponging miR-197-5p. Aging 2020, 12, 19352–19364. [Google Scholar] [CrossRef]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef]
- Kim, L.C.; Cook, R.S.; Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef]
- Rostamzadeh, D.; Yousefi, M.; Haghshenas, M.R.; Ahmadi, M.; Dolati, S.; Babaloo, Z. mTOR Signaling pathway as a master regulator of memory CD8+ T-cells, Th17, and NK cells development and their functional properties. J. Cell Physiol. 2019, 234, 12353–12368. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, L.A.; Murray, P.J. Proliferating Helper T Cells Require Rictor/mTORC2 Complex to Integrate Signals from Limiting Environmental Amino Acids. J. Biol. Chem. 2016, 291, 25815–25822. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Liu, H.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S. MHC II-PI(3)K/Akt/mTOR Signaling Pathway Regulates Intestinal Immune Response Induced by Soy Glycinin in Hybrid Grouper: Protective Effects of Sodium Butyrate. Front. Immunol. 2020, 11, 615980. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Li, M.; Chen, P.; Zhang, B.; Guo, H.; Cao, W.; Wei, X.; Cao, X.; Hao, X.; et al. mTOR complex component Rictor interacts with PKCzeta and regulates cancer cell metastasis. Cancer Res. 2010, 70, 9360–9370. [Google Scholar] [CrossRef]
- Li, H.; Lin, J.; Wang, X.; Yao, G.; Wang, L.; Zheng, H.; Yang, C.; Jia, C.; Liu, A.; Bai, X. Targeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancer. Breast Cancer Res. Treat. 2012, 134, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Marcais, A.; Blevins, R.; Graumann, J.; Feytout, A.; Dharmalingam, G.; Carroll, T.; Amado, I.F.; Bruno, L.; Lee, K.; Walzer, T.; et al. microRNA-mediated regulation of mTOR complex components facilitates discrimination between activation and anergy in CD4 T cells. J. Exp. Med. 2014, 211, 2281–2295. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef]
- Dancey, J. mTOR signaling and drug development in cancer. Nat. Rev. Clin. Oncol. 2010, 7, 209–219. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Yu, X.; Yuan, Z.; Yang, Y.; Liu, Y. Whole-Transcriptome Analysis Reveals Potential CeRNA Regulatory Mechanism in Takifugu rubripes against Cryptocaryon irritans Infection. Biology 2024, 13, 788. https://doi.org/10.3390/biology13100788
Xia Y, Yu X, Yuan Z, Yang Y, Liu Y. Whole-Transcriptome Analysis Reveals Potential CeRNA Regulatory Mechanism in Takifugu rubripes against Cryptocaryon irritans Infection. Biology. 2024; 13(10):788. https://doi.org/10.3390/biology13100788
Chicago/Turabian StyleXia, Yuqing, Xiaoqing Yu, Zhen Yuan, Yi Yang, and Ying Liu. 2024. "Whole-Transcriptome Analysis Reveals Potential CeRNA Regulatory Mechanism in Takifugu rubripes against Cryptocaryon irritans Infection" Biology 13, no. 10: 788. https://doi.org/10.3390/biology13100788
APA StyleXia, Y., Yu, X., Yuan, Z., Yang, Y., & Liu, Y. (2024). Whole-Transcriptome Analysis Reveals Potential CeRNA Regulatory Mechanism in Takifugu rubripes against Cryptocaryon irritans Infection. Biology, 13(10), 788. https://doi.org/10.3390/biology13100788





