The Rice YL4 Gene Encoding a Ribosome Maturation Domain Protein Is Essential for Chloroplast Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
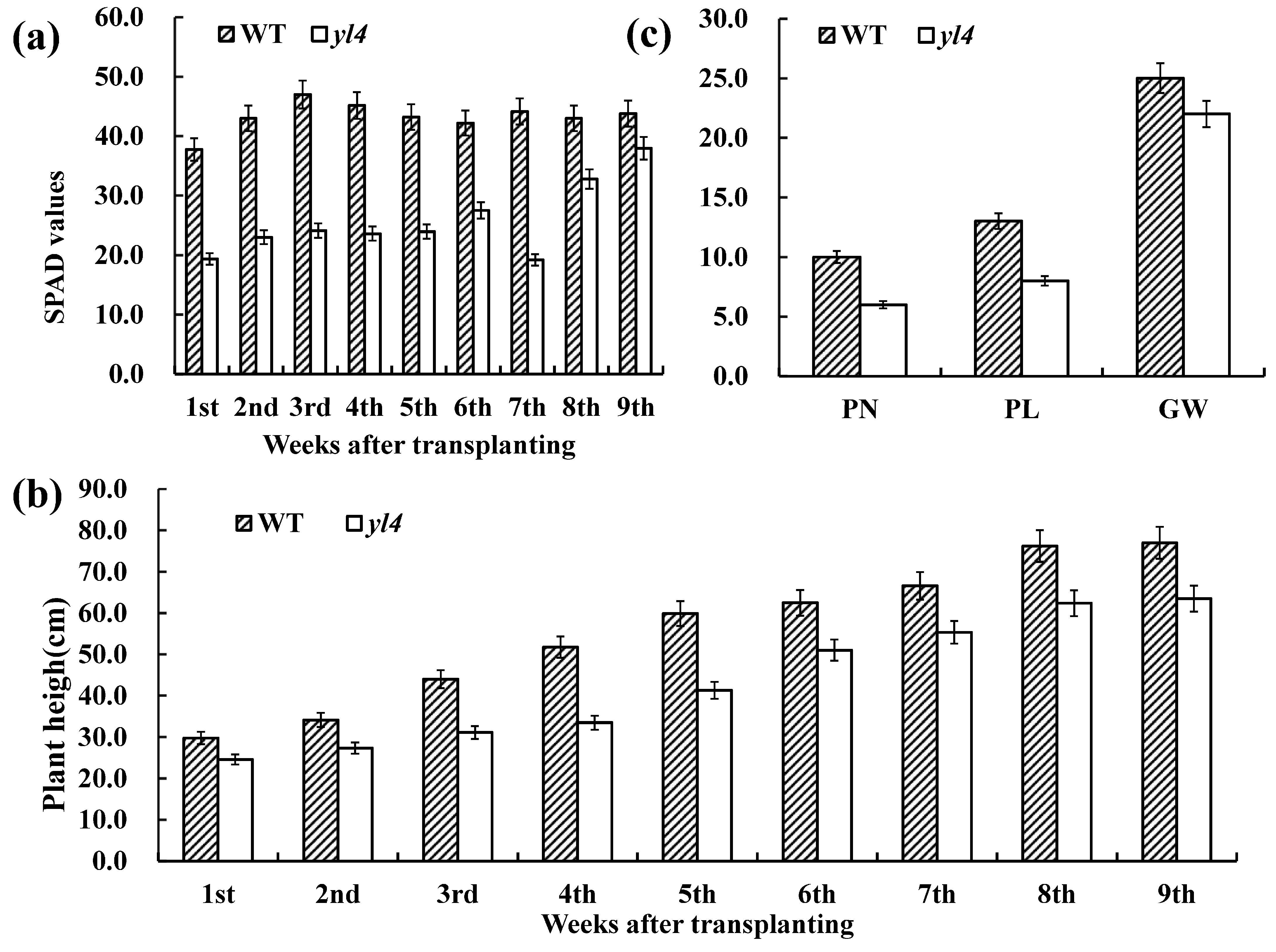
2.2. Phenotype Observation and Photosynthetic Pigment Measurements
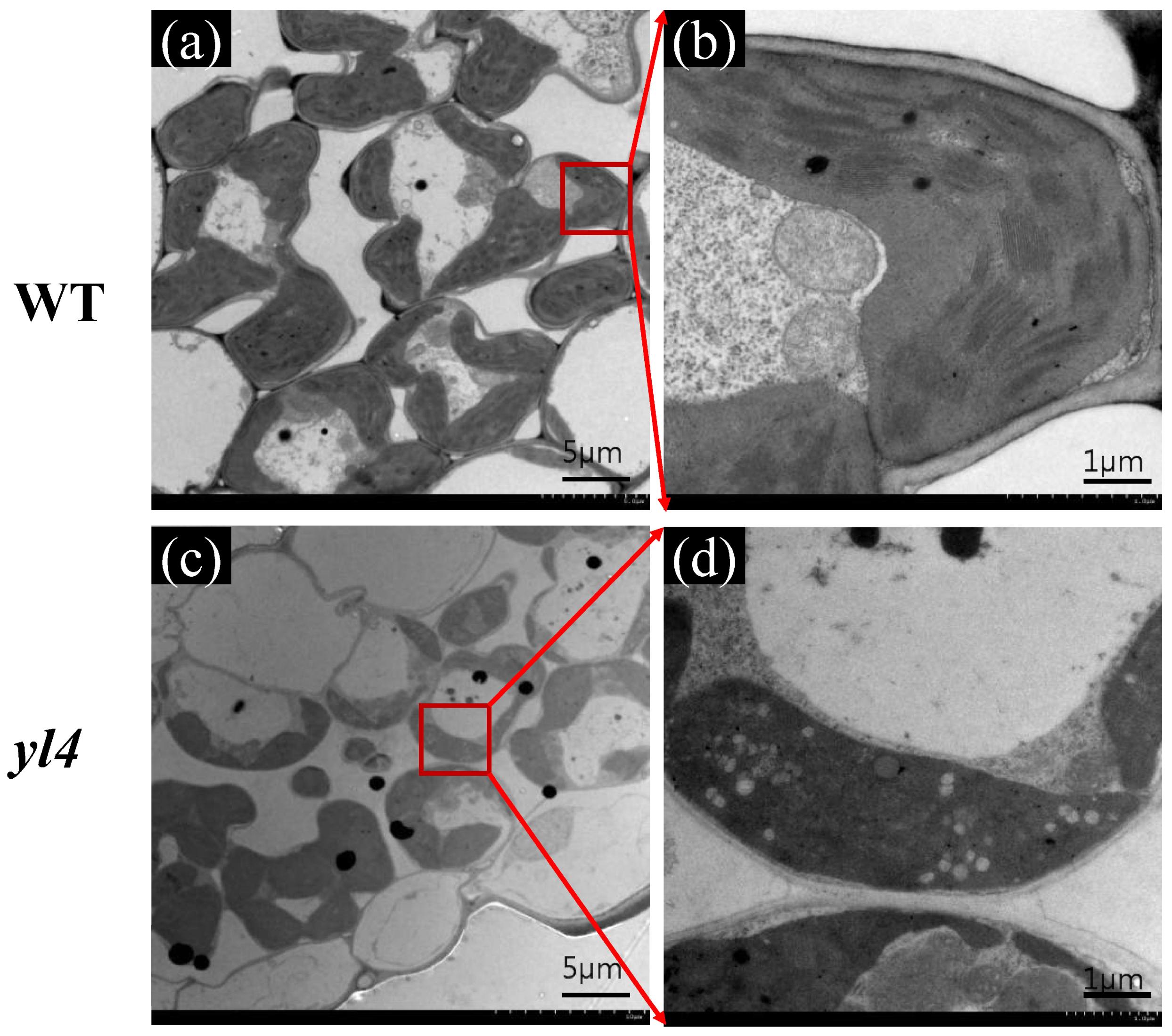
2.3. Transmission Electron Microscopy (TEM) Analysis
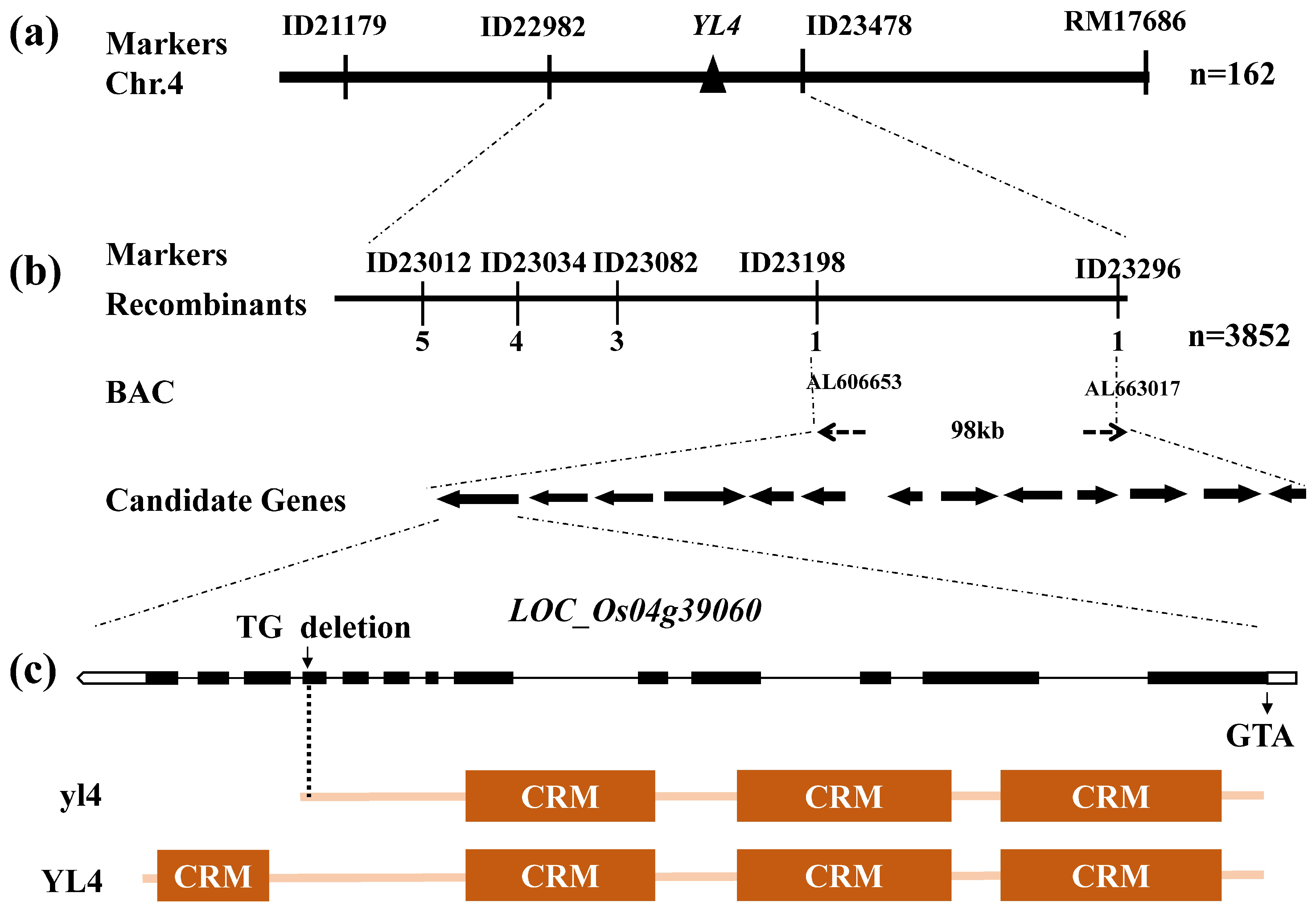
2.4. Map-Based Cloning of YL4
2.5. Knockout of YL4
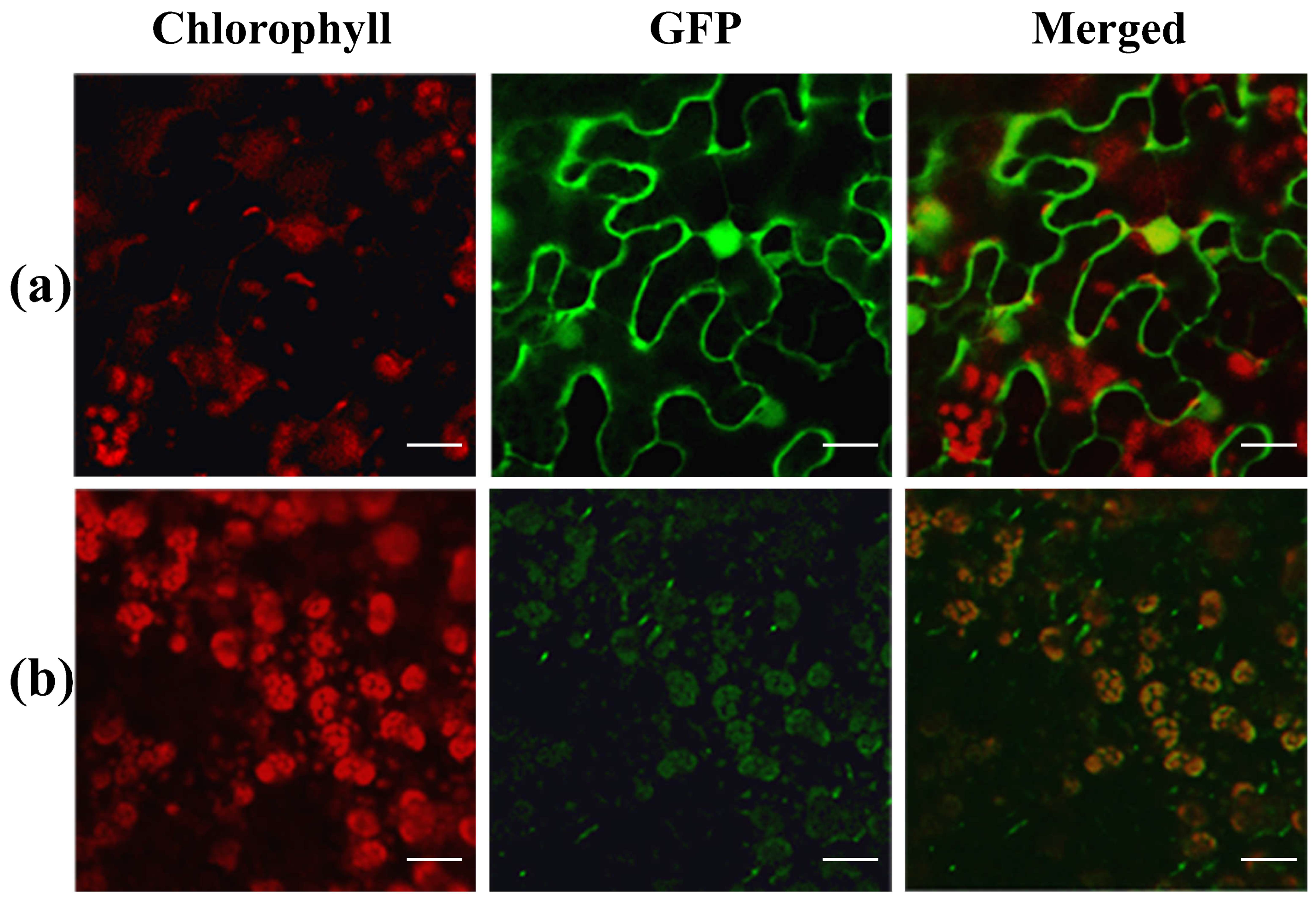
2.6. Subcellular Localization of YL4
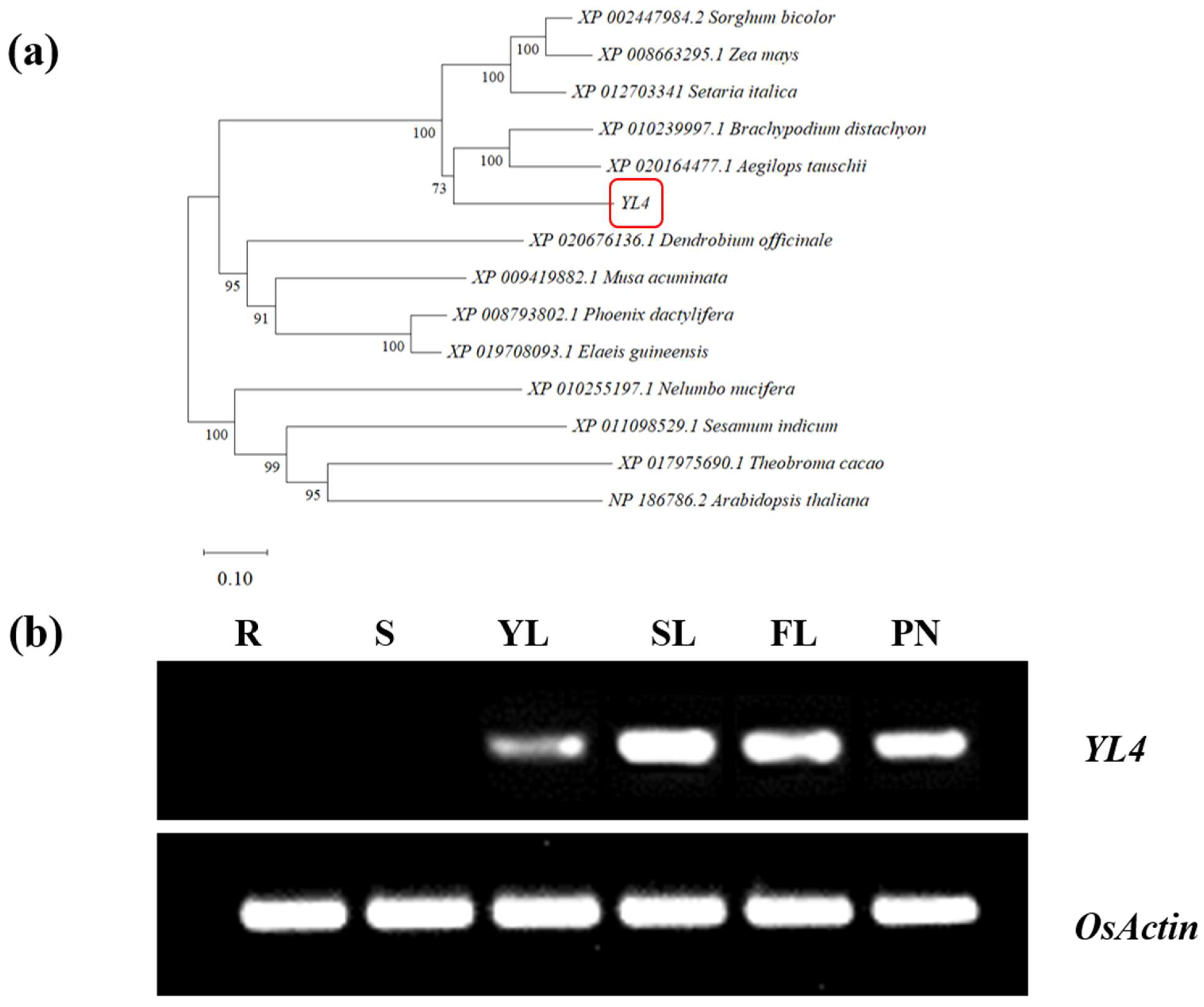
2.7. Sequence Alignment and Phylogenetic Analysis
2.8. RNA Extraction, RT-PCR, and Quantitative Real-Time PCR
3. Results
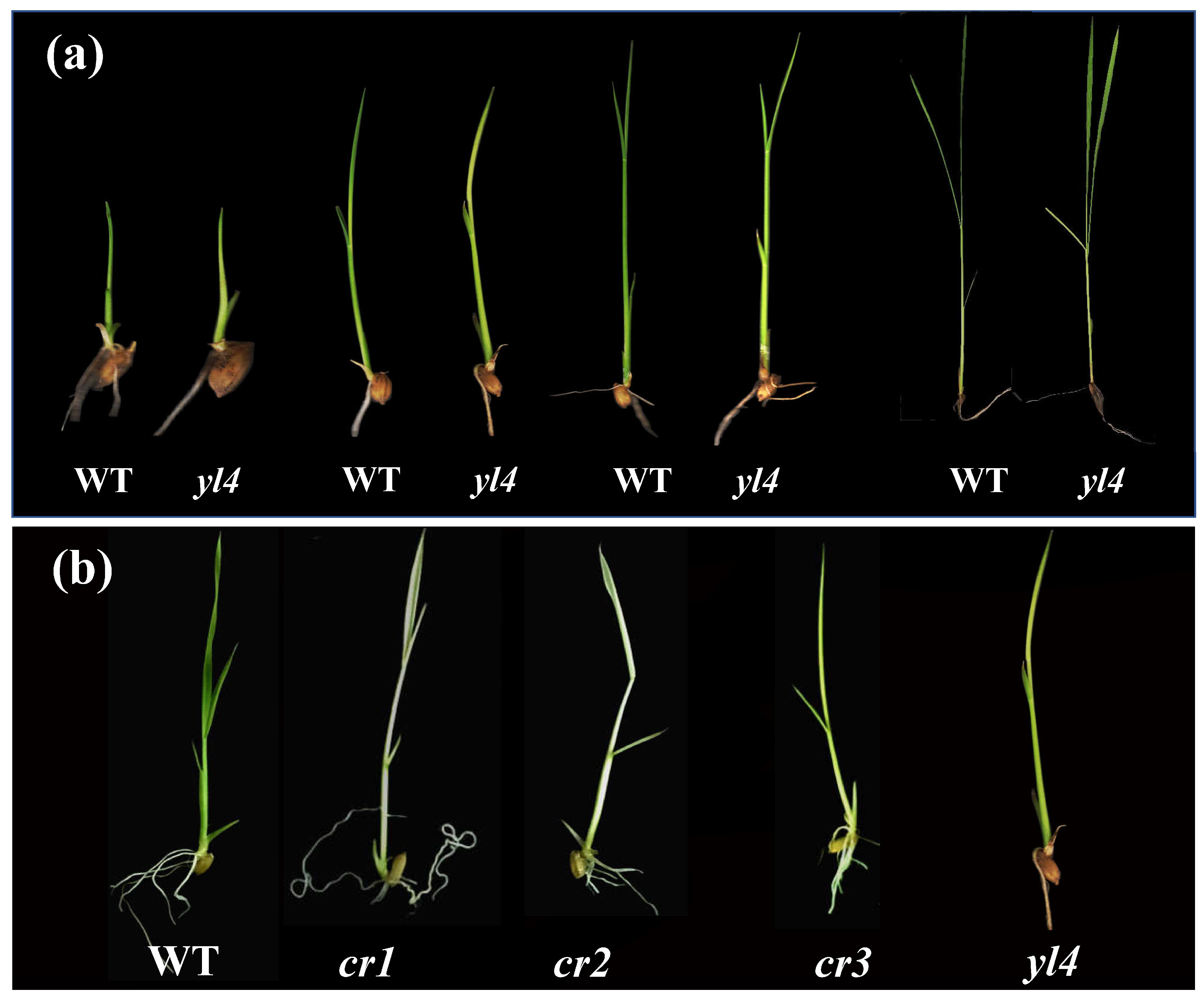
3.1. Characterization of the yl4 Mutant
3.2. Map-Based Cloning of YL4
3.3. Characterization of YL4 Protein
3.4. Expression Pattern and Subcellular Localization of YL4
3.5. The Transcript Expression of Related Genes in the yl4 Mutants
4. Discussion
4.1. YL4 Acts during the First Step of Chloroplast Development
4.2. Multiple Functions of YL4 in Chloroplast Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yagi, Y.; Shiina, T. Recent advances in the study of chloroplast gene expression and its evolution. Front. Plant Sci. 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Börner, T.; Aleynikova, A.Y.; Zubo, Y.O.; Kusnetsov, V.V. Chloroplast RNA polymerases: Role in chloroplast biogenesis. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M. New Perspectives on Chloroplast Protein Import. Plant Cell Physiol. 2018, 59, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- De Longevialle, A.F.; Small, I.D.; Lurin, C. Nuclearly Encoded Splicing Factors Implicated in RNA Splicing in Higher Plant Organelles. Mol. Plant 2010, 3, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, R.; Mohr, G.; Belfort, M.; Lambowitz, A.M. Group I and group II introns. FASEB J. 1993, 7, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ostheimer, G.J.; Williams Carrier, R.; Belcher, S.; Osborne, E.; Gierke, J.; Barkan, A. Group II intron splicing factors derived by diversification of an ancient RNA-binding domain. EMBO J. 2003, 22, 3919–3929. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Small, I. Pentatricopeptide Repeat Proteins in Plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Schmitz Linneweber, C.; Till, B.; Williams Carrier, R. CRS1 is a novel group II intron splicing factor that was derived from a domain of ancient origin. RNA 2001, 7, 1227–1238. [Google Scholar] [CrossRef]
- Ostersetzer, O.; Cooke, A.M.; Watkins, K.P.; Barkan, A. CRS1, a Chloroplast Group II Intron Splicing Factor, Promotes Intron Folding through Specific Interactions with Two Intron Domains. Plant Cell 2005, 17, 241–255. [Google Scholar] [CrossRef]
- Asakura, Y.; Barkan, A. A CRM Domain Protein Functions Dually in Group I and Group II Intron Splicing in Land Plant Chloroplasts. Plant Cell 2007, 19, 3864–3875. [Google Scholar] [CrossRef]
- Barkan, A.; Klipcan, L.; Ostersetzer, O.; Kawamura, T.; Asakura, Y.; Watkins, K.P. The CRM domain: An RNA binding module derived from an ancient ribosome-associated protein. RNA 2007, 13, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.B.; Clermont, M.G.; Hanson, M.R. Chloroplast RNA Metabolism. Annu. Rev. Plant Biol. 2010, 61, 125–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shen, L.; Ren, D.; Hu, J.; Zhu, L.; Gao, Z.; Zhang, G.; Guo, L.; Zeng, D.; Qian, Q. Characterization of the CRM Gene Family and Elucidating the Function of OsCFM2 in Rice. Biomolecules 2020, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, H.J.; Kim, D.H.; Jeon, Y.; Pai, H.-S.; Kang, H. A nuclear-encoded chloroplast protein harboring a single CRM domain plays an important role in the Arabidopsis growth and stress response. BMC Plant Biol. 2014, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, S.J.; Park, Y.-I.; Kang, H. CFM9, a Mitochondrial CRM Protein, Is Crucial for Mitochondrial Intron Splicing, Mitochondria Function and Arabidopsis Growth and Stress Responses. Plant Cell Physiol. 2019, 60, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Keren, I.; Klipcan, L.; Bezawork-Geleta, A.; Kolton, M.; Shaya, F.; Ostersetzer-Biran, O. Characterization of the Molecular Basis of Group II Intron RNA Recognition by CRS1-CRM Domains. J. Biol. Chem. 2008, 283, 23333–23342. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Laza, M.R.C.; Garcia, F.V.; Cassman, K.G. Chlorophyll meter estimates leaf area-based nitrogen concentration of rice. Commun. Soil Sci. Plant Anal. 1995, 26, 927–935. [Google Scholar] [CrossRef]
- Yuan, Z.; Ata Ul Karim, S.T.; Cao, Q.; Lu, Z.; Cao, W.; Zhu, Y.; Liu, X. Indicators for diagnosing nitrogen status of rice based on chlorophyll meter readings. Field Crops Res. 2016, 185, 12–20. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Goff, S.A.; Ricke, D.; Lan, T.H.; Presting, G.; Wang, R.; Dunn, M.; Glazebrook, J.; Sessions, A.; Oeller, P.; Varma, H.; et al. A Draft Sequence of the Rice Genome (Oryza sativa L. ssp. japonica). Science 2002, 296, 92–100. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Y.; Tan, J.; Hu, S.; Yu, J.; Xue, Q. A Genome-wide Microsatellite Polymorphism Database for the Indica and Japonica Rice. DNA Res. 2007, 14, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, S.; Wang, J.; Li, S.; Wong, K.-S.G.; Liu, B.; Deng, Y.; Dai, L.; Zhou, Y.; Zhang, X.; et al. A draft sequence of the rice (Oryza sativa ssp. indica) genome. Chin. Sci. Bull. 2001, 46, 1937–1942. [Google Scholar] [CrossRef]
- McCouch, S.R.; Teytelman, L.; Xu, Y.; Lobos, K.B.; Clare, K.; Walton, M.; Fu, B.; Maghirang, R.; Li, Z.; Xing, Y.; et al. Development and Mapping of 2240 New SSR Markers for Rice (Oryza sativa L.). DNA Res. 2002, 9, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, H.J.; Yu, J.; Bae, S.; Cho, Y.G.; Kang, K.K. Transcriptomic and physiological analysis of OsCAO1 knockout lines using the CRISPR/Cas9 system in rice. Plant Cell Rep. 2021, 40, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, J.j.; Wang, Y.y.; Gu, J.w.; Xie, X.z. Positive Regulation of Phytochrome B on Chlorophyll Biosynthesis and Chloroplast Development in Rice. Rice Sci. 2013, 20, 243–248. [Google Scholar] [CrossRef]
- Kyozuka, J.; McElroy, D.; Hayakawa, T.; Xie, Y.; Wu, R.; Shimamoto, K. Light-Regulated and Cell-Specific Expression of Tomato rbcS-gusA and Rice rbcS-gusA Fusion Genes in Transgenic Rice. Plant Physiol. 1993, 102, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Dietzel, L.; Schröter, Y.; Fey, V.; Wagner, R.; Pfannschmidt, T. The Role of Phosphorylation in Redox Regulation of Photosynthesis Genes psaA and psbA during Photosynthetic Acclimation of Mustard. Mol. Plant 2009, 2, 416–429. [Google Scholar] [CrossRef]
- Kusumi, K.; Sakata, C.; Nakamura, T.; Kawasaki, S.; Yoshimura, A.; Iba, K. A plastid protein NUS1 is essential for build-up of the genetic system for early chloroplast development under cold stress conditions. Plant J. 2011, 68, 1039–1050. [Google Scholar] [CrossRef]
- Yoo, S.C.; Cho, S.H.; Sugimoto, H.; Li, J.; Kusumi, K.; Koh, H.J.; Iba, K.; Paek, N.C. Rice Virescent3 and Stripe1 Encoding the Large and Small Subunits of Ribonucleotide Reductase Are Required for Chloroplast Biogenesis during Early Leaf Development. Plant Physiol. 2009, 150, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Little, M.C.; Hallick, R.B. Chloroplast rpoA, rpoB, and rpoC genes specify at least three components of a chloroplast DNA-dependent RNA polymerase active in tRNA and mRNA transcription. J. Biol. Chem. 1988, 263, 14302–14307. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.H.; Boynton, J.E.; Gillham, N.W. Chloroplast ribosomes and protein synthesis. Microbiol. Rev. 1994, 58, 700–754. [Google Scholar] [CrossRef] [PubMed]
- Vitha, S.; McAndrew, R.S.; Osteryoung, K.W. Ftsz Ring Formation at the Chloroplast Division Site in Plants. J. Cell Biol. 2001, 153, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Kusumi, K.; Noguchi, K.; Yano, M.; Yoshimura, A.; Iba, K. The rice nuclear gene, VIRESCENT 2, is essential for chloroplast development and encodes a novel type of guanylate kinase targeted to plastids and mitochondria. Plant J. 2007, 52, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Leister, D. Chloroplast research in the genomic age. Trends Genet. 2003, 19, 47–56. [Google Scholar] [CrossRef]
- Kusumi, K.; Iba, K. Establishment of the chloroplast genetic system in rice during early leaf development and at low temperatures. Front. Plant Sci. 2014, 5, 386. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liu, Y.; Zhang, Y.; Lin, D.; Pan, X.; Dong, Y. The Rice YL4 Gene Encoding a Ribosome Maturation Domain Protein Is Essential for Chloroplast Development. Biology 2024, 13, 580. https://doi.org/10.3390/biology13080580
Sun Y, Liu Y, Zhang Y, Lin D, Pan X, Dong Y. The Rice YL4 Gene Encoding a Ribosome Maturation Domain Protein Is Essential for Chloroplast Development. Biology. 2024; 13(8):580. https://doi.org/10.3390/biology13080580
Chicago/Turabian StyleSun, Yunguang, Yanxia Liu, Youze Zhang, Dongzhi Lin, Xiaobiao Pan, and Yanjun Dong. 2024. "The Rice YL4 Gene Encoding a Ribosome Maturation Domain Protein Is Essential for Chloroplast Development" Biology 13, no. 8: 580. https://doi.org/10.3390/biology13080580
APA StyleSun, Y., Liu, Y., Zhang, Y., Lin, D., Pan, X., & Dong, Y. (2024). The Rice YL4 Gene Encoding a Ribosome Maturation Domain Protein Is Essential for Chloroplast Development. Biology, 13(8), 580. https://doi.org/10.3390/biology13080580






