Simulating Habitat Suitability Changes of Threadfin Porgy (Evynnis cardinalis) in the Northern South China Sea Using Ensemble Models Under Medium-to-Long-Term Future Climate Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.1.1. Occurrence Data of E. cardinalis
2.1.2. Environmental Data Sources
2.2. Model Construction and Simulation of Potential Habitats of E. cardinalis
2.2.1. Selection and Construction of Single Models
2.2.2. Selection and Construction of Ensemble Models
2.2.3. Evaluation of the Accuracy of Single and Ensemble Models
2.2.4. Alterations in the Habitat Within Future Climate Scenarios for the Projection of E. cardinalis
3. Results
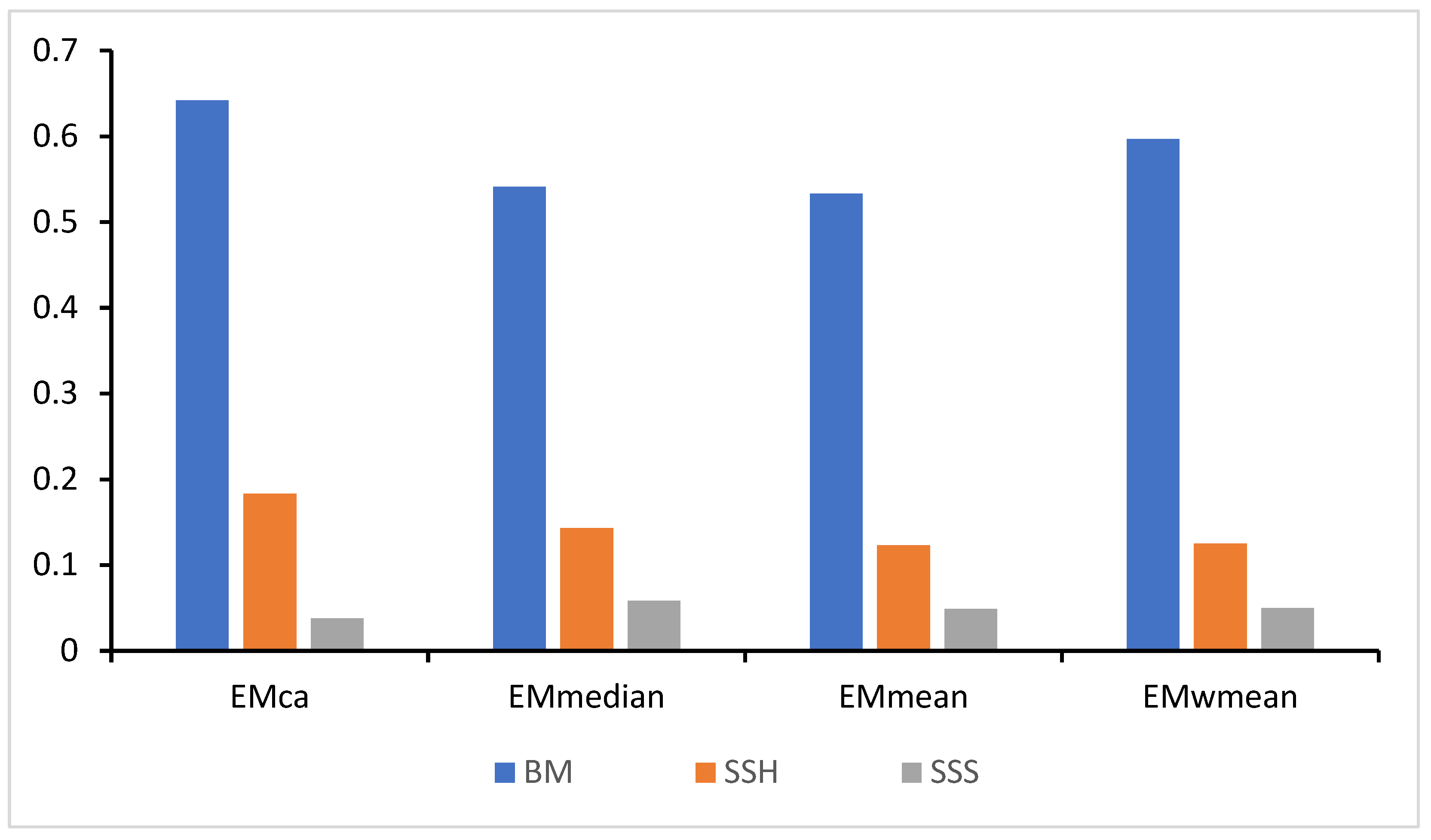
3.1. Evaluation of Single and Ensemble Models Performance
3.1.1. Assessment of the Performance of Single Models
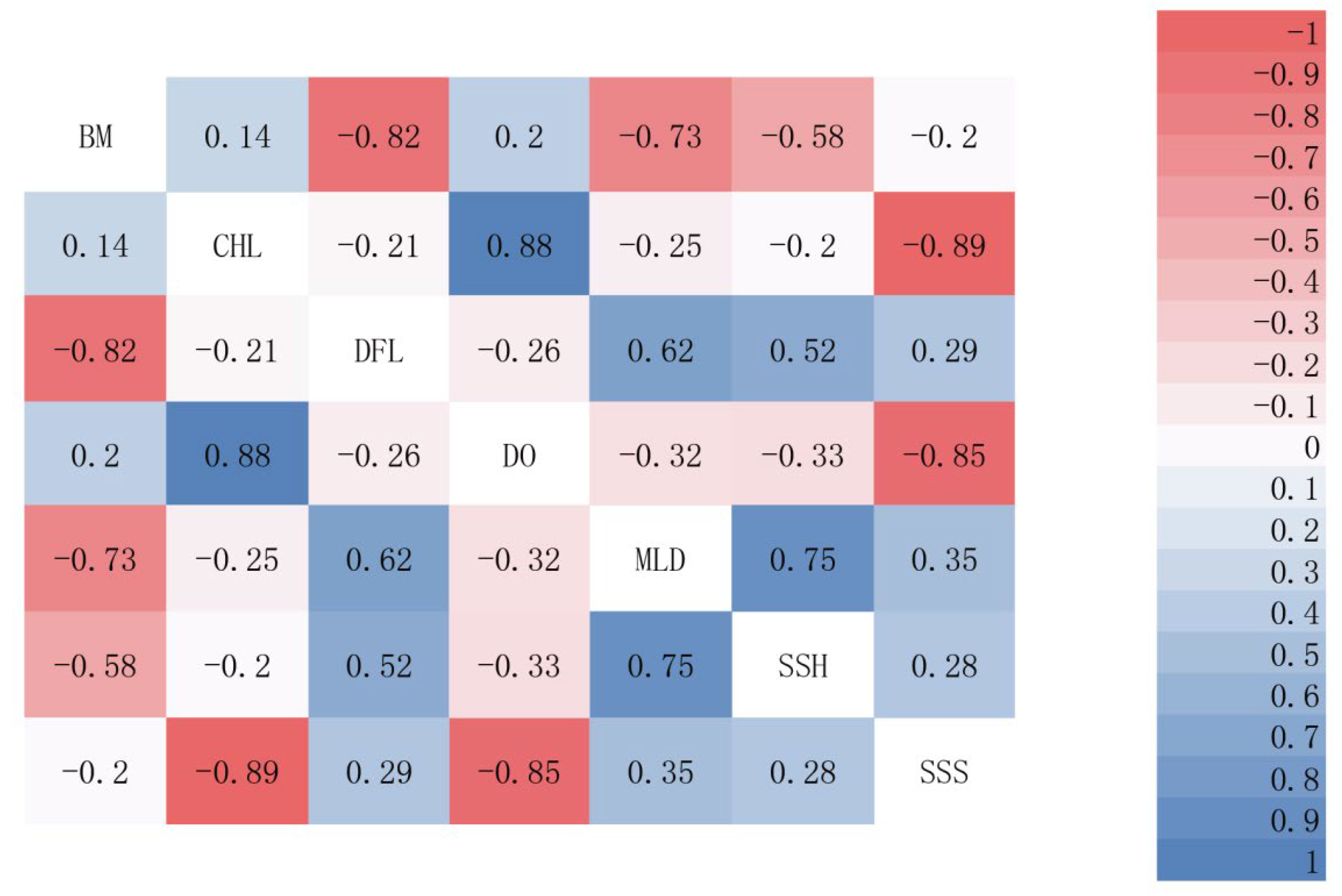
3.1.2. Evaluation of the Significance of Environmental Factors
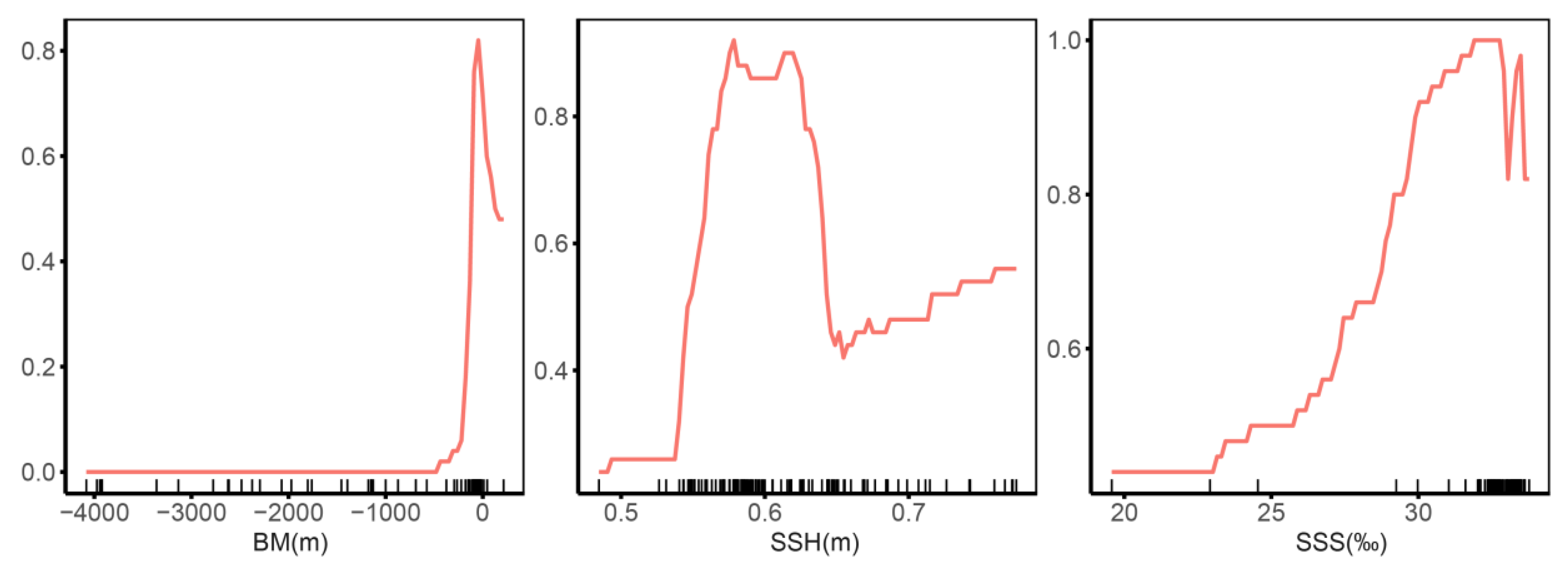
3.2. Contributions of Environmental Variables to Models and Response Curves
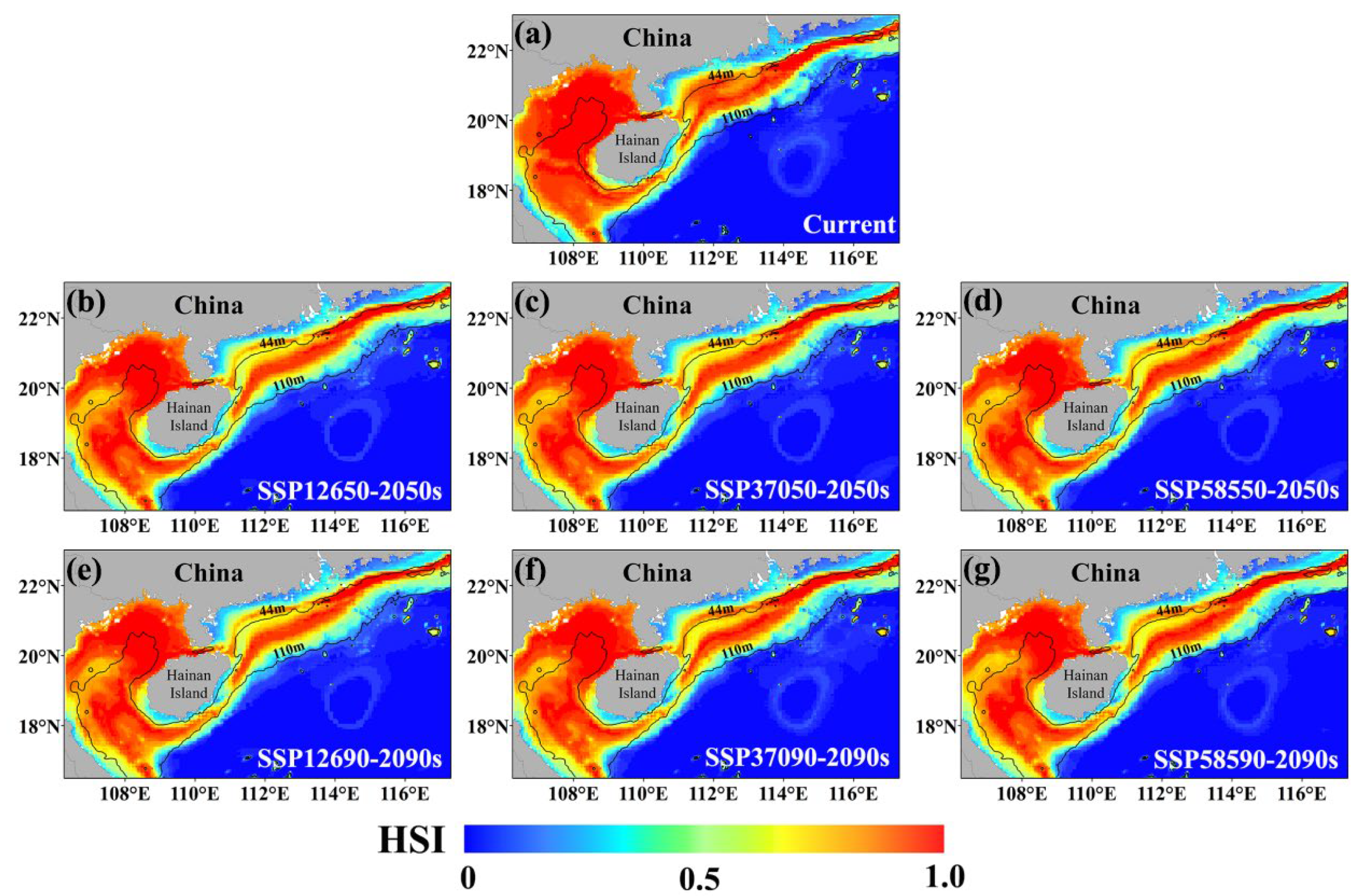
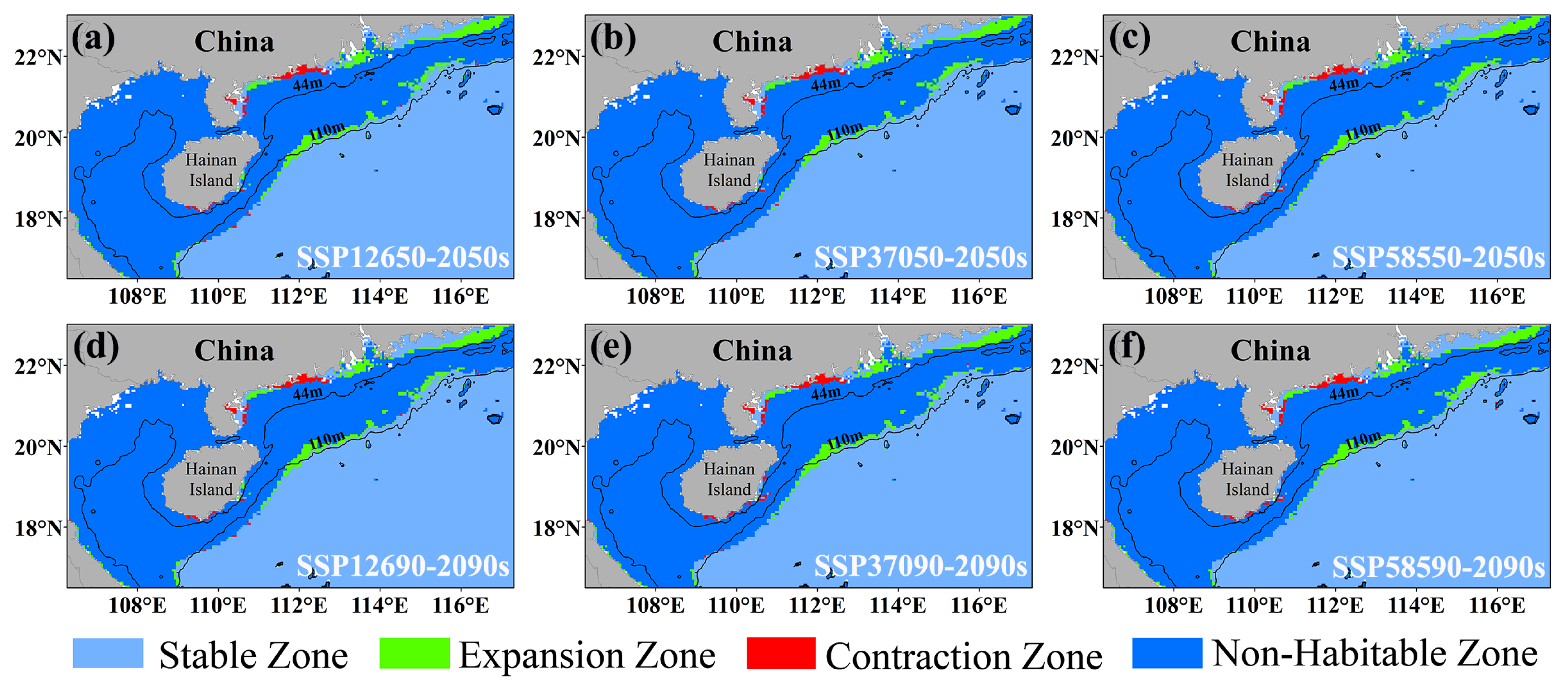
3.3. Distribution of the Current Habitat and Alterations in Future Habitat for E. cardinalis
4. Discussion
4.1. Accuracy Accessment of the Model and Present Distribution of E. cardinalis Habitats
4.2. The Influence of Future Climate Scenarios on the Habitat of E. cardinalis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciardini, V.; Contessa, G.M.; Falsaperla, R.; Gómez-Amo, J.L.; Meloni, D.; Monteleone, F.; Pace, G.; Piacentino, S.; Sferlazzo, D.; di Sarra, A. Global and Mediterranean climate change: A short summary. Ann. Dell’ist. Super. Sanita 2016, 52, 325–337. [Google Scholar] [CrossRef]
- Zhu, Q.; Guo, H.D.; Zhang, L.; Liang, D.; Wu, Z.R.; Liu, Y.M.; Dou, X.Y.; Du, X.B. Analysis of continuous calving front retreat and the associated influencing factors of the Thwaites Glacier using high-resolution remote sensing data from 2015 to 2023. Int. J. Digit. Earth 2024, 17, 2390438. [Google Scholar] [CrossRef]
- Qu, Y.; Jevrejeva, S.; Jackson, L.P.; Moore, J.C. Coastal Sea level rise around the China Seas. Glob. Planet. Change 2019, 172, 454–463. [Google Scholar] [CrossRef]
- Lee, S.; Park, M.S.; Kwon, M.; Park, Y.G.; Kim, Y.H.; Choi, N. Rapidly Changing East Asian Marine Heatwaves Under a Warming Climate. J. Geophys. Res. Ocean. 2023, 128, e2023JC019761. [Google Scholar] [CrossRef]
- Munday, P.L. New perspectives in ocean acidification research: Editor’s introduction to the special feature on ocean acidification. Biol. Lett. 2017, 13, 20170438. [Google Scholar] [CrossRef]
- Stelmakh, L.; Gorbunova, T. Effect of phytoplankton adaptation on the distribution of its biomass and chlorophyll a concentration in the surface layer of the Black Sea. Oceanol. Hydrobiol. Stud. 2019, 48, 404–414. [Google Scholar] [CrossRef]
- McCormick, L.R.; Levin, L.A. Physiological and ecological implications of ocean deoxygenation for vision in marine organisms. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2017, 375, 20160322. [Google Scholar] [CrossRef]
- Reynolds, S.D.; Franklin, C.E.; Norman, B.M.; Richardson, A.J.; Everett, J.D.; Schoeman, D.S.; White, C.R.; Lawson, C.L.; Pierce, S.J.; Rohner, C.A.; et al. Effects of climate warming on energetics and habitat of the world’s largest marine ectotherm. Sci. Total Environ. 2024, 951, 175832. [Google Scholar] [CrossRef]
- Xiong, P.L.; Cai, Y.C.; Jiang, P.W.; Xu, Y.W.; Sun, M.S.; Fan, J.T.; Chen, Z.Z. Impact of climate change on the distribution of Trachurus japonicus in the Northern South China Sea. Ecol. Indic. 2024, 160, 111758. [Google Scholar] [CrossRef]
- Free, C.M.; Thorson, J.T.; Pinsky, M.L.; Oken, K.L.; Wiedenmann, J.; Jensen, O.P. Impacts of historical warming on marine fisheries production. Science 2019, 363, 979–983. [Google Scholar] [CrossRef]
- Hu, W.J.; Du, J.G.; Su, S.K.; Tan, H.J.; Yang, W.; Ding, L.K.; Dong, P.; Yu, W.W.; Zheng, X.Q.; Chen, B. Effects of climate change in the seas of China: Predicted changes in the distribution of fish species and diversity. Ecol. Indic. 2022, 134, 108489. [Google Scholar] [CrossRef]
- Jones, M.C.; Cheung, W.W.L. Multi-model ensemble projections of climate change effects on global marine biodiversity. ICES J. Mar. Sci. 2015, 72, 741–752. [Google Scholar] [CrossRef]
- Genner, M.J.; Sims, D.W.; Southward, A.J.; Budd, G.C.; Masterson, P.; McHugh, M.; Rendle, P.; Southall, E.J.; Wearmouth, V.J.; Hawkins, S.J. Body size-dependent responses of a marine fish assemblage to climate change and fishing over a century-long scale. Glob. Change Biol. 2010, 16, 517–527. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The Impact of Climate Change on the World’s Marine Ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, C.L.; Zou, X.Q.; Feng, Z.Y.; Yao, Y.L.; Wang, T.; Zhang, C.C. Occurrence of polycyclic aromatic hydrocarbons (PAHs) in coral reef fish from the South China Sea. Mar. Pollut. Bull. 2019, 139, 339–345. [Google Scholar] [CrossRef]
- Xu, Y.W.; Zhang, P.; Panhwar, S.K.; Li, J.; Yan, L.; Chen, Z.Z.; Zhang, K. The initial assessment of an important pelagic fish, Mackerel Scad, in the South China Sea using data-poor length-based methods. Mar. Coast. Fish. 2023, 15, e210258. [Google Scholar] [CrossRef]
- Diao, C.Y.; Jia, H.; Guo, S.J.; Hou, G.; Xian, W.W.; Zhang, H. Biodiversity exploration in autumn using environmental DNA in the South China sea. Environ. Res. 2022, 204, 112357. [Google Scholar] [CrossRef]
- Qiu, Y.S.; Lin, Z.J.; Wang, Y.Z. Responses of fish production to fishing and climate variability in the northern South China Sea. Prog. Oceanogr. 2010, 85, 197–212. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.J.; Hou, G.; Huang, Z.R.; Shi, D.F.; Chen, Z.Z.; Qiu, Y.S. Length-Based Assessment of Fish Stocks in a Data-Poor, Jointly Exploited (China and Vietnam) Fishing Ground, Northern South China Sea. Front. Mar. Sci. 2021, 8, 718052. [Google Scholar] [CrossRef]
- Teng, W.; Chunhou, L.; Yong, L.; Ren, Z. Biodiversity and Conservation of Fish in the Beibu Gulf. Pak. J. Zool. 2024, 56, 429–490. [Google Scholar] [CrossRef]
- Peng, S.; Wang, X.H.; Du, F.Y.; Sun, D.R.; Wang, Y.Z.; Chen, P.M.; Qiu, Y.S. Variations in fish community composition and trophic structure under multiple drivers in the Beibu Gulf. Front. Mar. Sci. 2023, 10, 1159602. [Google Scholar] [CrossRef]
- Yan, Y.R.; Hou, G.; Chen, J.L.; Lu, H.S.; Jin, X.S. Feeding ecology of hairtail Trichiurus margarites and largehead hairtail Trichiurus lepturus in the Beibu Gulf, the South China Sea. Chin. J. Oceanol. Limnol. 2011, 29, 174–183. [Google Scholar] [CrossRef]
- Du, J.G.; Lu, Z.B.; Chen, M.R. Changes in ecological parameters of Parargyrops edita population in southern Taiwan Strait. J. Oceanogr. Taiwan Strait 2008, 27, 190–196. [Google Scholar]
- Ye, S.Z. The Distribution in Time and Space of Parargyrops edita Tanaka Population in Minnan-Taiwan Bank Fishing Ground. J. Fujian Fish. 2004, 4, 36–39. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Qiu, Y.S. Esitimation of growth and mortality parameters of Parargyrops edita Tanaka in Beibu Bay. J. Fish. China 2003, 27, 251–257. [Google Scholar]
- Chen, Z.Z.; Qiu, Y.S. Stock variation of Parargyrops edita Tanaka in Beibu Gulf. S. China Fish. Sci. 2005, 1, 26–31. [Google Scholar]
- Zhang, Y.M.; Dai, C.T.; Yan, Y.R.; Yang, Y.L.; Lu, H.S. Feeding habits and trophic level of crimson sea bream, (Parargyrops edita Tanaka) in the Beibu Gulf. J. Fish. China 2014, 38, 265–273. [Google Scholar]
- Zhang, Q.Y.; Yang, G.L. Study on feeding habits of lizard fishes in south FuJian and TaiWan bank fishing ground. J. Fish. China 1986, 2, 213–222. [Google Scholar]
- Hou, G.; Feng, Y.T.; Chen, Y.Y.; Wang, J.R.; Wang, J.S.; Zhao, H. Spatiotemporal Distribution of Threadfin Porgy Evynnis cardinalis in Beibu Gulf and Its Relationship with Environmental Factors. J. Guangdong Ocean. Univ. 2021, 41, 8–16. [Google Scholar]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.T.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Xiong, P.L.; Xu, Y.W.; Sun, M.S.; Zhou, X.X.; Jiang, P.W.; Chen, Z.Z.; Fan, J.T. The current and future seasonal geographic distribution of largehead hairtail Trichiurus japonicus in the Beibu Gulf, South China Sea. Front. Mar. Sci. 2023, 9, 14. [Google Scholar] [CrossRef]
- Robinson, L.M.; Elith, J.; Hobday, A.J.; Pearson, R.G.; Kendall, B.E.; Possingham, H.P.; Richardson, A.J. Pushing the limits in marine species distribution modelling: Lessons from the land present challenges and opportunities. Glob. Ecol. Biogeogr. 2011, 20, 789–802. [Google Scholar] [CrossRef]
- Fu, Y.G.; Zhou, X.H.; Zhou, D.X.; Li, J.; Zhang, W.J. Estimation of sea level variability in the South China Sea from satellite altimetry and tide gauge data. Adv. Space Res. 2021, 68, 523–533. [Google Scholar] [CrossRef]
- Brodie, S.; Hobday, A.J.; Smith, J.A.; Everett, J.D.; Taylor, M.D.; Gray, C.A.; Suthers, I.M. Modelling the oceanic habitats of two pelagic species using recreational fisheries data. Fish. Oceanogr. 2015, 24, 463–477. [Google Scholar] [CrossRef]
- Karp, M.A.; Brodie, S.; Smith, J.A.; Richerson, K.; Selden, R.L.; Liu, O.R.; Muhling, B.A.; Samhouri, J.F.; Barnett, L.A.K.; Hazen, E.L.; et al. Projecting species distributions using fishery-dependent data. Fish Fish. 2023, 24, 71–92. [Google Scholar] [CrossRef]
- Cushman, S.A.; Kilshaw, K.; Campbell, R.D.; Kaszta, Z.; Gaywood, M.; Macdonald, D.W. Comparing the performance of global, geographically weighted and ecologically weighted species distribution models for Scottish wildcats using GLM and Random Forest predictive modeling. Ecol. Model. 2024, 492, 110691. [Google Scholar] [CrossRef]
- Gómez-Ruiz, E.P.; Lacher, T.E. Climate change, range shifts, and the disruption of a pollinator-plant complex. Sci. Rep. 2019, 9, 14048. [Google Scholar] [CrossRef]
- Liu, X.X.; Wang, J.; Zhang, Y.L.; Yu, H.M.; Xu, B.D.; Zhang, C.L.; Ren, Y.P.; Xue, Y. Comparison between two GAMs in quantifying the spatial distribution of Hexagrammos otakii in Haizhou Bay, China. Fish. Res. 2019, 218, 209–217. [Google Scholar] [CrossRef]
- Wen, X.Y.; Fang, G.F.; Chai, S.Q.; He, C.J.; Sun, S.H.; Zhao, G.H.; Lin, X. Can ecological niche models be used to accurately predict the distribution of invasive insects? A case study of Hyphantria cunea in China. Ecol. Evol. 2024, 14, e11159. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, H.; Jiang, K.J.; Xiang, D.L.; Shi, Y.C.; Huang, S.S.; Li, Y.; Han, H.B. Simulating the changes of the habitats suitability of chub mackerel (Scomber japonicus) in the high seas of the North Pacific Ocean using ensemble models under medium to long-term future climate scenarios. Mar. Pollut. Bull. 2024, 207, 116873. [Google Scholar] [CrossRef]
- Gu, R.; Wei, S.P.; Li, J.R.; Zheng, S.H.; Li, Z.T.; Liu, G.L.; Fan, S.H. Predicting the impacts of climate change on the geographic distribution of moso bamboo in China based on biomod2 model. Eur. J. For. Res. 2024, 143, 1499–1512. [Google Scholar] [CrossRef]
- Chen, Y.L.; Shan, X.J.; Ovando, D.; Yang, T.; Dai, F.Q.; Jin, X.S. Predicting current and future global distribution of black rockfish (Sebastes schlegelii) under changing climate. Ecol. Indic. 2021, 128, 107799. [Google Scholar] [CrossRef]
- Schickele, A.; Leroy, B.; Beaugrand, G.; Goberville, E.; Hattab, T.; Francour, P.; Raybaud, V. Modelling European small pelagic fish distribution: Methodological insights. Ecol. Model. 2020, 416, 108902. [Google Scholar] [CrossRef]
- Dahms, C.; Killen, S.S. Temperature change effects on marine fish range shifts: A meta-analysis of ecological and methodological predictors. Glob. Change Biol. 2023, 29, 4459–4479. [Google Scholar] [CrossRef]
- Shi, W.; Wang, M.H. Tropical instability wave modulation of chlorophyll-a in the Equatorial Pacific. Sci. Rep. 2021, 11, 22517. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Shi, Y.C.; Kang, B.; Fan, W.; Xu, L.L.; Zhang, S.M.; Cui, X.S.; Dai, Y. Spatio-Temporal Variations in the Potential Habitat Distribution of Pacific Sardine (Sardinops sagax) in the Northwest Pacific Ocean. Fishes 2023, 8, 86. [Google Scholar] [CrossRef]
- Niittynen, P.; Luoto, M. The importance of snow in species distribution models of arctic vegetation. Ecography 2018, 41, 1024–1037. [Google Scholar] [CrossRef]
- Zubing, Y.; Yuming, L.; Lichuan, H.; Yifeng, L.; Shuo, W.; Songguang, X.; Yiqing, S. Habitat suitability of crown-of-thorns starfish and Titan triggerfish and their response to climate change based on ensemble species distribution model. S. China Fish. Sci. 2024, 20, 56–67. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Aguirre-Gutierrez, J.; Carvalheiro, L.G.; Polce, C.; van Loon, E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-purpose: Species distribution model performance depends on evaluation criteria—Dutch Hoverflies as a case study. PLoS ONE 2013, 8, e63708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Shan, X.J.; Gorfine, H.; Dai, F.Q.; Wu, Q.; Yang, T.; Shi, Y.Q.; Jin, X.S. Ensemble projections of fish distribution in response to climate changes in the Yellow and Bohai Seas, China. Ecol. Indic. 2023, 146, 109759. [Google Scholar] [CrossRef]
- Nisin, K.M.N.M.; Sreenath, K.R.; Sreeram, M.P. Change in habitat suitability of the invasive Snowflake coral (Carijoa riisei) during climate change: An ensemble modelling approach. Ecol. Inform. 2023, 76, 102145. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.R.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Metz, C.E. Basic Principles of Roc Analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Lawson, K.N.; Lang, K.M.; Rabaiotti, D.; Drew, J. Predicting climate change impacts on critical fisheries species in Fijian marine systems and its implications for protected area spatial planning. Divers. Distrib. 2023, 29, 1226–1244. [Google Scholar] [CrossRef]
- Ruta, D.; Gabrys, B. Classifier selection for majority voting. Inf. Fusion 2005, 6, 63–81. [Google Scholar] [CrossRef]
- Bakr, E.M.; El-Sallab, A.; Rashwan, M. EMCA: Efficient Multiscale Channel Attention Module. IEEE Access 2022, 10, 103447–103461. [Google Scholar] [CrossRef]
- Zuo, Z.K.; Wu, Z.J.; Sun, Y.Y.; Zhang, R.H.; Yan, L. Accelerating the generation of coefficient vectors in elite multi-parent crossover algorithm by using empirical probability density curve. Eng. J. Wuhan Univ. 2020, 53, 728–733. [Google Scholar] [CrossRef]
- Cai, Y.C.; Chen, Z.Z.; Xu, S.N.; Zhang, K. Tempo-spatial distribution of Evynnis cardinalis in Beibu Gulf. S. China Fish. Sci. 2017, 13, 1–10. [Google Scholar]
- Liu, Y.H.; Zhang, X.M.; Zong, S.X. Prediction of the Potential Distribution of Teinopalpus aureus Mell, 1923 (Lepidoptera, Papilionidae) in China Using Habitat Suitability Models. Forests 2024, 15, 828. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Zhang, K.; Xu, Y.; Xu, S.; Chen, Z. Spatial and Temporal Distribution of Habitat Pattern of Trichiurus japonicus in the Northern South China Sea Under Future Climate Scenarios. Fishes 2024, 9, 488. [Google Scholar] [CrossRef]
- Dietterich, T.G. Ensemble methods in machine learning. In Multiple Classifier Systems; Kittler, J., Roli, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 1857, pp. 1–15. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Reside, A.E.; Watson, I.; VanDerWal, J.; Kutt, A.S. Incorporating low-resolution historic species location data decreases performance of distribution models. Ecol. Model. 2011, 222, 3444–3448. [Google Scholar] [CrossRef]
- Kuemmerlen, M.; Schmalz, B.; Guse, B.; Cai, Q.; Fohrer, N.; Jaehnig, S.C. Integrating catchment properties in small scale species distribution models of stream macroinvertebrates. Ecol. Model. 2014, 277, 77–86. [Google Scholar] [CrossRef]
- Marshall, L.; Beckers, V.; Vray, S.; Rasmont, P.; Vereecken, N.J.; Dendoncker, N. High thematic resolution land use change models refine biodiversity scenarios: A case study with Belgian bumblebees. J. Biogeogr. 2021, 48, 345–358. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Case, T.J.; Holt, R.D.; McPeek, M.A.; Keitt, T.H. The community context of species’ borders: Ecological and evolutionary perspectives. Oikos 2005, 108, 28–46. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, K. On the niche stasis of intercontinental invasive plants. Biodivers. Sci. 2010, 18, 547–558. [Google Scholar]
- Einum, S.; Burton, T. Divergence in rates of phenotypic plasticity among ectotherms. Ecol. Lett. 2023, 26, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, A.I.T.; Hagger, V.; Greenville, A.C. Ecological forecasts to inform near-term management of threats to biodiversity. Glob. Change Biol. 2020, 26, 5816–5828. [Google Scholar] [CrossRef]
- Montoya, J.M.; Raffaelli, D. Climate change, biotic interactions and ecosystem services. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2013–2018. [Google Scholar] [CrossRef]
- Yang, T.Y.; Liu, X.Y.; Han, Z.Q. Predicting the Effects of Climate Change on the Suitable Habitat of Japanese Spanish Mackerel (Scomberomorus niphonius) Based on the Species Distribution Model. Front. Mar. Sci. 2022, 9, 927790. [Google Scholar] [CrossRef]
- Zhang, X.M.; Shi, Y.C.; Li, S.W.; Yang, Y.Y.; Xu, B.Q.; Wang, X.X.; Su, H.X.; Li, F. Climate change enables invasion of the portunid crab Charybdis bimaculata into the southern Bohai Sea. Front. Mar. Sci. 2024, 11, 1334896. [Google Scholar] [CrossRef]
- Giarolla, E.; Veiga, S.F.; Nobre, P.; Silva, M.B.; Capistrano, V.B.; Callegare, A.O. Sea surface height trends in the southern hemisphere oceans simulated by the Brazilian Earth System Model under RCP4.5 and RCP8.5 scenarios. J. South. Hemisph. Earth Syst. Sci. 2020, 70, 280–289. [Google Scholar] [CrossRef]
- Cheng, L.J.; Trenberth, K.E.; Gruber, N.; Abraham, J.P.; Fasullo, J.T.; Li, G.C.; Mann, M.E.; Zhao, X.M.; Zhu, J. Improved Estimates of Changes in Upper Ocean Salinity and the Hydrological Cycle. J. Clim. 2020, 33, 10357–10381. [Google Scholar] [CrossRef]
- Moltó, V.; Palmer, M.; Ospina-Alvarez, A.; Pérez-Mayol, S.; Benseddik, A.B.; Gatt, M.; Morales-Nin, B.; Alemany, F.; Catalán, I.A. Projected effects of ocean warming on an iconic pelagic fish and its fishery. Sci. Rep. 2021, 11, 8803. [Google Scholar] [CrossRef]
- Venegas, R.M.; Acevedo, J.; Treml, E.A. Three decades of ocean warming impacts on marine ecosystems: A review and perspective. Deep Sea Res. Part II Top. Stud. Oceanogr. 2023, 212, 105318. [Google Scholar] [CrossRef]
- Melbourne-Thomas, J. Climate shifts for krill predators. Nat. Clim. Change 2020, 10, 390–391. [Google Scholar] [CrossRef]
- Sun, D.R.; Lin, Z.J. Variations of major commercial fish stocks and strategies for fishery management in beibu gulf. J. Trop. Oceanogr. 2004, 2, 62–68. [Google Scholar]
- Brown, C.J.; Fulton, E.A.; Hobday, A.J.; Matear, R.J.; Possingham, H.P.; Bulman, C.; Christensen, V.; Forrest, R.E.; Gehrke, P.C.; Gribble, N.A.; et al. Effects of climate-driven primary production change on marine food webs: Implications for fisheries and conservation. Glob. Change Biol. 2010, 16, 1194–1212. [Google Scholar] [CrossRef]
- Braun, C.D.; Lezama-Ochoa, N.; Farchadi, N.; Arostegui, M.C.; Alexander, M.; Allyn, A.; Bograd, S.J.; Brodie, S.; Crear, D.P.; Curtis, T.H.; et al. Widespread habitat loss and redistribution of marine top predators in a changing ocean. Sci. Adv. 2023, 9, eadi2718. [Google Scholar] [CrossRef]
- McGreevy, S.R.; Rupprecht, C.D.D.; Niles, D.; Wiek, A.; Carolan, M.; Kallis, G.; Kantamaturapoj, K.; Mangnus, A.; Jehlicka, P.; Taherzadeh, O.; et al. Sustainable agrifood systems for a post-growth world. Nat. Sustain. 2022, 5, 1011–1017. [Google Scholar] [CrossRef]
- FAO. Blue Transformation—Roadmap 2022–2030; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Alam, M.S.; Yousuf, A. Fishermen’s community livelihood and socio-economic constraints in coastal areas: An exploratory analysis. Environ. Chall. 2024, 14, 100810. [Google Scholar] [CrossRef]
- Spalding, A.K.; Grorud-Colvert, K.; Allison, E.H.; Amon, D.J.; Collin, R.; de Vos, A.; Friedlander, A.M.; Johnson, S.M.; Mayorga, J.; Paris, C.B.; et al. Engaging the tropical majority to make ocean governance and science more equitable and effective. Npj Ocean Sustain. 2023, 2, 8. [Google Scholar] [CrossRef]
- Matovu, B.; Bleischwitz, R.; Alkoyak-Yildiz, M.; Arlikatti, S. Invigorating women’s empowerment in marine fishing to promote transformative cultures and narratives for sustainability in the blue economy: A scoping literature review from the Global South. Mitig. Adapt. Strateg. Glob. Change 2024, 29, 83. [Google Scholar] [CrossRef]
- Farmery, A.K.; Allison, E.H.; Andrew, N.L.; Troell, M.; Voyer, M.; Campbell, B.; Eriksson, H.; Fabinyi, M.; Song, A.M.; Steenbergen, D. Blind spots in visions of a “blue economy” could undermine the ocean’s contribution to eliminating hunger and malnutrition. One Earth 2021, 4, 28–38. [Google Scholar] [CrossRef]
- Bennett, N.J.; Blythe, J.; White, C.S.; Campero, C. Blue growth and blue justice: Ten risks and solutions for the ocean economy. Mar. Policy 2021, 125, 104387. [Google Scholar] [CrossRef]
- Sheeja, S.R.; Ajay, A. State led social security and inclusion of marine fisherfolk: Analyzing the case of Kerala, India. Mar. Policy 2023, 147, 105392. [Google Scholar] [CrossRef]
- Gopal, N.; Hapke, H.M.; Edwin, L. Technological transformation and changing social relations in the ring seine fishery of Kerala, India. Marit. Stud. 2023, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Matovu, B.; Brouwer, F.; Bleischwitz, R.; Aljanabi, F.; Alkoyak-Yildiz, M. Resource nexus perspectives in the Blue Economy of India: The case of sand mining in Kerala. Environ. Sci. Policy 2024, 151, 103617. [Google Scholar] [CrossRef]
- Matovu, B.; Lukambagire, I.; Mwabvu, B.; Manianga, A.; Alkoyak-Yildiz, M.; Niranjanaa, S.; Jabbi, B.; Etta, L.A. Co-designing transformative ocean sustainability narratives to address complex human-environmental challenges facing coastal fisherwomen: An evidence-based study. Environ. Chall. 2024, 15, 100923. [Google Scholar] [CrossRef]
- Cavaleri Gerhardinger, L.; Brodie Rudolph, T.; Gaill, F.; Mortyn, G.; Littley, E.; Vincent, A.; Firme Herbst, D.; Ziveri, P.; Jeanneau, L.; Laamanen, M.; et al. Bridging Shades of Blue: Co-constructing Knowledge with the International Panel for Ocean Sustainability. Coast. Manag. 2023, 51, 244–264. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Toki, T.; Shimabukuro, H.; Kubo, Y.; Takahashi, S.; Shinjo, R. Development and application of an environmental education tool (board game) for teaching integrated resource management of the water cycle on coral reef islands. Sustainability 2022, 14, 16562. [Google Scholar] [CrossRef]

| Evaluation | FDA | GAM | GLM | MARS | MAXNET |
|---|---|---|---|---|---|
| ROC | 0.957 | 0.954 | 0.898 | 0.967 | 0.942 |
| SD1 | 0.019 | 0.020 | 0.029 | 0.021 | 0.014 |
| TSS | 0.846 | 0.822 | 0.720 | 0.860 | 0.783 |
| SD2 | 0.052 | 0.073 | 0.053 | 0.060 | 0.038 |
| Assessment | EMca | EMmean | EMmedian | EMwmean |
|---|---|---|---|---|
| ROC | 0.97 | 0.962 | 0.961 | 0.962 |
| TSS | 0.85 | 0.85 | 0.833 | 0.85 |
| Period and Climate Scenarios | No Suitable Areas (0 < HSI ≤ 0.25) | Low Suitable Areas (0.25 < HSI ≤ 0.5) | Moderately Suitable Areas (0.5 < HSI ≤ 0.75) | Highly Suitable Areas (0.75 < HSI ≤ 1) |
|---|---|---|---|---|
| Current | 309,225 | 53,700 | 35,175 | 158,475 |
| SSP-126-2050 | 293,950 | 54,825 | 49,625 | 146,350 |
| SSP-126-2100 | 293,550 | 55,025 | 47,050 | 149,125 |
| SSP-370-2050 | 294,100 | 53,575 | 49,325 | 147,750 |
| SSP-370-2100 | 294,700 | 53,275 | 45,975 | 150,800 |
| SSP-585-2050 | 294,475 | 52,650 | 48,400 | 149,225 |
| SSP-585-2100 | 293,325 | 54,400 | 49,175 | 147,850 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, J.; Cai, Y.; Zhang, K.; Xu, Y.; Chen, Z.; Xu, S. Simulating Habitat Suitability Changes of Threadfin Porgy (Evynnis cardinalis) in the Northern South China Sea Using Ensemble Models Under Medium-to-Long-Term Future Climate Scenarios. Biology 2025, 14, 236. https://doi.org/10.3390/biology14030236
Zhang J, Li J, Cai Y, Zhang K, Xu Y, Chen Z, Xu S. Simulating Habitat Suitability Changes of Threadfin Porgy (Evynnis cardinalis) in the Northern South China Sea Using Ensemble Models Under Medium-to-Long-Term Future Climate Scenarios. Biology. 2025; 14(3):236. https://doi.org/10.3390/biology14030236
Chicago/Turabian StyleZhang, Junyi, Jiajun Li, Yancong Cai, Kui Zhang, Youwei Xu, Zuozhi Chen, and Shannan Xu. 2025. "Simulating Habitat Suitability Changes of Threadfin Porgy (Evynnis cardinalis) in the Northern South China Sea Using Ensemble Models Under Medium-to-Long-Term Future Climate Scenarios" Biology 14, no. 3: 236. https://doi.org/10.3390/biology14030236
APA StyleZhang, J., Li, J., Cai, Y., Zhang, K., Xu, Y., Chen, Z., & Xu, S. (2025). Simulating Habitat Suitability Changes of Threadfin Porgy (Evynnis cardinalis) in the Northern South China Sea Using Ensemble Models Under Medium-to-Long-Term Future Climate Scenarios. Biology, 14(3), 236. https://doi.org/10.3390/biology14030236








