Independent Effects of Dopant, Oxygen Vacancy, and Specific Surface Area on Crystal Phase of HfO2 Thin Films towards General Parameters to Engineer the Ferroelectricity
Abstract
:1. Introduction
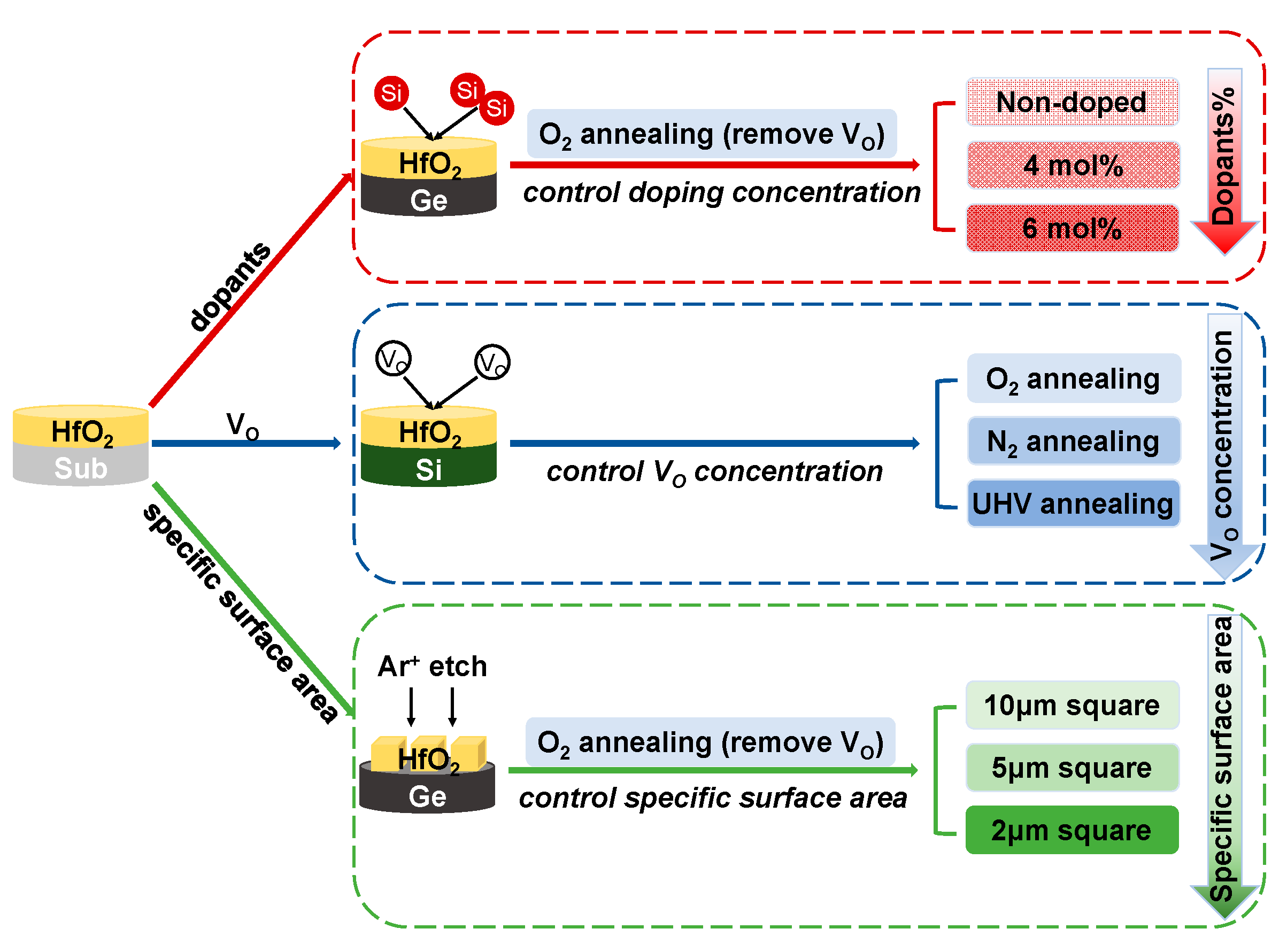
2. Experimental
3. Results
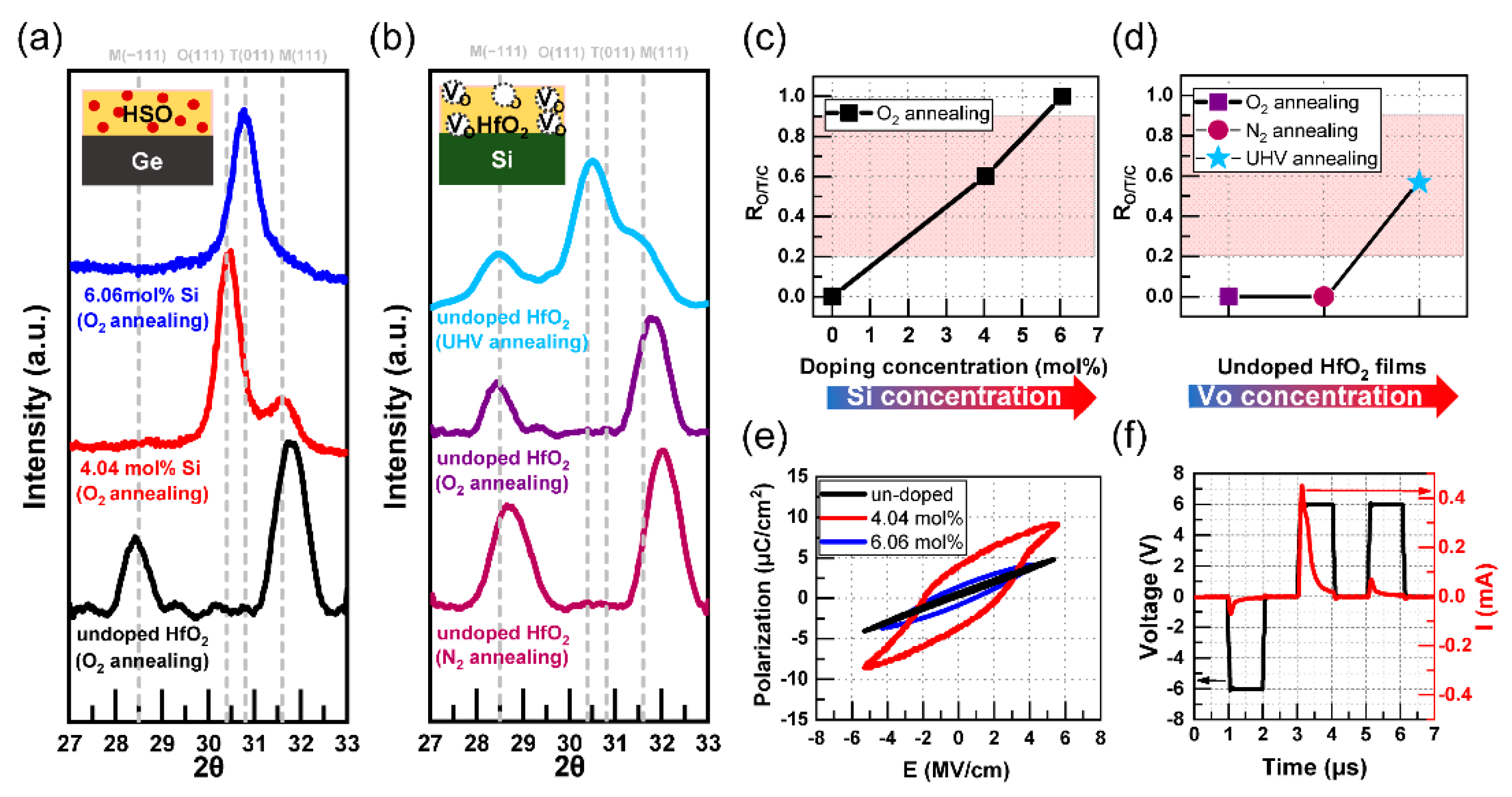
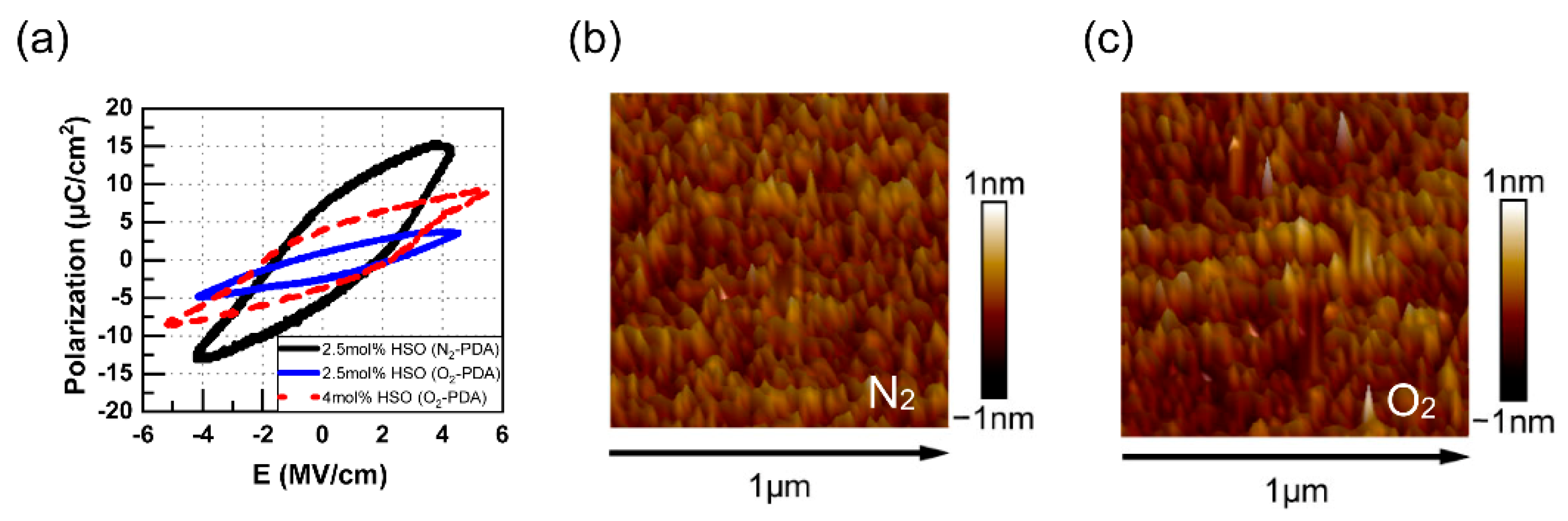
3.1. Independent and Common Effects of Dopants and VO
3.2. Effect of Specific Surface Area
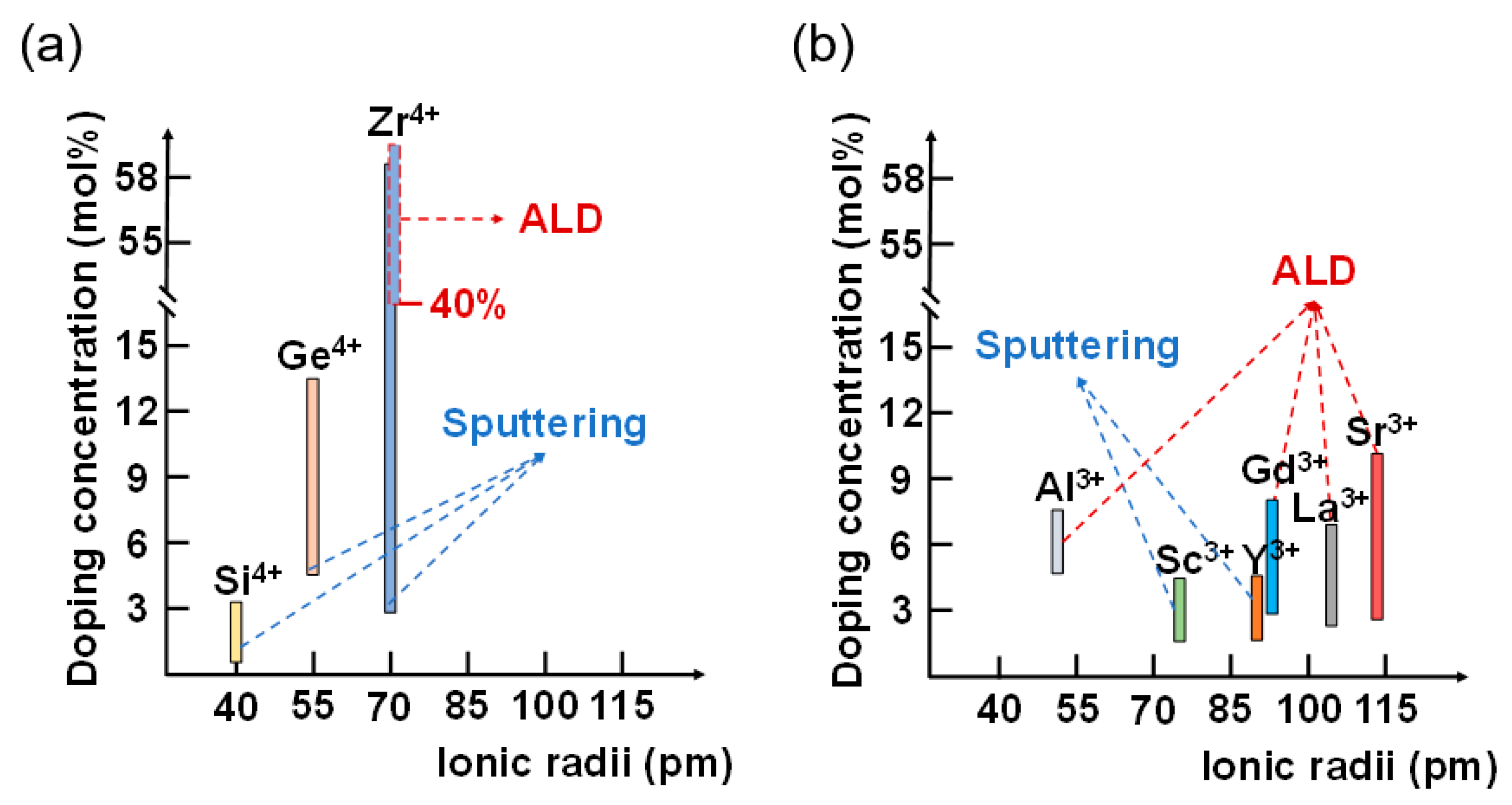
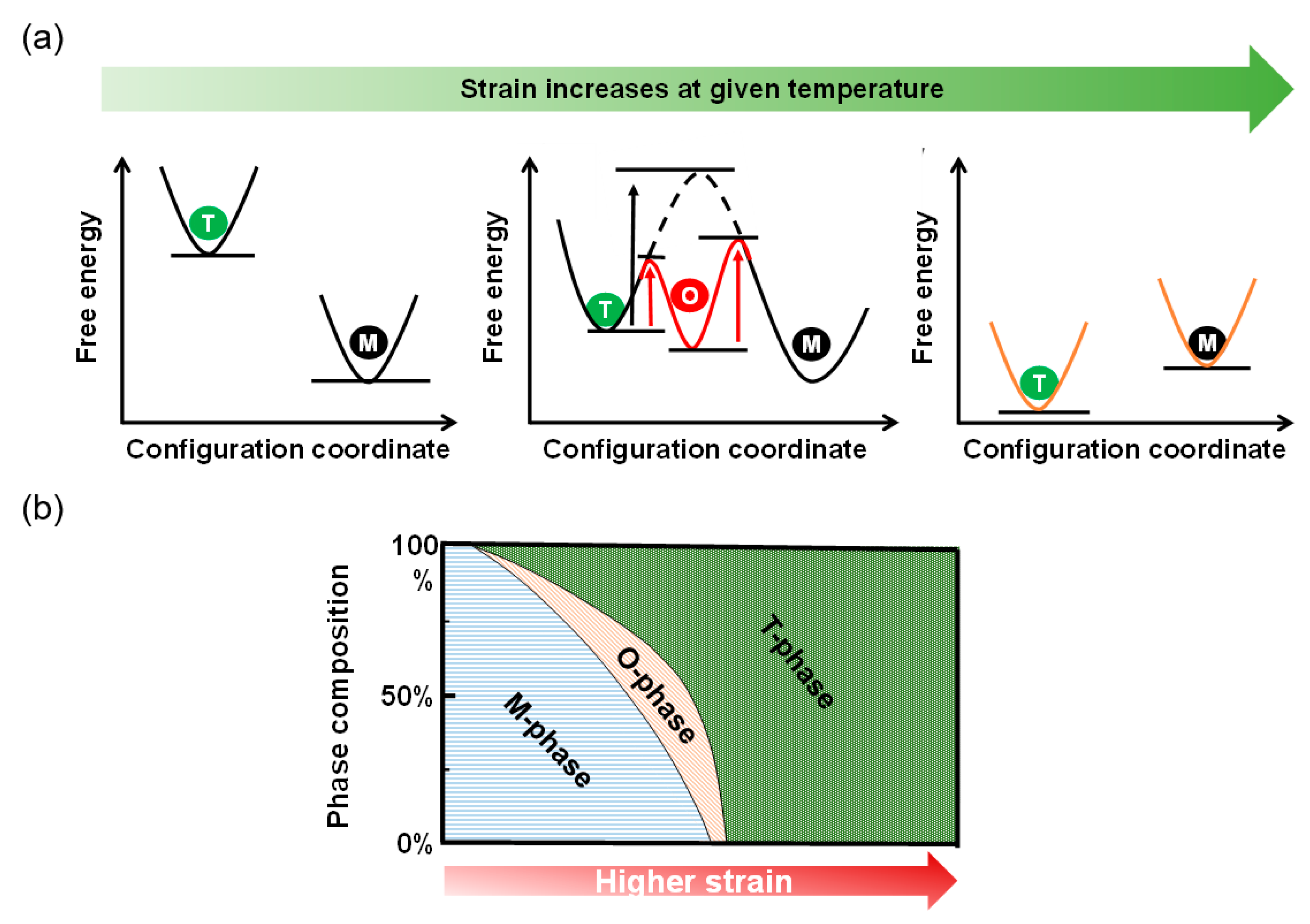
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohr, M.; Chau, R.; Ghani, T.; Mistry, K. The High-K Solution. IEEE Spectr. 2007, 44, 29. [Google Scholar] [CrossRef]
- Böscke, T.S.; Müller, J.; Bräuhaus, D.; Schröder, U.; Böttger, U. Ferroelectricity in hafnium oxide thin films. Appl. Phys. Lett. 2011, 99, 102903. [Google Scholar] [CrossRef]
- Müller, J.; Polakowski, P.; Müller, S.; Mikolajick, T. Ferroelectric Hafnium Oxide Based Materials and Devices: Assessment of Current Status and Future Prospects. ECS J. Solid State Sci. Technol. 2015, 4, N30–N35. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, Y.H.; Mikolajick, T.; Schroeder, U.; Hwang, C.S. Review and perspective on ferroelectric HfO2-based thin films for memory applications. MRS Commun. 2018, 8, 795. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Lee, Y.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Kim, K.D.; Müller, J.; Kersch, A.; Schroeder, U.; Mikolajick, T.; et al. Ferroelectricity and Antiferroelectricity of Doped Thin HfO2-Based Films. Adv. Mater. 2015, 27, 1811. [Google Scholar] [PubMed]
- Sang, X.; Grimley, E.D.; Schenk, T.; Schroeder, U.; Lebeau, J.M. On the structural origins of ferroelectricity in HfO2 thin films. Appl. Phys. Lett. 2015, 106, 162905. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, D.H.; Yang, K.; Park, J.-Y.; Yu, G.T.; Park, H.W.; Materano, M.; Mittmann, T.; Lomenzo, P.D.; Mikolajick, T.; et al. Review of defect chemistry in fluorite-structure ferroelectrics for future electronic devices. J. Mater. Chem. C 2020, 8, 10526. [Google Scholar] [CrossRef]
- Shiraishi, T.; Katayama, K.; Yokouchi, T.; Shimizu, T.; Oikawa, T.; Sakata, O.; Uchida, H.; Imai, Y.; Kiguchi, T.; Konno, T.J. Impact of mechanical stress on ferroelectricity in (Hf0.5Zr0.5)O2 thin films. Appl. Phys. Lett. 2016, 108, 262904. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Kim, K.; Hwang, C.S. Study on the degradation mechanism of the ferroelectric properties of thin Hf0.5Zr0.5O2 films on TiN and Ir electrodes. Appl. Phys. Lett. 2014, 105, 072901. [Google Scholar]
- Batra, R.; Tran, H.D.; Ramprasad, R. Stabilization of metastable phases in hafnia owing to surface energy effects. Appl. Phys. Lett. 2016, 108, 172902. [Google Scholar]
- Park, M.H.; Lee, Y.H.; Kim, H.J.; Schenk, T.; Lee, W.; Kim, K.D.; Fengler, F.P.G.; Mikolajick, T.; Schroeder, U.; Hwang, C.S. Surface and grain boundary energy as the key enabler of ferroelectricity in nanoscale hafnia-zirconia: A comparison of model and experiment. Nanoscale 2017, 9, 9973. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.D.; Park, M.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Lee, Y.H.; Hyun, S.D.; Gwon, T.; Hwang, C.S. Ferroelectricity in undoped-HfO2 thin films induced by deposition temperature control during atomic layer deposition. J. Mater. Chem. C 2016, 4, 6864. [Google Scholar] [CrossRef]
- Materlik, R.; Künneth, C.; Kersch, A. The Origin of Ferroelectricity in Hf1−xZrxO2: A Computational Investigation and a Surface Energy Model. J. Appl. Phys. 2015, 117, 134109. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, W.; Pancotti, A.; Lubin, C.; Tortech, L.; Richter, C.; Mikolajick, T.; Schroeder, U.; Barrett, N. Physical chemistry of the TiN/Hf0.5Zr0.5O2 interface. J. Appl. Phys. 2020, 127, 064105. [Google Scholar] [CrossRef]
- Schroeder, U.; Materano, M.; Mittmann, T.; Lomenzo, P.D.; Mikolajick, T.; Toriumi, A. Recent progress for obtaining the ferroelectric phase in hafnium oxide based films: Impact of oxygen and zirconium. Jpn. J. Appl. Phys. 2019, 58, SL0801. [Google Scholar] [CrossRef]
- Szyjka, T.; Baumgarten, L.; Mittmann, T.; Matveyev, Y.; Schlueter, C.; Mikolajick, T.; Schroeder, U.; Müller, M. Chemical Stability of IrO2 Top Electrodes in Ferroelectric Hf0.5Zr0.5O2-Based Metal–Insulator–Metal Structures: The Impact of Annealing Gas. Phys. Stat. Solidi Rapid Res. Lett. 2021, 15, 2100027. [Google Scholar] [CrossRef]
- Mittmann, T.; Michailow, M.; Lomenzo, P.D.; Gärtner, J.; Falkowski, M.; Kersch, A.; Mikolajick, T.; Schroeder, U. Stabilizing the ferroelectric phase in HfO2-based films sputtered from ceramic targets under ambient oxygen. Nanoscale 2021, 13, 912. [Google Scholar] [CrossRef] [PubMed]
- Lomenzo, P.D.; Takmeel, Q.; Zhou, C.; Fancher, C.M.; Lambers, E.; Rudawski, N.G.; Jones, J.L.; Moghaddam, S.; Nishida, T. TaN interface properties and electric field cycling effects on ferroelectric Si-doped HfO2 thin films. J. Appl. Phys. 2015, 117, 134105. [Google Scholar] [CrossRef]
- Nishimura, T.; Xu, L.; Shibayama, S.; Yajima, T.; Migita, S.; Toriumi, A. Ferroelectricity of nondoped thin HfO2 films in TiN/HfO2/TiN stacks. Jpn. J. Appl. Phys. 2016, 55, 08PB01. [Google Scholar] [CrossRef]
- Mittmann, T.; Materano, M.; Lomenzo, P.D.; Park, M.H.; Stolichnov, I.; Cavalieri, M.; Zhou, C.; Chung, C.C.; Jones, J.L.; Szyjka, T.; et al. Origin of Ferroelectric Phase in Undoped HfO2 Films Deposited by Sputtering. Adv. Mater. Interfaces 2019, 6, 1900042. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Shibayama, S.; Nishimura, T.; Yajima, T.; Migita, S.; Toriumi, A. Evolution of ferroelectric HfO2 in ultrathin region down to 3 nm. Appl. Phys. Lett. 2018, 112, 102902. [Google Scholar] [CrossRef]
- Mueller, S.; Mueller, J.; Singh, A.; Riedel, S.; Sundqvist, J.; Schroeder, U.; Mikolajick, T. Incipient Ferroelectricity in Al-Doped HfO2 Thin Films. Adv. Funct. Mater. 2012, 22, 2412. [Google Scholar] [CrossRef]
- Xu, L.; Shibayama, S.; Izukashi, K.; Nishimura, T.; Yajima, T.; Migita, S.; Toriumi, A. General relationship for cation and anion doping effects on ferroelectric HfO2 formation. In Proceedings of the 2016 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 3–7 December 2016; p. 25. [Google Scholar]
- Garvie, R.C. The occurrence of metastable tetragonal zirconia as a crystallite size effect. J. Phys. Chem. 1965, 69, 1238–1243. [Google Scholar] [CrossRef]
- Xu, L.; Nishimura, T.; Shibayama, S.; Yajima, T.; Migita, S.; Toriumi, A. Kinetic pathway of the ferroelectric phase formation in doped HfO2 films. J. Appl. Phys. 2017, 122, 124104. [Google Scholar] [CrossRef]
- Schroeder, U.; Yurchuk, E.; Muller, J.; Martin, D.; Schenk, T.; Polakowski, P.; Adelmann, C.; Popovici, M.I.; Kalinin, S.V.; Mikolajick, T. Impact of different dopants on the switching properties of ferroelectric hafnium oxide. Jpn. J. Appl. Phys. 2014, 53, 08LE02. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and x-ray studies of Ce1−xRexO2−y, where Re = La, Pr, Nd, Eu, Gd, Tb. J. Appl. Phys. 1994, 76, 2435. [Google Scholar] [CrossRef]
- Jena, S.; Tokas, R.B.; Misal, J.S.; Rao, K.D.; Udupa, D.V.; Thakur, S.; Sahoo, N.K. Effect of O2/Ar gas flow ratio on the optical properties and mechanical stress of sputtered HfO2 thin films. Thin Solid Film. 2015, 592, 135. [Google Scholar] [CrossRef]
- Lukyanchuk, I.A.; Schilling, A.; Gregg, J.M.; Catalan, G.; Scott, J.F. Origin of ferroelastic domains in free-standing single-crystal ferroelectric films. Phys. Rev. B 2009, 79, 144111. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Hwang, C.S. The effects of crystallographic orientation and strain of thin Hf0.5Zr0.5O2 film on its ferroelectricity. Appl. Phys. Lett. 2014, 104, 072901. [Google Scholar] [CrossRef]
- Katayama, K.; Shimizu, T.; Sakata, O.; Shiraishi, T.; Nakamura, S.; Kiguchi, T.; Akama, A.; Konno, T.J.; Uchida, H.; Funakubo, H. Orientation control and domain structure analysis of {100}-oriented epitaxial ferroelectric orthorhombic HfO2-based thin films. J. Appl. Phys. 2016, 119, 134101. [Google Scholar] [CrossRef]
- Kisi, E.H. Influence of Hydrostatic Pressure on the t→o Transformation in Mg-PSZ Studied by In Situ Neutron Diffraction. J. Am. Ceram. Soc. 1998, 81, 741–745. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, Y.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Kim, K.D.; Hyun, S.D.; Mikolajick, T.; Schroeder, U.; Hwang, C.S. Understanding the formation of the metastable ferroelectric phase in hafnia–zirconia solid solution thin films. Nanoscale 2018, 10, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.H.; Lee, Y.H.; Mikolajick, T.; Schroeder, U.; Hwang, C.S. Thermodynamic and Kinetic Origins of Ferroelectricity in Fluorite Structure Oxides. Adv. Electron. Mater. 2019, 5, 1800522. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, T.; Zhu, L.; Chen, D.; Fan, Y.; Liu, J.; Li, X. Independent Effects of Dopant, Oxygen Vacancy, and Specific Surface Area on Crystal Phase of HfO2 Thin Films towards General Parameters to Engineer the Ferroelectricity. Electronics 2022, 11, 2369. https://doi.org/10.3390/electronics11152369
Cui T, Zhu L, Chen D, Fan Y, Liu J, Li X. Independent Effects of Dopant, Oxygen Vacancy, and Specific Surface Area on Crystal Phase of HfO2 Thin Films towards General Parameters to Engineer the Ferroelectricity. Electronics. 2022; 11(15):2369. https://doi.org/10.3390/electronics11152369
Chicago/Turabian StyleCui, Tianning, Liping Zhu, Danyang Chen, Yuyan Fan, Jingquan Liu, and Xiuyan Li. 2022. "Independent Effects of Dopant, Oxygen Vacancy, and Specific Surface Area on Crystal Phase of HfO2 Thin Films towards General Parameters to Engineer the Ferroelectricity" Electronics 11, no. 15: 2369. https://doi.org/10.3390/electronics11152369
APA StyleCui, T., Zhu, L., Chen, D., Fan, Y., Liu, J., & Li, X. (2022). Independent Effects of Dopant, Oxygen Vacancy, and Specific Surface Area on Crystal Phase of HfO2 Thin Films towards General Parameters to Engineer the Ferroelectricity. Electronics, 11(15), 2369. https://doi.org/10.3390/electronics11152369




