Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere
Abstract
:1. Introduction
2. Battery Samples and Experimental Methods
2.1. Battery Samples and Experimental Pretreatment
2.2. Experimental Methods
- (a)
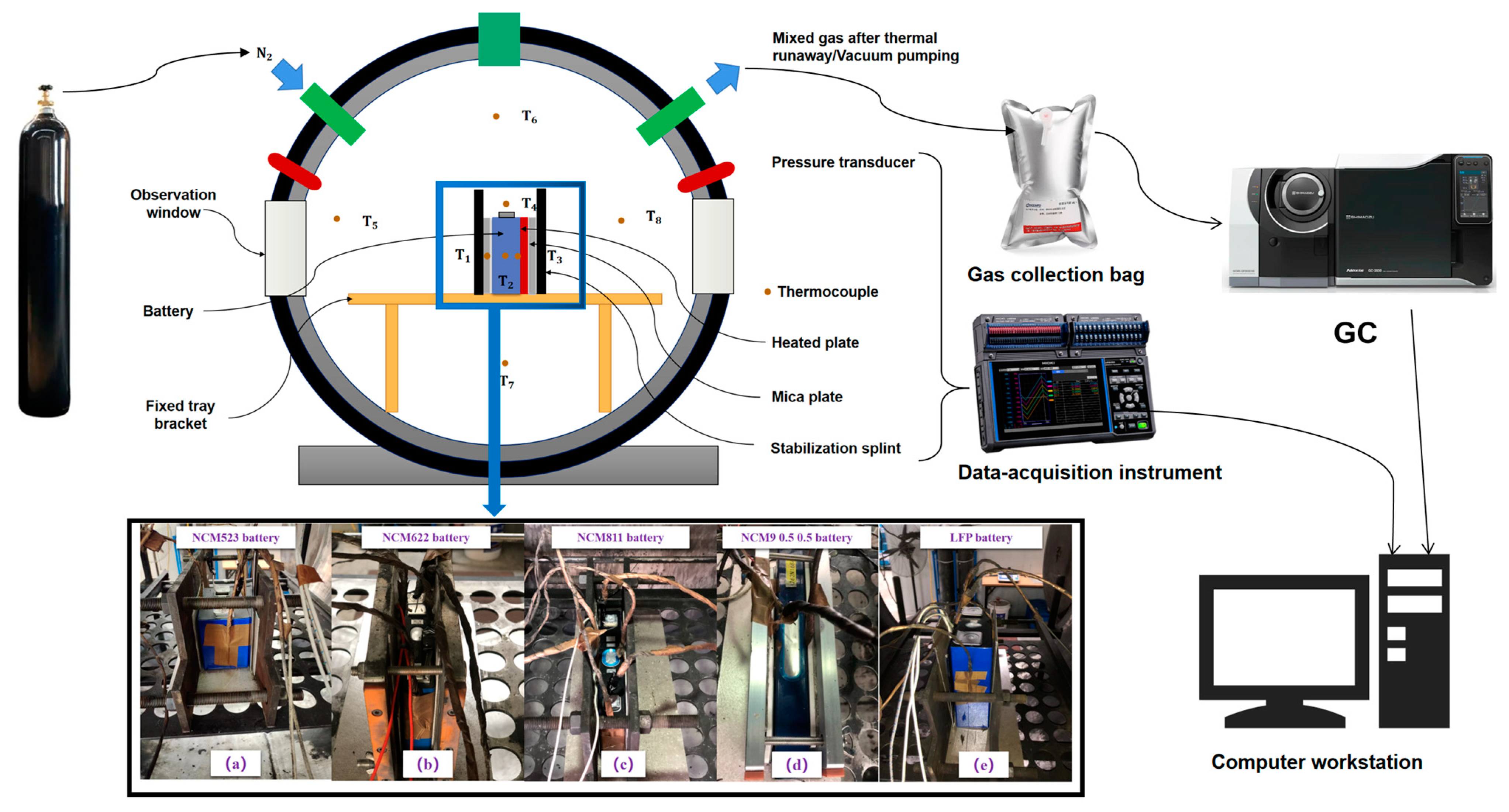
- The battery is centrally located within the AEC cabin with a thermocouple affixed to its surface using aluminum tape. Two additional thermocouples (T1 and T3) are positioned at the center of the battery’s large surface, while a third thermocouple (T2) is located on the battery’s side. Four more thermocouples (T5–T8) are evenly placed around the battery in four directions (down, left, right, and ambient) to measure the temperature within the cabin. Additionally, a thermocouple (T4) is situated 30 mm above the safety valve. The heating plate used in the experiment has the same size and arrangement as the battery, as indicated in Figure 2a–e.
- (b)
- The battery, heating plate, and mica plate are secured using clamps, with the bolt’s tightening force determining the preload.
- (c)
- After arranging the battery, the AEC cabin door is closed, and the gas within is vacuumed three times to reach a pressure of −90 KPa. The AEC is then refilled with N2 to reach normal pressure, resulting in a 1% reduction in oxygen composition within the AEC. The experiment is allowed to stand for 10 min to ensure that the internal gas is stable and meets the necessary conditions for the next step.
- (d)
- The heating plate is then turned on, and the battery’s surface temperature, voltage, internal pressure, and other parameters are carefully monitored for any changes. The voltage sag is used as an indication of the start of battery TR, as per previous studies [8,22,36,39,40,41,42,45]. Once the battery voltage drops, the heating plate is turned off, and the experiment is allowed to continue until the battery undergoes TR spontaneously.
- (e)
- The judgment basis for the end of battery TR release flue gas is determined by monitoring the AEC cabin’s internal pressure fluctuation rate |dp/dt|, which must be less than 0.2 KPa/s and must last for more than 30 s after the occurrence of TR. The experiment ends when the battery surface temperature drops below 80 °C, and the data is saved.
- (f)
- At the end of the experiment, the battery’s TR product, including particulate matter and electrolyte, is collected and analyzed for its internal gas composition.
3. Experimental Results and Data Analysis
3.1. TR Temperature Characteristics of Batteries with Different Cathode Materials
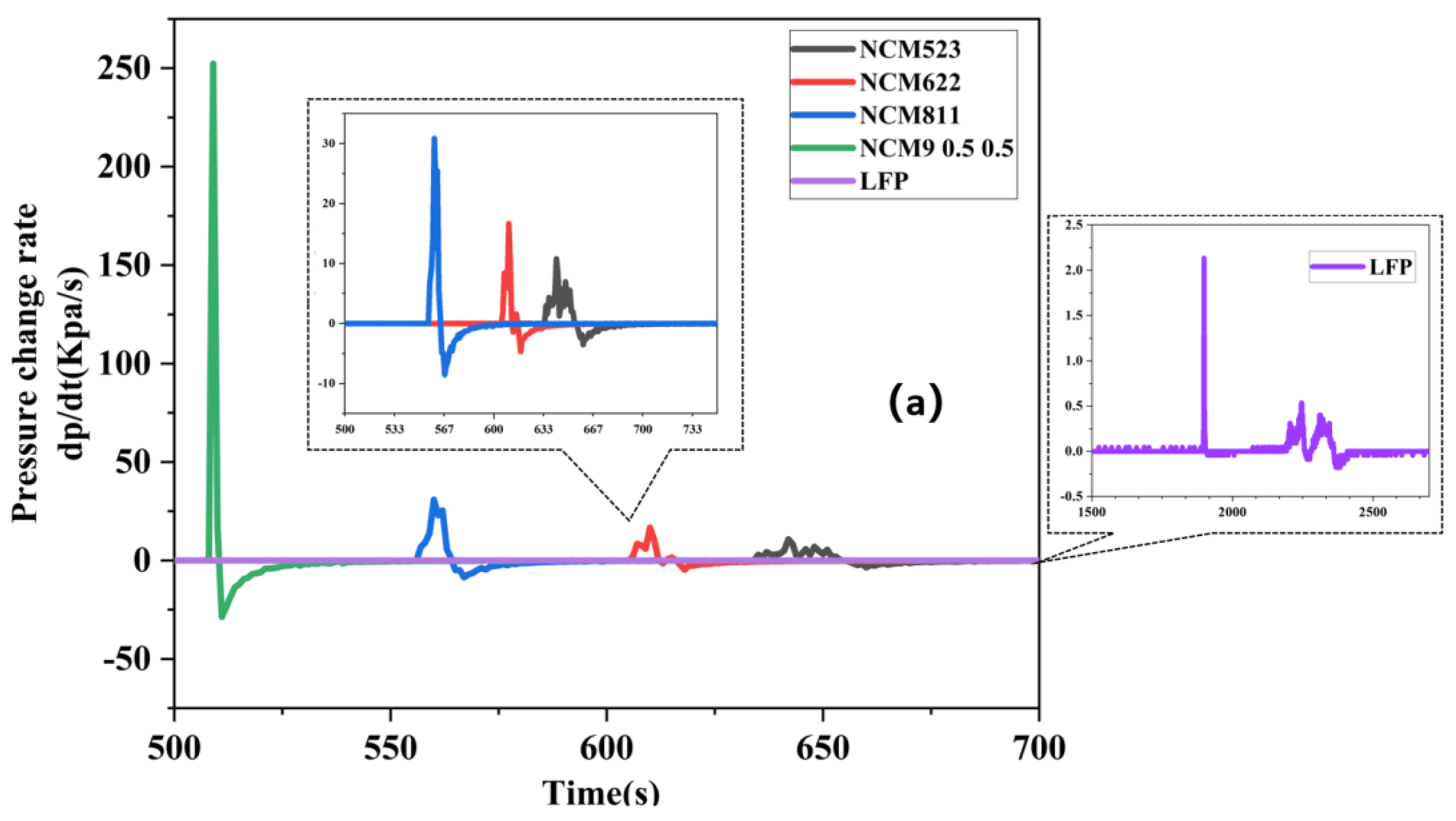
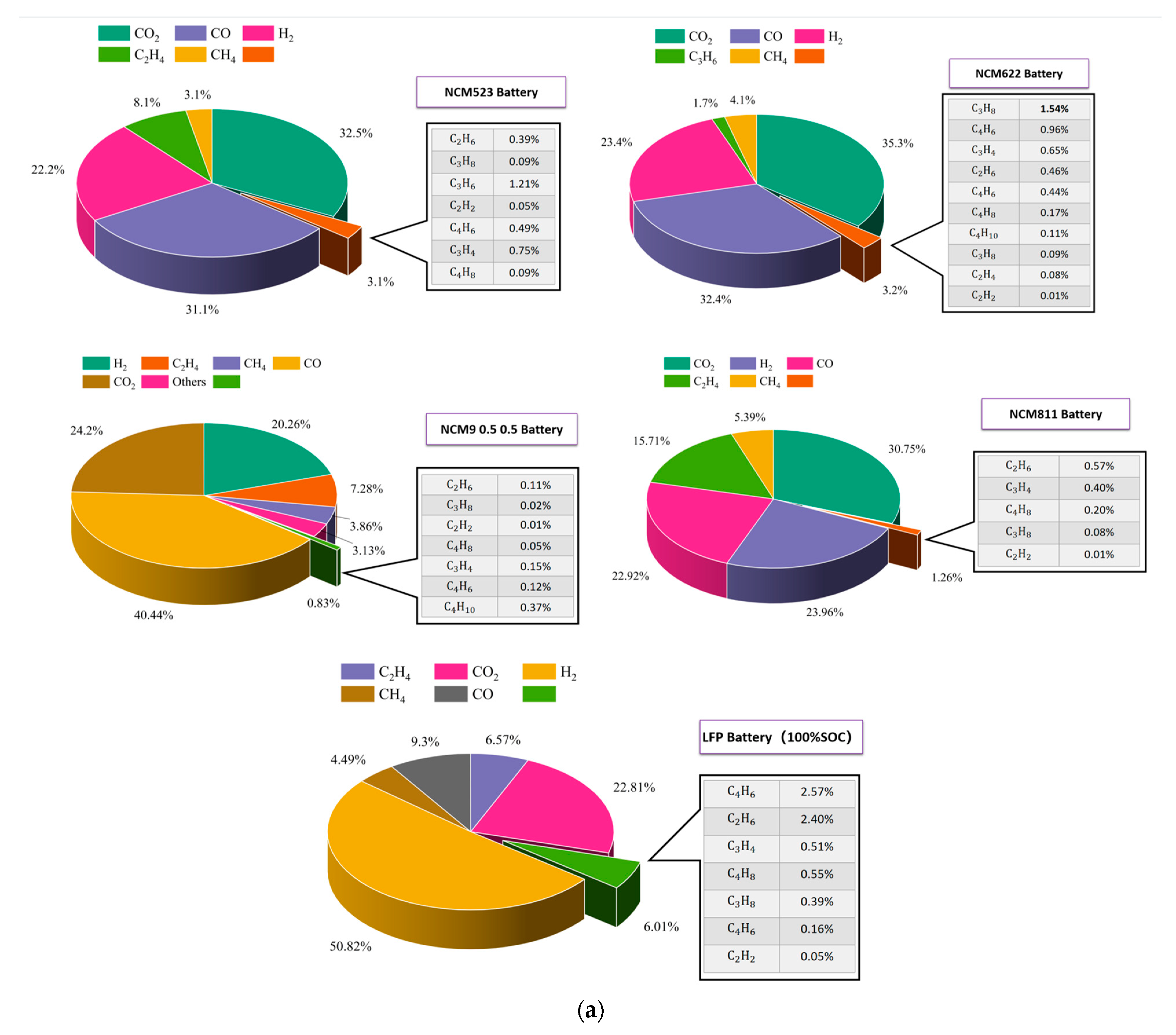
3.2. TR Gas Release Characteristics of Batteries with Different Cathode Materials
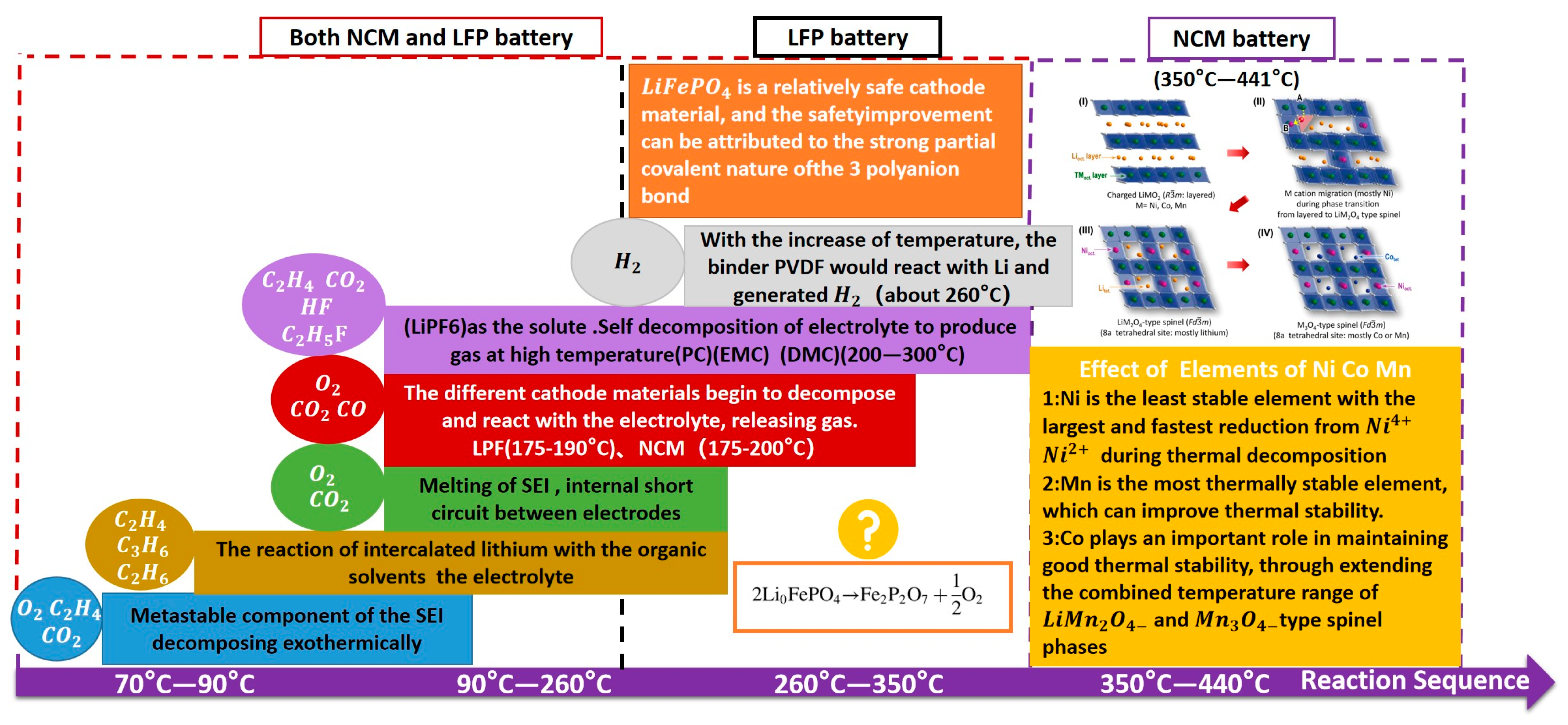
3.3. Battery TR Gas Release Characteristics and TR Manifestations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbols | |
| SOC | State of charge |
| LFP | |
| NCM | |
| SEI | Solid electrolyte interphase |
| DMC | Dimethyl carbonate |
| EMC | Ethyl methyl carbonate |
| PC | Propylene carbonate |
| ARC | Accelerating rate calorimeter |
| AEC | Adiabatic explosion chamber |
| DSC | Differential scanning calorimeter |
| GC | Gas chromatographyy |
| PVDF | Polyvinylidene fluoride |
| UFL | Upper flammable limit |
| LFL | Lower flammable limit |
| TR | Thermal runaway |
| AEC internal volume | |
| Internal pressure of AEC after experiment | |
| Back pressure of AEC before experiment | |
| AEC internal ambient temperature after experiment | |
| Internal ambient temperature of AEC before experiment | |
| Gases generated | |
| Gas constant | |
| Time normalization | |
| Temperature normalization | |
| Flammability limit of the gas | |
| Volume percentage | |
| Flammability limit of combustible component i in battery | |
| Gas–solid ratio | |
| Initial weight of battery | |
| Battery residual weight | |
| Particulate matter mass | |
| C-rate | Battery charge and discharge rate |
| LFP | |
| LIBs | Lithium-ion batteries |
References
- Wang, X.; Wei, X.; Zhu, J.; Dai, H.; Zheng, Y.; Xu, X.; Chen, Q. A review of modeling, acquisition, and application of lithium-ion battery impedance for onboard battery management. eTransportation 2021, 7, 100093. [Google Scholar] [CrossRef]
- Gao, W.; Hu, Z.; Huang, H.; Xu, L.; Fang, C.; Li, J.; Wang, C.; Ouyang, M. All-condition economy evaluation method for fuel cell systems: System efficiency contour map. eTransportation 2021, 9, 100127. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Zhao, S.; Li, H.; Yu, H. Oxygen anionic redox activated high-energy cathodes: Status and prospects. eTransportation 2021, 8, 100118. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. An overview of modification strategies to improve LiNi0·8Co0·1Mn0·1O2 (NCM811) cathode performance for automotive lithium-ion batteries. eTransportation 2021, 7, 100105. [Google Scholar] [CrossRef]
- Wildfeuer, L.; Lienkamp, M. Quantifiability of inherent cell-to-cell variations of commercial lithium-ion batteries. eTransportation 2021, 9, 100129. [Google Scholar] [CrossRef]
- Piao, N.; Gao, X.; Yang, H.; Guo, Z.; Hu, G.; Cheng, H.-M.; Li, F. Challenges and development of lithium-ion batteries for low temperature environments. eTransportation 2021, 11, 100145. [Google Scholar] [CrossRef]
- Meng, X.; Li, S.; Fu, W.; Chen, Y.; Duan, Q.; Wang, Q. Experimental study of intermittent spray cooling on suppression for lithium iron phosphate battery fires. eTransportation 2021, 11, 100142. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Li, W.; Gao, Z.; Zhang, B.; Ouyang, M. Experimental study on the cell-jet temperatures of abused prismatic Ni-rich automotive batteries under medium and high states of charge. Appl. Therm. Eng. 2021, 202, 117859. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; de Persis, S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Sloop, S.E.; Kerr, J.B.; Kinoshita, K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge. J. Power Sources 2003, 119–121, 330–337. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Eshetu, G.G.; Mathiron, D.; Ribière, P.; Armand, M.; Laruelle, S. Thermal behaviour of the lithiated-graphite/electrolyte interface through GC/MS analysis. Electrochim. Acta 2012, 83, 402–409. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Di Michiel, M.; Offer, G.J.; Hinds, G.; et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 2015, 6, 6924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, Y.; Li, C.; Li, W.; Ouyang, M. Thermal runaway product ejection process in lithium-ion power batteries under medium state of charge. Energy Storage Sci. Technol. 2019, 6, 1076–1081. [Google Scholar]
- Zhang, Y.; Wang, H.; Li, W.; Li, C.; Ouyang, M. Size distribution and elemental composition of vent particles from abused prismatic Ni-rich automotive lithium-ion batteries. J. Energy Storage 2019, 26, 100991. [Google Scholar] [CrossRef]
- Bolshova, T.; Shvartsberg, V.; Shmakov, A. Synergism of trimethylphosphate and carbon dioxide in extinguishing premixed flames. Fire Saf. J. 2021, 125, 103406. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wang, Y.; Li, C.; Liu, Y.; Ouyang, M. Thermal abusive experimental research on the large-format lithium-ion battery using a buried dual-sensor. J. Energy Storage 2021, 33, 102156. [Google Scholar] [CrossRef]
- Qin, P.; Sun, J.; Wang, Q. A new method to explore thermal and venting behavior of lithium-ion battery thermal runaway. J. Power Sources 2020, 486, 229357. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Jhu, C.-Y.; Wang, Y.-W.; Chiang, C.-C.; Shu, C.-M. Thermal runaway features of 18650 lithium-ion batteries for LiFePO4 cathode material by DSC and VSP2. J. Therm. Anal. Calorim. 2012, 109, 1297–1302. [Google Scholar] [CrossRef]
- Kupper, C.; Spitznagel, S.; Döring, H.; Danzer, M.A.; Gutierrez, C.; Kvasha, A.; Bessler, W.G. Combined modeling and experimental study of the high-temperature behavior of a lithium-ion cell: Differential scanning calorimetry, accelerating rate calorimetry and external short circuit. Electrochim. Acta 2019, 306, 209–219. [Google Scholar] [CrossRef]
- Yuan, L.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental study on thermal runaway and vented gases of lithium-ion cells. Process Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
- Gachot, G.; Ribière, P.; Mathiron, D.; Grugeon, S.; Armand, M.; Leriche, J.-B.; Pilard, S.; Laruelle, S. Gas Chromatography/Mass Spectrometry As a Suitable Tool for the Li-Ion Battery Electrolyte Degradation Mechanisms Study. Anal. Chem. 2010, 83, 478–485. [Google Scholar] [CrossRef]
- Spinner, N.S.; Field, C.R.; Hammond, M.H.; Williams, B.A.; Myers, K.M.; Lubrano, A.L.; Rose-Pehrsson, S.L.; Tuttle, S.G. Physical and chemical analysis of lithium-ion battery cell-to-cell failure events inside custom fire chamber. J. Power Sources 2015, 279, 713–721. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2013, 4, 3633–3642. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Lu, S.; Li, K.; Liu, C.; Cheng, X.; Zhang, H. An experimental study on burning behaviors of 18650 lithium ion batteries using a cone calorimeter. J. Power Sources 2015, 273, 216–222. [Google Scholar] [CrossRef]
- Ping, P. Lithium-Ion Battery Thermal Runway and Fire Risk Analysis and the Development on the Safer Battery System. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2014. [Google Scholar]
- Ribière, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ. Sci. 2011, 5, 5271–5280. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Xu, C.; Jin, C.; Li, K.; Du, Z.; Wang, Q.; Ouyang, M.; Feng, X. Dynamic thermophysical modeling of thermal runaway propagation and parametric sensitivity analysis for large format lithium-ion battery modules. J. Power Sources 2021, 520, 230724. [Google Scholar] [CrossRef]
- Liu, P.; Liu, C.; Yang, K.; Zhang, M.; Gao, F.; Mao, B.; Li, H.; Duan, Q.; Wang, Q. Thermal runaway and fire behaviors of lithium iron phosphate battery induced by over heating. J. Energy Storage 2020, 31, 101714. [Google Scholar] [CrossRef]
- Zhou, Z.; Ju, X.; Zhou, X.; Yang, L.; Cao, B. A comprehensive study on the impact of heating position on thermal runaway of prismatic lithium-ion batteries. J. Power Sources 2022, 520, 230919. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.-E. Toxic fluoride gas emissions from lithium-ion battery fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somandepalli, V.; Marr, K.; Horn, Q. Quantification of Combustion Hazards of Thermal Runaway Failures in Lithium-Ion Batteries. SAE Int. J. Altern. Powertrains 2014, 3, 98–104. [Google Scholar] [CrossRef]
- Wang, H.; Shi, W.; Hu, F.; Wang, Y.; Hu, X.; Li, H. Over-heating triggered thermal runaway behavior for lithium-ion battery with high nickel content in positive electrode. Energy 2021, 224, 120072. [Google Scholar] [CrossRef]
- Abraham, D.; Roth, E.; Kostecki, R.; McCarthy, K.; MacLaren, S.; Doughty, D. Diagnostic examination of thermally abused high-power lithium-ion cells. J. Power Sources 2006, 161, 648–657. [Google Scholar] [CrossRef]
- Hoelle, S.; Scharner, S.; Asanin, S.; Hinrichsen, O. Analysis on Thermal Runaway Behavior of Prismatic Lithium-Ion Batteries with Autoclave Calorimetry. J. Electrochem. Soc. 2021, 168, 120515. [Google Scholar] [CrossRef]
- Kondo, H.; Baba, N.; Makimura, Y.; Itou, Y.; Kobayashi, T. Model validation and simulation study on the thermal abuse behavior of LiNi0.8Co0.15Al0.05O2-based batteries. J. Power Sources 2019, 448, 227464. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, S.; Li, K.; Zhao, M.; Qiu, Z.; Han, D.; Zhang, G.; Habetler, T.G. Hazard analysis of thermally abused lithium-ion batteries at different state of charges. J. Energy Storage 2019, 27, 101065. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Zhang, P. Effects of the battery enclosure on the thermal behaviors of lithium-ion battery module during TR propagation by external-heating. J. Energy Storage 2022, 48, 104002. [Google Scholar] [CrossRef]
- Teng, X.; Zhan, C.; Bai, Y.; Ma, L.; Liu, Q.; Wu, C.; Wu, F.; Yang, Y.; Lu, J.; Amine, K. In Situ Analysis of Gas Generation in Lithium-Ion Batteries with Different Carbonate-Based Electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 22751–22755. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Shin, J.-S.; Han, C.-H.; Jung, U.-H.; Lee, S.-I.; Kim, H.-J.; Kim, K. Effect of Li2CO3 additive on gas generation in lithium-ion batteries. J. Power Sources 2002, 109, 47–52. [Google Scholar] [CrossRef]
- Zou, K.; Lu, S.; Chen, X.; Gao, E.; Cao, Y.; Bi, Y. Thermal and gas characteristics of large-format LiNi0.8Co0.1Mn0.1O2 pouch power cell during thermal runaway. J. Energy Storage 2021, 39, 102609. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, C.; Li, H.; Peng, W.; Zhang, Z.; Wang, Q. Experimental study on thermal runaway and its propagation in the large format lithium ion battery module with two electrical connection modes. Energy 2020, 205, 117906. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Han, Q.; Su, Q.; Chen, Y.; Li, J.; Lei, T.; Chen, Y.; Chen, J. Effect of high-temperature crystallization on the electrochemical properties of LiNi0.5Co0.2Mn0.3O2 synthesized from a lithiated transition metal oxide precursor. Ionics 2018, 24, 2957–2963. [Google Scholar] [CrossRef]
- Wang, S.I.; Bi, M.S.; Li, Y. Industrial multivariate mixed gas explosion limit calculating. Chem. Equip. Technol. 2000, 28–30. [Google Scholar] [CrossRef]
- Bak, S.-M.; Hu, E.; Zhou, Y.; Yu, X.; Senanayake, S.D.; Cho, S.-J.; Kim, K.-B.; Chung, K.Y.; Yang, X.-Q.; Nam, K.-W. Structural Changes and Thermal Stability of Charged LiNixMnyCozO2 Cathode Materials Studied by Combined In Situ Time-Resolved XRD and Mass Spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Konishi, H.; Yuasa, T.; Yoshikawa, M. Thermal stability of Li1−yNixMn(1−x)/2Co(1−x)/2O2 layer-structured cathode materials used in Li-Ion batteries. J. Power Sources 2011, 196, 6884–6888. [Google Scholar] [CrossRef]
- Röder, P.; Baba, N.; Friedrich, K.A.; Wiemhöfer, H.-D. Impact of delithiated Li0FePO4 on the decomposition of LiPF6-based electrolyte studied by accelerating rate calorimetry. J. Power Sources 2013, 236, 151–157. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Y.-C.; Jenkins, D.M.; Chernova, N.A.; Chung, Y.; Radhakrishnan, B.; Chu, I.-H.; Fang, J.; Wang, Q.; Omenya, F.; et al. Thermal Stability and Reactivity of Cathode Materials for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 7013–7021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ping, P.; Wang, Q.; Huang, P.; Li, K.; Sun, J.; Kong, D.; Chen, C. Study of the fire behavior of high-energy lithium-ion batteries with full-scale burning test. J. Power Sources 2015, 285, 80–89. [Google Scholar] [CrossRef]
- Yang, H.; Shen, X.-D. Dynamic TGA–FTIR studies on the thermal stability of lithium/graphite with electrolyte in lithium-ion cell. J. Power Sources 2007, 167, 515–519. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Zhang, Y.; Ouyang, M. Flammability characteristics of the battery vent gas: A case of NCA and LFP lithium-ion batteries during external heating abuse. J. Energy Storage 2019, 24, 100775. [Google Scholar] [CrossRef]
- Liu, B. Study on explosion limit prediction of mixed combustible gas. Guangdong Chem. Ind. 2018, 45, 119–121. [Google Scholar]
- Röder, P.; Baba, N.; Wiemhöfer, H.-D. A detailed thermal study of a Li[Ni0.33Co0.33Mn0.33]O2/LiMn2O4-based lithium ion cell by accelerating rate and differential scanning calorimetry. J. Power Sources 2013, 248, 978–987. [Google Scholar] [CrossRef]
- Richard, M.N.; Dahn, J.R. Accelerating Rate Calorimetry Study on the Thermal Stability of Lithium Intercalated Graphite in Electrolyte. I. Experimental. J. Electrochem. Soc. 1999, 146, 2068–2077. [Google Scholar] [CrossRef]
- Jiang, J.; Dahn, J.R. ARC studies of the thermal stability of three different cathode materials: LiCoO2; Li[Ni0.1Co0.8Mn0.1]O2; and LiFePO4, in LiPF6 and LiBoB EC/DEC electrolytes. Electrochem. Commun. 2004, 6, 39–43. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes—Impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef] [Green Version]
- Gnanaraj, J.S.; Zinigrad, E.; Asraf, L.; Gottlieb, H.E.; Sprecher, M.; Aurbach, D.; Schmidt, M. The use of accelerating rate calorimetry (ARC) for the study of the thermal reactions of Li-ion battery electrolyte solutions. J. Power Sources 2003, 119–121, 794–798. [Google Scholar] [CrossRef]
- Belharouak, I.; Lu, W.; Vissers, D.; Amine, K. Safety characteristics of Li(Ni0.8Co0.15Al0.05)O2 and Li(Ni1/3Co1/3Mn1/3)O2. Electrochem. Commun. 2006, 8, 329–335. [Google Scholar] [CrossRef]
- Kawamura, T.; Kimura, A.; Egashira, M.; Okada, S.; Yamaki, J.-I. Thermal stability of alkyl carbonate mixed-solvent electrolytes for lithium ion cells. J. Power Sources 2002, 104, 260–264. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zhang, Z.; Wang, Q.; Jin, C.; Wu, C.; Xu, C.; Hao, J.; Sun, L.; Du, Z.; et al. Fire and explosion characteristics of vent gas from lithium-ion batteries after thermal runaway: A comparative study. eTransportation 2022, 13, 100190. [Google Scholar] [CrossRef]






| Related Researchers | Research Object | Test Instrument | Test Result |
|---|---|---|---|
| Zhang et al. [8] | Type: Square battery Capacity: 50 Ah Anode: Graphite | Adiabatic test chamber | 1: TR can occur when the jet temperature at the vent valve position increases. 2: The maximum temperature can reach up to 701 °C. 3: As the SOC of the battery increases, the jet velocity and temperature also increase. |
| Qin et al. [20] | Capacity: 2.6 Ah Anode: Graphite | ARC | 1: The rate of temperature increase in the battery before the second stage of TR, known as ‘Tsc’, does not have a linear relationship with the gas production rate. 2: The rise in internal battery pressure is caused by the gas generated during the redox reaction occurring inside the battery. |
| Yuan et al. [22] | (1) Capacity: 3.8 Ah Anode: Graphite (2) Capacity: 1.3 Ah Anode: Graphite (3) Capacity: 3.2 Ah Cathode: NCM Anode: Graphite | ARC DSC GC | 1: The NCM battery exhibits a low initial temperature for TR, but a relatively high maximum temperature for gas production and TR. 2: The LTO battery experiences a low maximum temperature during TR and produces less gas. 3: The LFP battery has a relatively high initial temperature for TR, while the maximum temperature and gas production rate are between those of the LTO and NCM batteries. |
| Wang et al. [35] | Type: Cylindrical battery Capacity: 4.6 Ah Anode: Graphite | Self-made experimental device TR comparison experiment | 1: NCM811 compared to NCM111, NCM532, and NCM622, increasing the nickel content in the positive electrode amplifies the damage caused by TR in the battery. |
| Abraham et al. [36] | Capacity: 1 Ah Anode: Mag-10 Graphite | Microscope, Spectrometer, diffraction method, ARC | 1: The research provides evidence for the sequence of events leading to battery TR and the corresponding sequence of gas generation sources. |
| S. Hoelle et al. [37] | Capacity: 8–145 Ah Cathode: NCM, NCA, LMO Anode: Graphite | Battery needle test bench | 1: The gas production of LIBs with different ampere hours was examined and standardized, and the findings revealed that the range of gas production was between 1.6 L/Ah and 2.8 L/Ah. |
| Kondo et al. [38] | Capacity: 0.5 Ah Anode: Graphite | Combining DSC and simulation | 1: The thermal properties of the battery were determined via DSC experiments. 2: A simulation was carried out to examine the thermal abuse of the battery. |
| Liao [39] | Capacity: 2.4 Ah Anode: Graphite | Self-made 24 L sealed high pressure vessel | 1: The maximum temperature during TR of a battery increases linearly with the SOC. . 3: This process also generates harmful environmental substances such as benzene. |
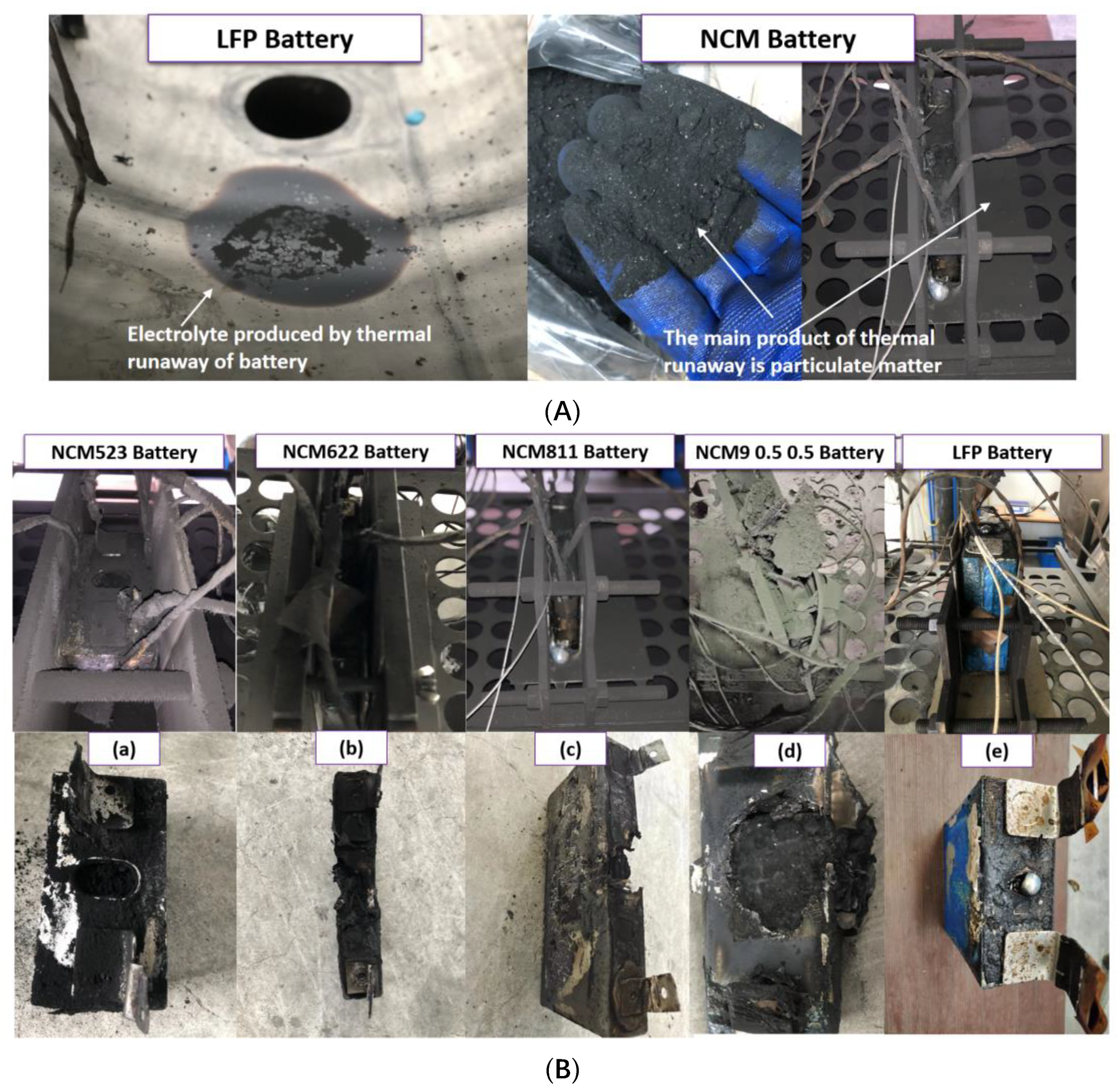
| This study | (1) Capacity: 304 Ah Square battery Anode: Graphite (2) Capacity: 118 Ah Square battery Anode: Graphite (3) Capacity: 50 Ah Square battery Anode: Graphite (4) Capacity: 153 Ah Square battery Anode: Graphite (5) Capacity: 165 Ah Square battery Anode: Graphite | 1: Inert atmosphere 2: GC-MS | 1: The normalized gas production of NCM batteries ranges from 1.8 to 2.8 L/Ah, while that of LFP batteries is only 0.569 L/Ah. 2: Based on gas production, the degree of harm caused by TR is ranked as follows: NCM 9 0.5 0.5 > NCM 811 > NCM 622 > NCM 523 > LFP. 3: LFP battery TR produces a large amount of electrolyte, while NCM battery generates a large number of particles. are the main gas components generated during TR of NCM and LFP batteries. 5: The flammability limit of the TR gas of the battery was calculated, and the risk of TR of LFP and NCM batteries was re-evaluated from the perspective of flammability limit. |
| Cell | LFP | NCM523 | NCM622 | NCM811 | NCM 9 0.5 0.5 |
|---|---|---|---|---|---|
| Shape | Square | Square | Square | Square | Square |
| Cathode | |||||
| Anode | Graphite | Graphite | Graphite | Graphite | Graphite |
| Specific energy (Wh/kg) | 172.51 | 247.43 | 234.03 | 273.06 | 324.95 |
| Weight (g) | 5639 | 2628 | 908 | 1815 | 2158 |
| Upper limit cut-off voltage (V) | 3.65 | 4.3 | 4.3 | 4.3 | 4.3 |
| Lower cut-off voltage (V) | 2.5 | 2.8 | 2.8 | 2.8 | 2.8 |
| Wrapper Material | Al Alloy | Al Alloy | Al Alloy | Al Alloy | Al Alloy |
| Capacity (Ah) | 304 | 153 | 50 | 118 | 165 |
| Max discharge current | 2C | 2C | 2C | 1C | 1C |
| Temperature range for normal Operation (°C) | −40~55 | −40~50 | −40~45 | −40~55 | −40~50 |
| Jellyroll | 2 | 2 | 2 | 2 | 2 |
| SOC | 100% | 100% | 100% | 100% | 100% |
| (°C) | T1 | T2 | T3 | ||
|---|---|---|---|---|---|
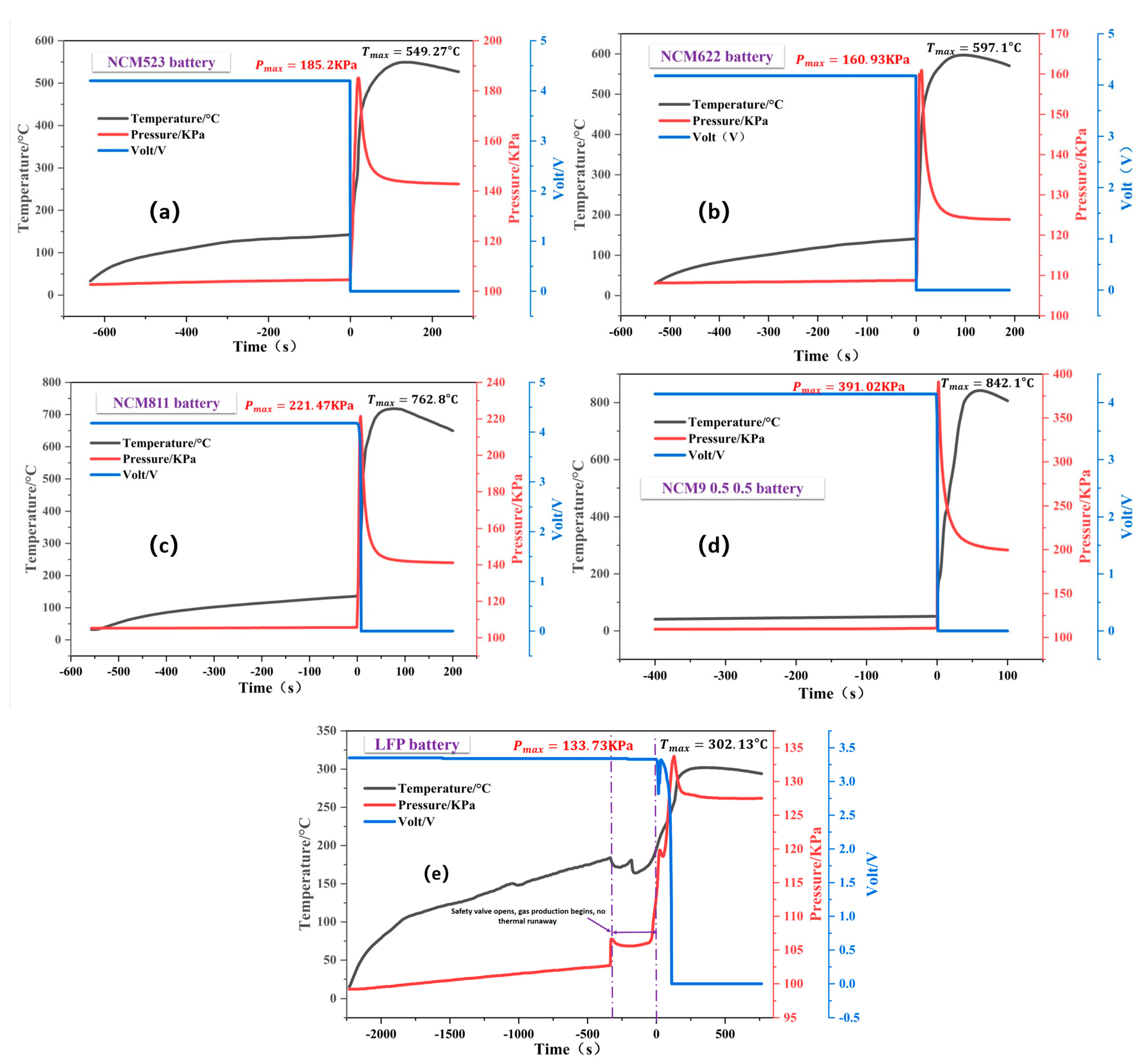
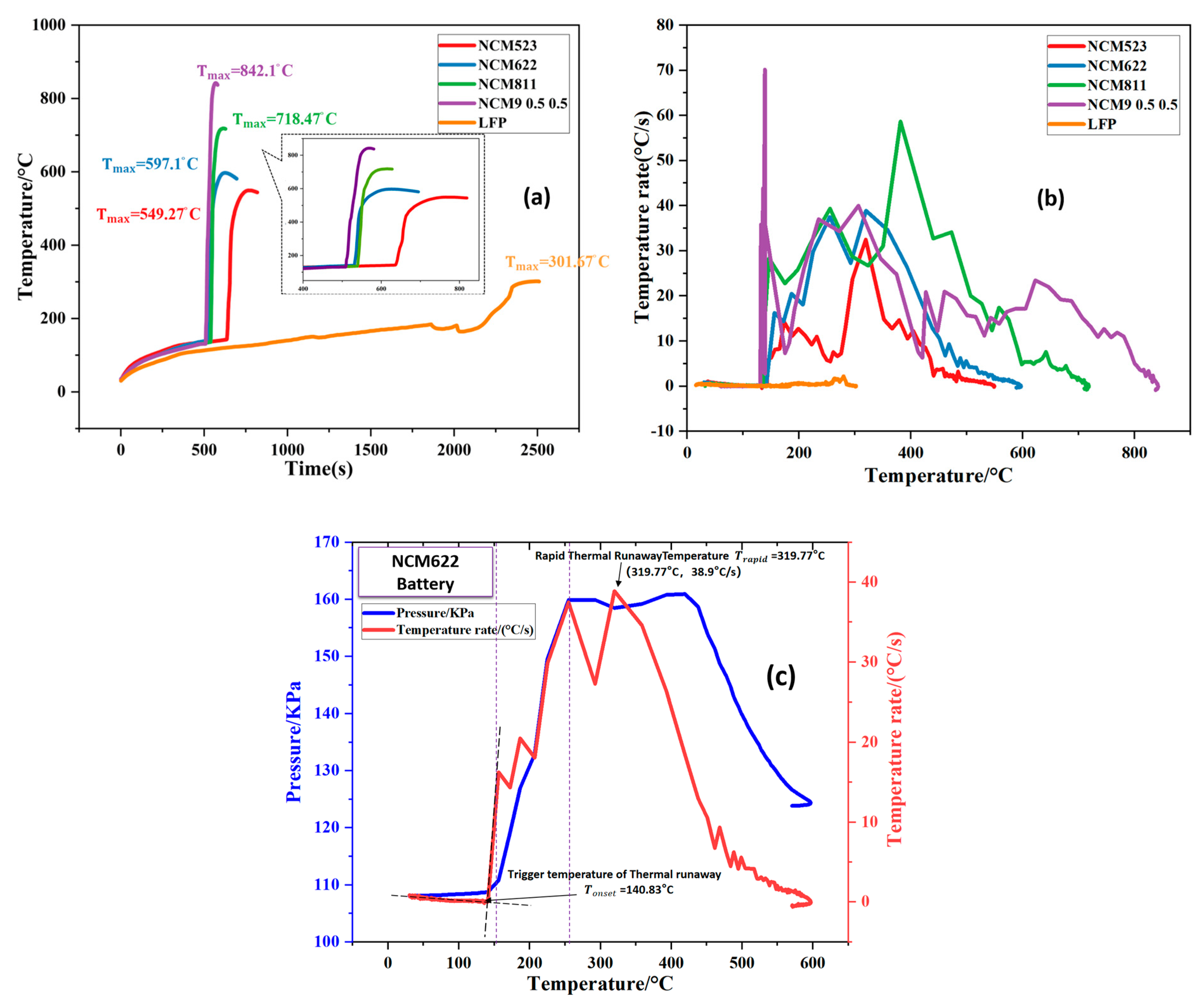
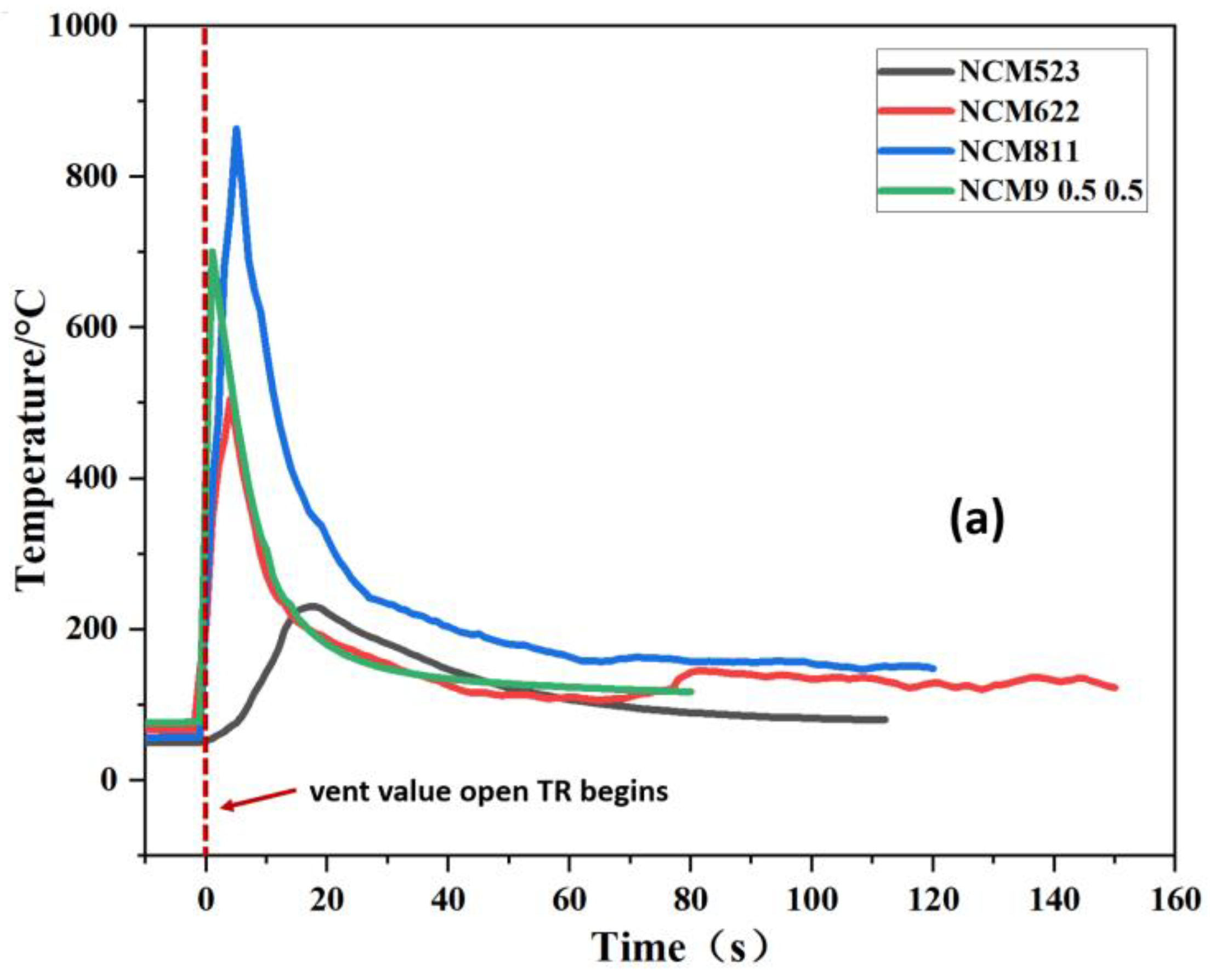
| NCM523 | 370.6 | 589.3 | 695.5 | 549.3 | 142.7 |
| NCM622 | 555.9 | 504.8 | 600.6 | 597.1 | 140.8 |
| NCM811 | 564.2 | 767.6 | 826.1 | 762.8 | 135.6 |
| NCM9 0.5 0.5 | 843.5 | 903.7 | 943.9 | 842.1 | 130.6 |
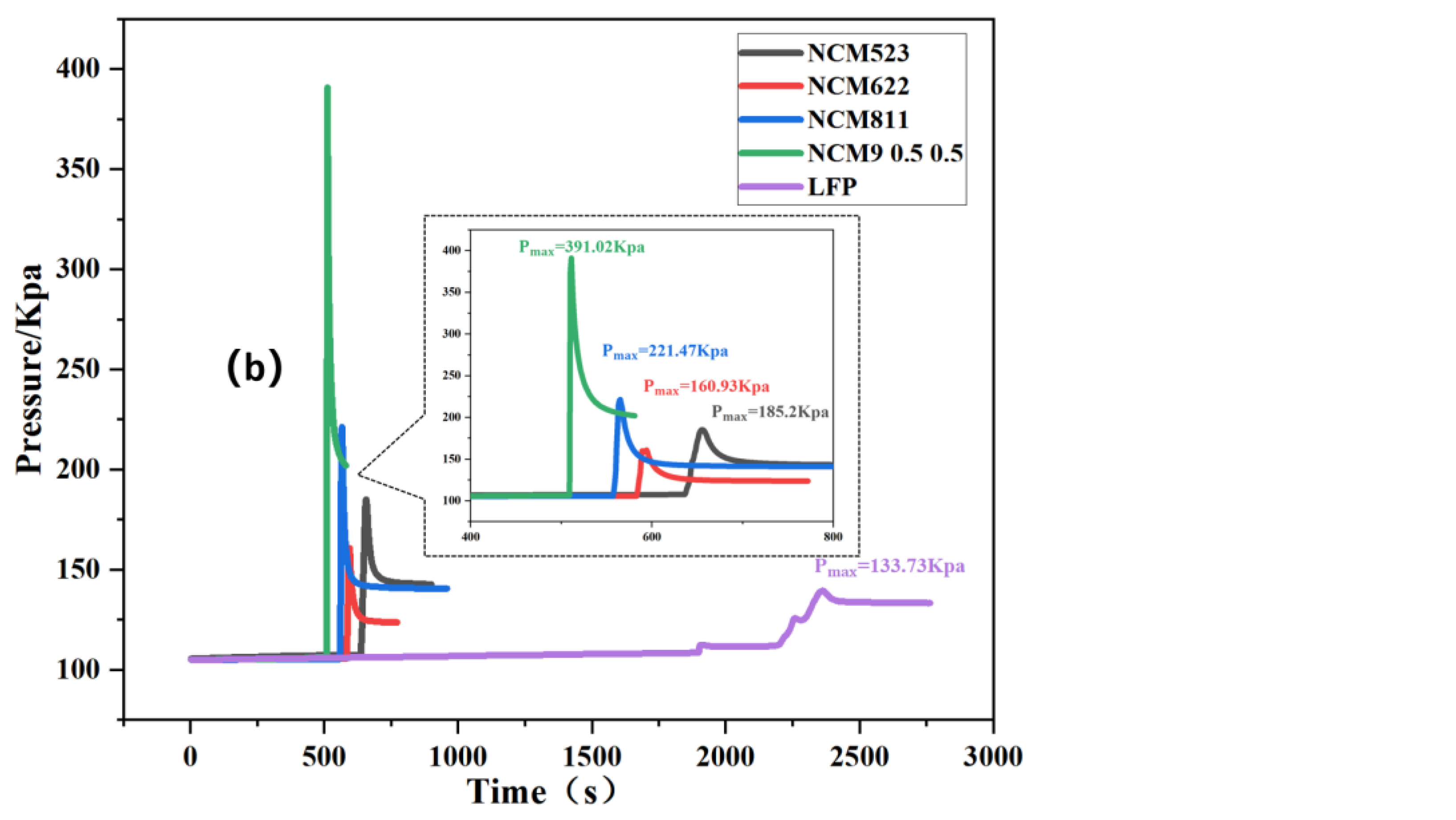
| LFP | 170.9 | 306.6 | 559.2 | 302.1 | 184.0 |
| Cell | n (mol) | L/Ah |
|---|---|---|
| NCM523 | 12.39 | 1.814 |
| NCM622 | 4.99 | 2.236 |
| NCM811 | 12.09 | 2.295 |
| NCM9 0.5 0.5 | 20.27 | 2.752 |
| LFP | 7.72 | 0.569 |
| Gas Type | ||
|---|---|---|
| 74 | 12.5 | |
| 75.6 | 4 | |
| 74.1 | 4.2 | |
| 15 | 5 | |
| 36 | 2.7 | |
| 13 | 2.9 | |
| 1.3- | 16.3 | 1.1 |
| 10.3 | 2.4 | |
| 9.5 | 2.2 |
| Cell | Mass Loss Rate (%) | |
|---|---|---|
| NCM523 | 1.614 | 37.84 |
| NCM622 | 0.518 | 40.36 |
| NCM811 | 1.034 | 48.67 |
| NCM9 0.5 0.5 | 0.910 | 62.89 |
| LFP | 50.619 | 19.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Wang, H.; Li, M.; Li, C.; Zhang, Y.; Li, Y.; Yang, X.; Feng, X.; Ouyang, M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics 2023, 12, 1603. https://doi.org/10.3390/electronics12071603
Shen H, Wang H, Li M, Li C, Zhang Y, Li Y, Yang X, Feng X, Ouyang M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics. 2023; 12(7):1603. https://doi.org/10.3390/electronics12071603
Chicago/Turabian StyleShen, Hengjie, Hewu Wang, Minghai Li, Cheng Li, Yajun Zhang, Yalun Li, Xinwei Yang, Xuning Feng, and Minggao Ouyang. 2023. "Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere" Electronics 12, no. 7: 1603. https://doi.org/10.3390/electronics12071603
APA StyleShen, H., Wang, H., Li, M., Li, C., Zhang, Y., Li, Y., Yang, X., Feng, X., & Ouyang, M. (2023). Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics, 12(7), 1603. https://doi.org/10.3390/electronics12071603







