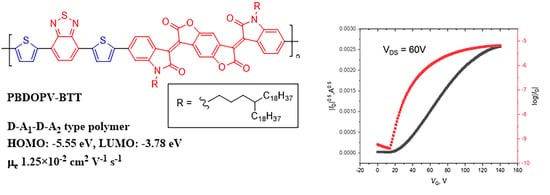
N-Type Charge Carrier Transport Properties of BDOPV-Benzothiadiazole-Based Semiconducting Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. General Measurements
2.3. Thin Film Transistors
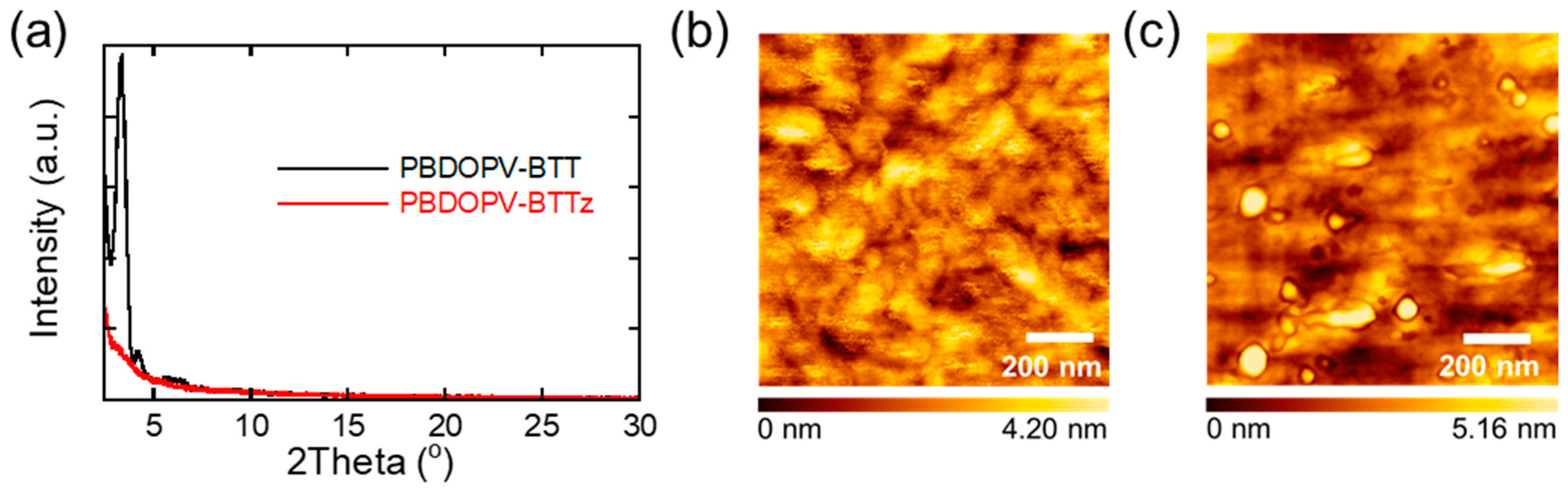
2.4. X-ray Diffraction
2.5. Atomic Force Microscopy
3. Results and Discussion
3.1. Synthesis
3.2. Optical and Electrochemical Properties
3.3. Computational Calculations
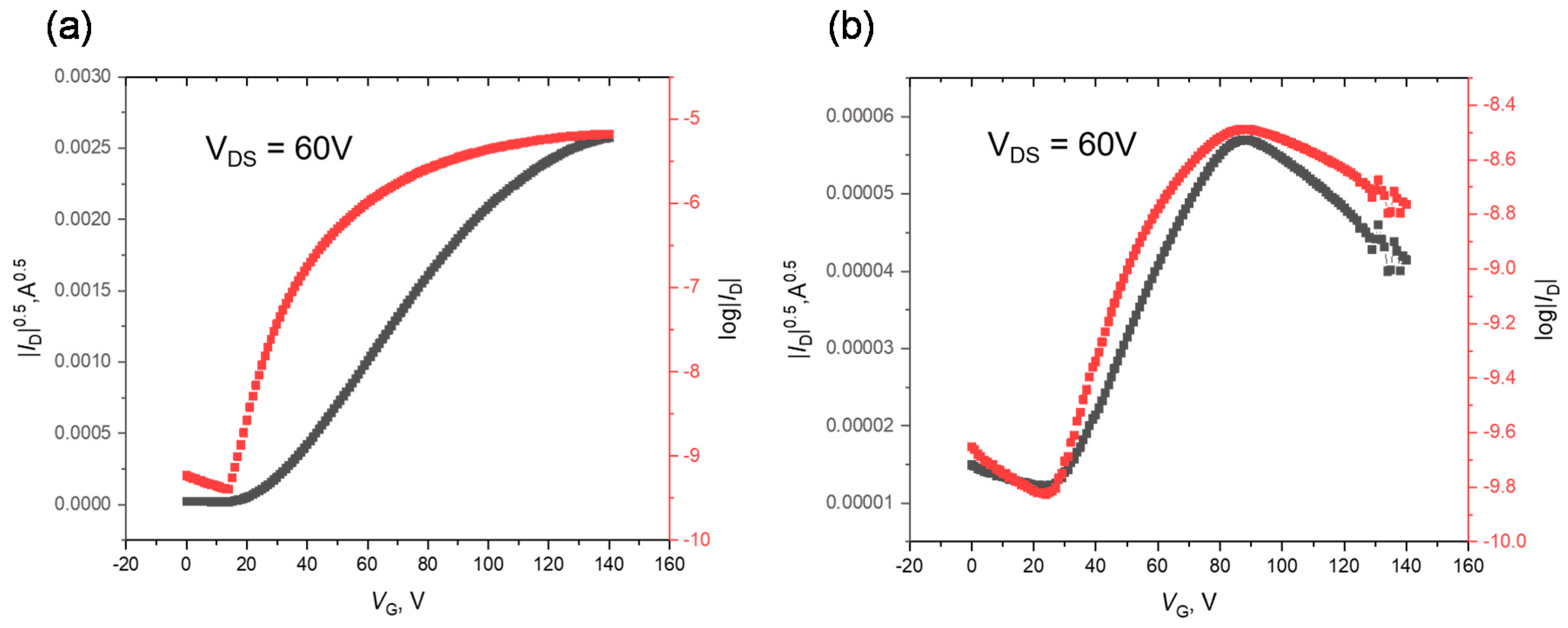
3.4. Thin Film Transistor Performances
3.5. Thin Film Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao, Y.; Dong, H.; Hu, W. Charge Transport in Organic and Polymeric Semiconductors for Flexible and Stretchable Devices. Adv. Mater. 2016, 28, 4513–4523. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, Y.; Liu, Y. Engineering of Amorphous Polymeric Insulators for Organic Field-Effect Transistors. Adv. Electron. Mater. 2017, 3, 1700157. [Google Scholar] [CrossRef]
- Shi, L.; Guo, Y.; Hu, W.; Liu, Y. Design and effective synthesis methods for high-performance polymer semiconductors in organic field-effect transistors. Mater. Chem. Front. 2017, 1, 2423–2456. [Google Scholar] [CrossRef]
- Wang, B.; Huang, W.; Chi, L.; Al-Hashimi, M.; Marks, T.J.; Facchetti, A. High-k Gate Dielectrics for Emerging Flexible and Stretchable Electronics. Chem. Rev. 2018, 118, 5690–5754. [Google Scholar] [CrossRef] [PubMed]
- Nketia-Yawson, B.; Noh, Y.-Y. Recent Progress on High-Capacitance Polymer Gate Dielectrics for Flexible Low-Voltage Transistors. Adv. Funct. Mater. 2018, 28, 1802201. [Google Scholar] [CrossRef]
- Wang, Y.; Michinobu, T. Rational design strategies for electron-deficient semiconducting polymers in ambipolar/n-channel organic transistors and all-polymer solar cells. J. Mater. Chem. C 2018, 6, 10390–10410. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Chen, X.; Bao, Z.; Someya, T. Materials and structural designs of stretchable conductors. Chem. Soc. Rev. 2019, 48, 2946–2966. [Google Scholar] [CrossRef]
- Sun, H.; Guo, X.; Facchetti, A. High-Performance n-Type Polymer Semiconductors: Applications, Recent Development, and Challenges. Chem 2020, 6, 1310–1326. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Dubal, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef]
- Kimpel, J.; Michinobu, T. Conjugated polymers for functional applications: Lifetime and performance of polymeric organic semiconductors in organic field-effect transistors. Polym. Int. 2020. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.-R.; Zhou, Y.; Han, S.-T. Ambipolar polymers for transistor applications. Polym. Int. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Sonar, P.; Murphy, L.; Hong, W. High mobility diketopyrrolopyrrole (DPP)-based organic semiconductor materials for organic thin film transistors and photovoltaics. Energy Environ. Sci. 2013, 6, 1684–1710. [Google Scholar] [CrossRef]
- Wang, E.; Mammo, W.; Andersson, M.R. 25th Anniversary Article: Isoindigo-Based Polymers and Small Molecules for Bulk Heterojunction Solar Cells and Field Effect Transistors. Adv. Mater. 2014, 26, 1801–1826. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Wang, J.-Y.; Pei, J. Design, Synthesis, and Structure-Property Relationships of Isoindigo-Based Conjugated Polymers. Acc. Chem. Res. 2014, 47, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Stalder, R.; Mei, J.; Graham, K.R.; Estrada, L.A.; Reynolds, J.R. Isoindigo, a Versatile Electron-Deficient Unit for High-Performance Organic Electronics. Chem. Mater. 2014, 26, 664–678. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, S.; Liu, Y. Design of High-Mobility Diketopyrrolopyrrole-Based-Conjugated Copolymers for Organic Thin-Film Transistors. Adv. Mater. 2015, 27, 3589–3606. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hendriks, K.H.; Wienk, M.M.; Janssen, R.A.J. Diketopyrrolopyrrole Polymers for Organic Solar Cells. Acc. Chem. Res. 2016, 49, 78–85. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, 1903882. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Kohara, A.; Matsumoto, H.; Manzhos, S.; Feron, K.; Bottle, S.E.; Michinobu, T.; Sonar, P. Tuning the Charge Carrier Polarity of Organic Transistors by Varying the Electron Affinity of the Flanked Units in Diketopyrrolopyrrole-Based Copolymers. Adv. Funct. Mater. 2020, 30, 1907452. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Ren, Y.; Kohara, A.; Matsumoto, H.; Chen, Y.; Manzhos, S.; Feron, K.; Bottle, S.E.; Bell, J.; et al. Diketopyrrolopyrrole-Based Dual-Acceptor Copolymers to Realize Tunable Charge Carrier Polarity of Organic Field-Effect Transistors and High-Performance Nonvolatile Ambipolar Flash Memories. ACS Appl. Electron. Mater. 2020, 2, 1609–1618. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 57, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hu, Y. Development of n-type organic semiconductors for thin film transistors: A viewpoint of molecular design. J. Mater. Chem. C 2014, 2, 3099–3117. [Google Scholar] [CrossRef]
- Wang, Y.; Michinobu, T. Benzothiadiazole and its π-extended, heteroannulated derivatives: Useful acceptor building blocks for high-performance donor-acceptor polymers in organic electronics. J. Mater. Chem. C 2016, 4, 6200–6214. [Google Scholar] [CrossRef]
- Wang, Y.; Hasegawa, T.; Matsumoto, H.; Mori, T.; Michinobu, T. D-A1-D-A2 Backbone Strategy for Benzobisthiadiazole Based n-Channel Organic Transistors: Clarifying the Selenium-Substitution Effect on the Molecular Packing and Charge Transport Properties in Electron-Deficient Polymers. Adv. Funct. Mater. 2017, 27, 1701486. [Google Scholar] [CrossRef]
- Jia, H.; Lei, T. Emerging research directions for n-type conjugated polymers. J. Mater. Chem. C 2019, 7, 12809–12821. [Google Scholar] [CrossRef]
- Wang, Y.; Hasegawa, T.; Matsumoto, H.; Michinobu, T. Significant Improvement of Unipolar n-Type Transistor Performances by Manipulating the Coplanar Backbone Conformation of Electron-Deficient Polymers via Hydrogen Bonding. J. Am. Chem. Soc. 2019, 141, 3566–3575. [Google Scholar] [CrossRef]
- Wang, Y.; Hasegawa, T.; Matsumoto, H.; Michinobu, T. Significant Difference in Semiconducting Properties of Isomeric All-Acceptor Polymers Synthesized via Direct Arylation Polycondensation. Angew. Chem. Int. Ed. 2019, 58, 11893–11902. [Google Scholar] [CrossRef]
- Sui, Y.; Deng, Y.; Du, T.; Shi, Y.; Geng, Y. Design strategies of n-type conjugated polymers for organic thin-film transistors. Mater. Chem. Front. 2019, 3, 1932–1951. [Google Scholar] [CrossRef]
- Said, A.A.; Xie, J.; Wang, Y.; Wang, Z.; Zhou, Y.; Zhao, K.; Gao, W.-B.; Michinobu, T.; Zhang, Q. Efficient Inverted Perovskite Solar Cells by Employing N-Type (D-A1-D-A2) Polymers as Electron Transporting Layer. Small 2019, 15, 1803339. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, S.W.; Lee, J.; Matsumoto, H.; Kim, B.J.; Michinobu, T. Dual Imide-Functionalized Unit-Based Regioregular D-A1-D-A2 Polymers for Efficient Unipolar n-Channel Organic Transistors and All-Polymer Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 22583–22594. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Wang, Y.; You, H.; Lee, W.; Michinobu, T.; Kim, B.J. Impact of Incorporating Nitrogen Atoms in Naphthalenediimide-Based Polymer Acceptors on the Charge Generation, Device Performance, and Stability of All-Polymer Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 35896–35903. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, Y.; Mori, T.; Michinobu, T. Improving the air-stability of n-type organic thin-film transistors by polyacrylonitrile additive. Jpn. J. Appl. Phys. 2020, 59, SDDC05. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Chen, H.; Zheng, L.; Zhu, C.; Zhang, L.; Tan, S.; Wang, H.; Guo, Y.; Tang, Q.; et al. High- Performance, Air-Stable Field-Effect Transistors Based on Heteroatom-Substituted Naphthalenediimide-Benzothiadiazole Copolymers Exhibiting Ultrahigh Electron Mobility up to 8.5 cm V-1 s-1. Adv. Mater 2017, 29, 1602410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hasegawa, T.; Matsumoto, H.; Mori, T.; Michinobu, T. High-Performance n-Channel Organic Transistors Using High-Molecular-Weight Electron-Deficient Copolymers and Amine-Tailed Self-Assembled Monolayers. Adv. Mater. 2018, 30, 1707164. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Sun, B.; Li, Y. Novel stable (3E,7E)-3,7-bis(2-oxoindolin-3-ylidene)-benzo [1,2-b:4,5-b′]difuran-2,6(3H,7H)-dione based donor-acceptor polymer semiconductors for n-type organic thin film transistors. Chem. Commun. 2013, 49, 3790–3792. [Google Scholar] [CrossRef]
- Lei, T.; Dou, J.-H.; Cao, X.-Y.; Wang, J.-Y.; Pei, J. A BDOPV-Based Donor-Acceptor Polymer for High-Performance n-Type and Oxygen-Doped Ambipolar Field-Effect Transistors. Adv. Mater. 2013, 25, 6589–6593. [Google Scholar] [CrossRef]
- Lei, T.; Dou, J.-H.; Cao, X.-Y.; Wang, J.-Y.; Pei, J. Electron-Deficient Poly(p-phenylene vinylene) Provides Electron Mobility over 1 cm2 V-1 s-1 under Ambient Conditions. J. Am. Chem. Soc. 2013, 135, 12168–12171. [Google Scholar] [CrossRef]
- Lei, T.; Xia, X.; Wang, J.-Y.; Liu, C.-J.; Pei, J. “Conformation Locked” Strong Electron-Deficient Poly(p-Phenylene Vinylene) Derivatives for Ambient-Stable n-Type Field-Effect Transistors: Synthesis, Properties, and Effects of Fluorine Substitution Position. J. Am. Chem. Soc. 2014, 136, 2135–2141. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, F.; Di, C.-A.; Yan, T.-W.; Zou, Y.; Zhou, X.; Zhu, D.; Wang, J.-Y.; Pei, J. Toward High Performance n-Type Thermoelectric Materials by Rational Modification of BDPPV Backbones. J. Am. Chem. Soc. 2015, 137, 6979–6982. [Google Scholar] [CrossRef]
- Zhou, X.; Ai, N.; Guo, Z.-H.; Zhuang, F.-D.; Jiang, Y.-S.; Wang, J.-Y.; Pei, J. Balanced Ambipolar Organic Thin-Film Transistors Operated under Ambient Conditions: Role of the Donor Moiety in BDOPV-Based Conjugated Copolymers. Chem. Mater. 2015, 27, 1815–1820. [Google Scholar]
- Zheng, Y.-Q.; Lei, T.; Dou, J.-H.; Xia, X.; Wang, J.-Y.; Liu, C.-J.; Pei, J. Strong Electron-Deficient Polymers Lead to High Electron Mobility in Air and Their Morphology-Dependent Transport Behaviors. Adv. Mater. 2016, 28, 7213–7219. [Google Scholar] [PubMed]
- Zhao, X.; Madan, D.; Cheng, Y.; Zhou, J.; Li, H.; Thon, S.M.; Bragg, A.E.; DeCoster, M.E.; Hopkins, P.E.; Katz, H.E. High Conductivity and Electron-Transfer Validation in an n-Type Fluoride-Anion-Doped Polymer for Thermoelectrics in Air. Adv. Mater. 2017, 29, 1606928. [Google Scholar]
- Zheng, Y.-Q.; Yao, Z.-F.; Lei, T.; Dou, J.-H.; Yang, C.-Y.; Zou, L.; Meng, X.; Ma, W.; Wang, J.-Y.; Pei, J. Unraveling the Solution-State Supramolecular Structures of Donor-Acceptor Polymers and their Influence on Solid-State Morphology and Charge-Transport Properties. Adv. Mater. 2017, 29, 1701072. [Google Scholar]
- Shi, K.; Zhang, W.; Gao, D.; Zhang, S.; Lin, Z.; Zou, Y.; Wang, L.; Yu, G. Well-Balanced Ambipolar Conjugated Polymers Featuring Mild Glass Transition Temperatures Toward High-Performance Flexible Field-Effect Transistors. Adv. Mater. 2018, 30, 1705286. [Google Scholar]
- Wang, F.; Dai, Y.; Wang, W.; Lu, H.; Qiu, L.; Ding, Y.; Zhang, G. Incorporation of Heteroatoms in Conjugated Polymers Backbone toward Air-Stable, High-Performance n-Channel Unencapsulated Polymer Transistors. Chem. Mater. 2018, 30, 5451–5459. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]

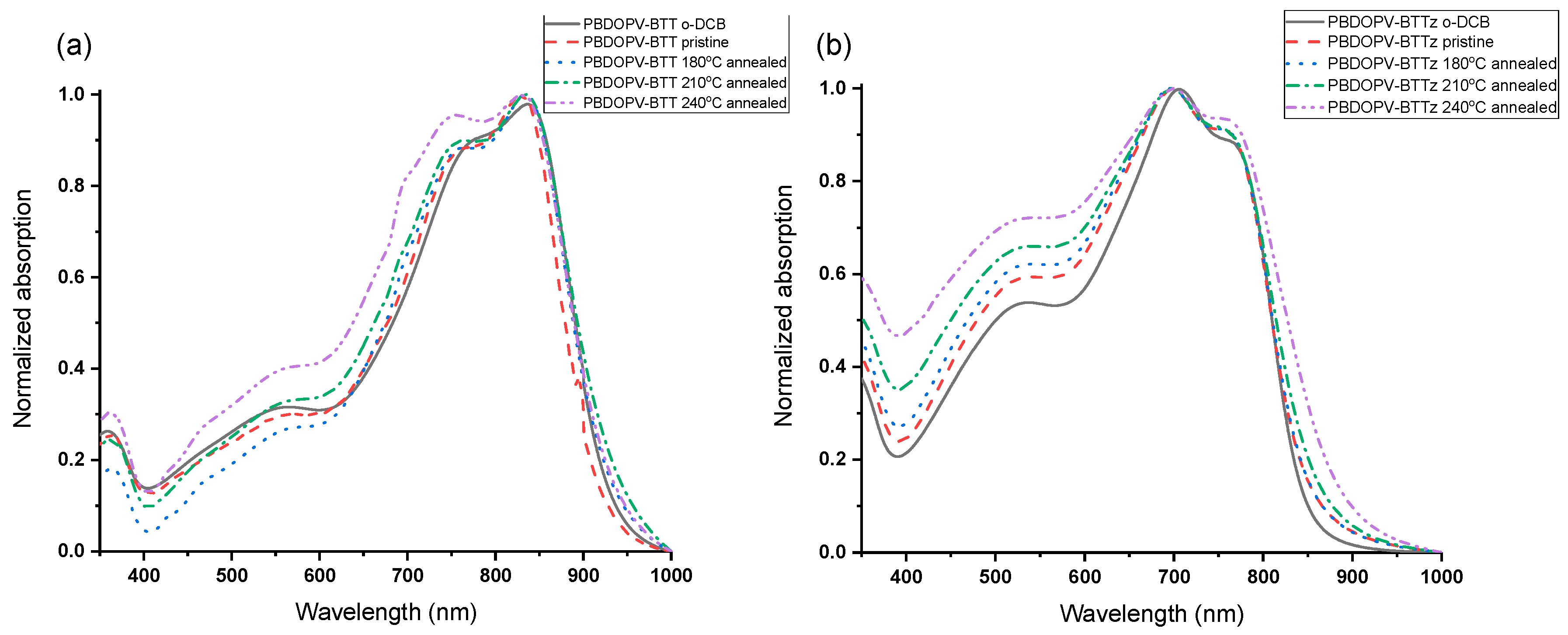


| λmax_sol (nm) 1 | λmax_film (nm) | λonset (nm) | Egopt (eV) 3 | Eox (V) | Ered (V) | EHOMO (eV) 4 | ELUMO (eV) 4 | EgCV (eV) | |
|---|---|---|---|---|---|---|---|---|---|
| PBDOPV-BTT | 836 | 828 | 930 | 1.33 | 0.85 | −0.92 | −5.55 | −3.78 | 1.72 |
| PBDOPV-BTTz | 762 2 | 757 2 | 865 | 1.43 | 1.10 | −0.90 | −5.80 | −3.80 | 2.00 |
| Annealing Temp. (°C) | μe max (cm2 V−1 s−1) | μe average (cm2 V−1 s−1) 1 | Ion/Ioff | Vth (V) | |
|---|---|---|---|---|---|
| PBDOPV-BTT | - | 5.00 × 10−3 | (2.92 ± 1.19) × 10−3 | 102–103 | 45 ± 7 |
| 150 | 5.21 × 10−3 | (3.61 ± 1.27) × 10−3 | 103–105 | 41 ± 6 | |
| 180 | 6.44 × 10−3 | (5.02 ± 1.37) × 10−3 | 102–104 | 27 ± 7 | |
| 210 | 3.97 × 10−3 | (2.24 ± 0.78) × 10−3 | 103–104 | 36 ± 9 | |
| 240 | 1.25 × 10−2 | (1.02 ± 0.14) × 10−2 | 103–105 | 18 ± 12 | |
| PBDOPV-BTTz | - | 4.09 × 10−6 | (2.88 ± 0.79) × 10−6 | 10–102 | 12 ± 8 |
| 150 | 4.33 × 10−6 | (3.13 ± 0.98) × 10−6 | 10 | 4 ± 7 | |
| 180 | 7.40 × 10−6 | (4.86 ± 1.30) × 10−6 | 10–102 | 13 ± 7 | |
| 210 | 1.06 × 10−5 | (9.54 ± 0.73) × 10−6 | 10–102 | 13 ± 10 | |
| 240 | 6.81 × 10−6 | (4.42 ± 1.70) × 10−6 | 10 | 25 ± 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Otep, S.; Kimpel, J.; Mori, T.; Michinobu, T. N-Type Charge Carrier Transport Properties of BDOPV-Benzothiadiazole-Based Semiconducting Polymers. Electronics 2020, 9, 1604. https://doi.org/10.3390/electronics9101604
Wang S, Otep S, Kimpel J, Mori T, Michinobu T. N-Type Charge Carrier Transport Properties of BDOPV-Benzothiadiazole-Based Semiconducting Polymers. Electronics. 2020; 9(10):1604. https://doi.org/10.3390/electronics9101604
Chicago/Turabian StyleWang, Siyu, Sultan Otep, Joost Kimpel, Takehiko Mori, and Tsuyoshi Michinobu. 2020. "N-Type Charge Carrier Transport Properties of BDOPV-Benzothiadiazole-Based Semiconducting Polymers" Electronics 9, no. 10: 1604. https://doi.org/10.3390/electronics9101604
APA StyleWang, S., Otep, S., Kimpel, J., Mori, T., & Michinobu, T. (2020). N-Type Charge Carrier Transport Properties of BDOPV-Benzothiadiazole-Based Semiconducting Polymers. Electronics, 9(10), 1604. https://doi.org/10.3390/electronics9101604