Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease
Abstract
1. Osteoporosis in CKD
2. Sclerostin as Marker of Bone Turnover in CKD
3. Serum Sclerostin Is Increased in CKD
4. Serum Sclerostin in CKD: Bone or Vessels?
5. Sclerostin and Cardiovascular Events
6. Sclerostin and Patient Outcome in CKD
7. Sclerostin, BMD and Fractures in CKD
8. Anti-Sclerostin Therapy
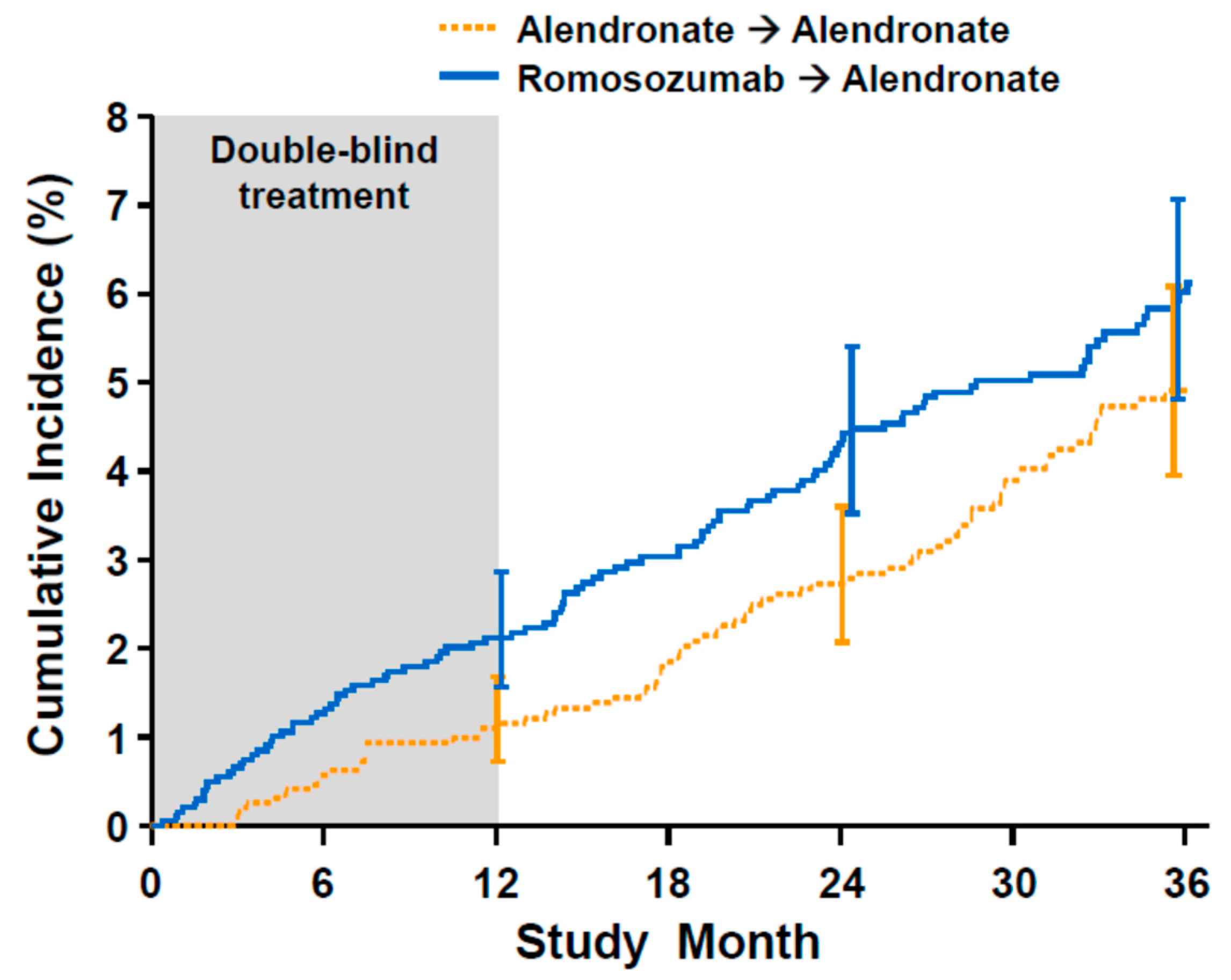
9. Romosozumab and Cardiovascular Events
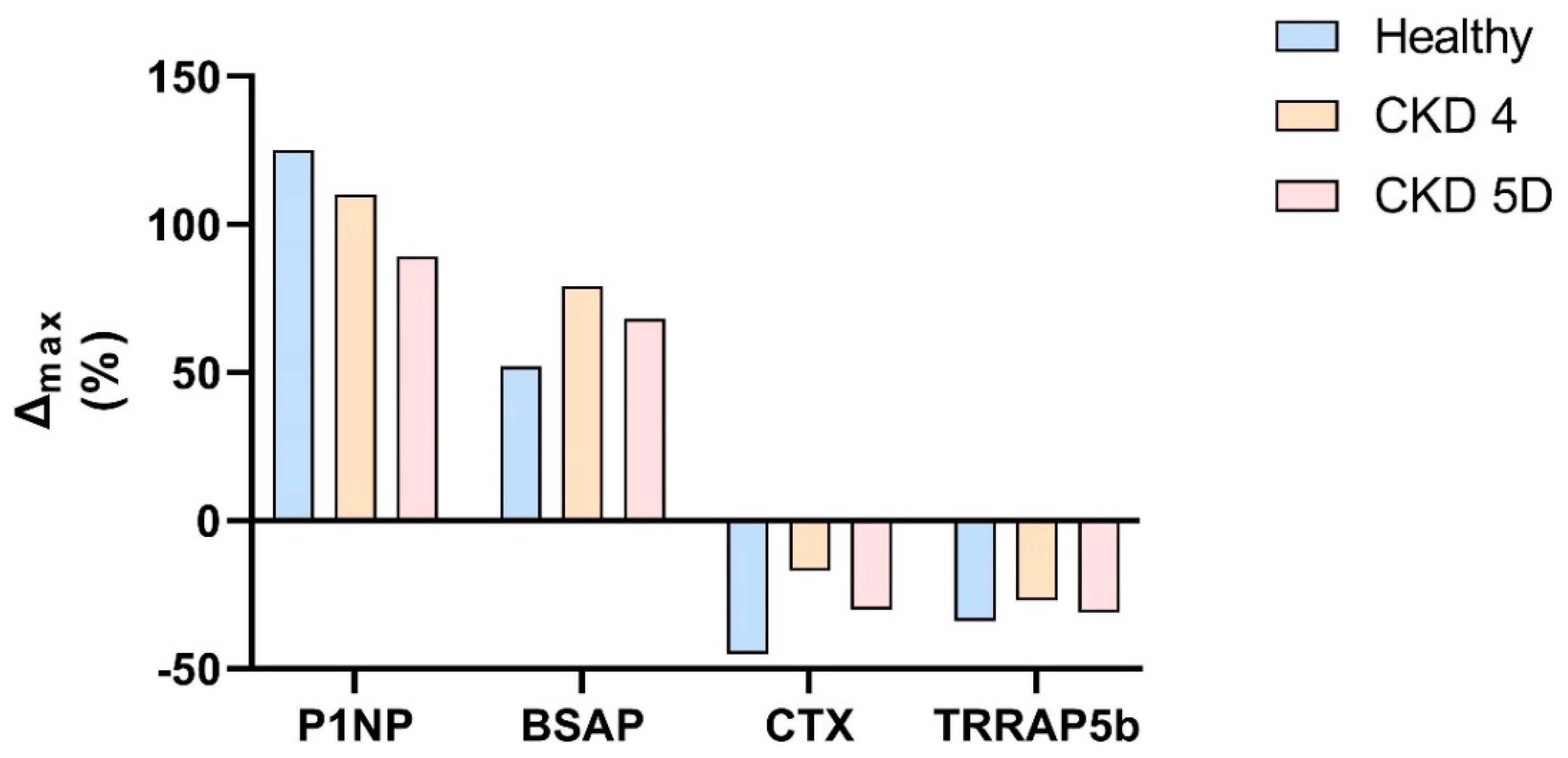
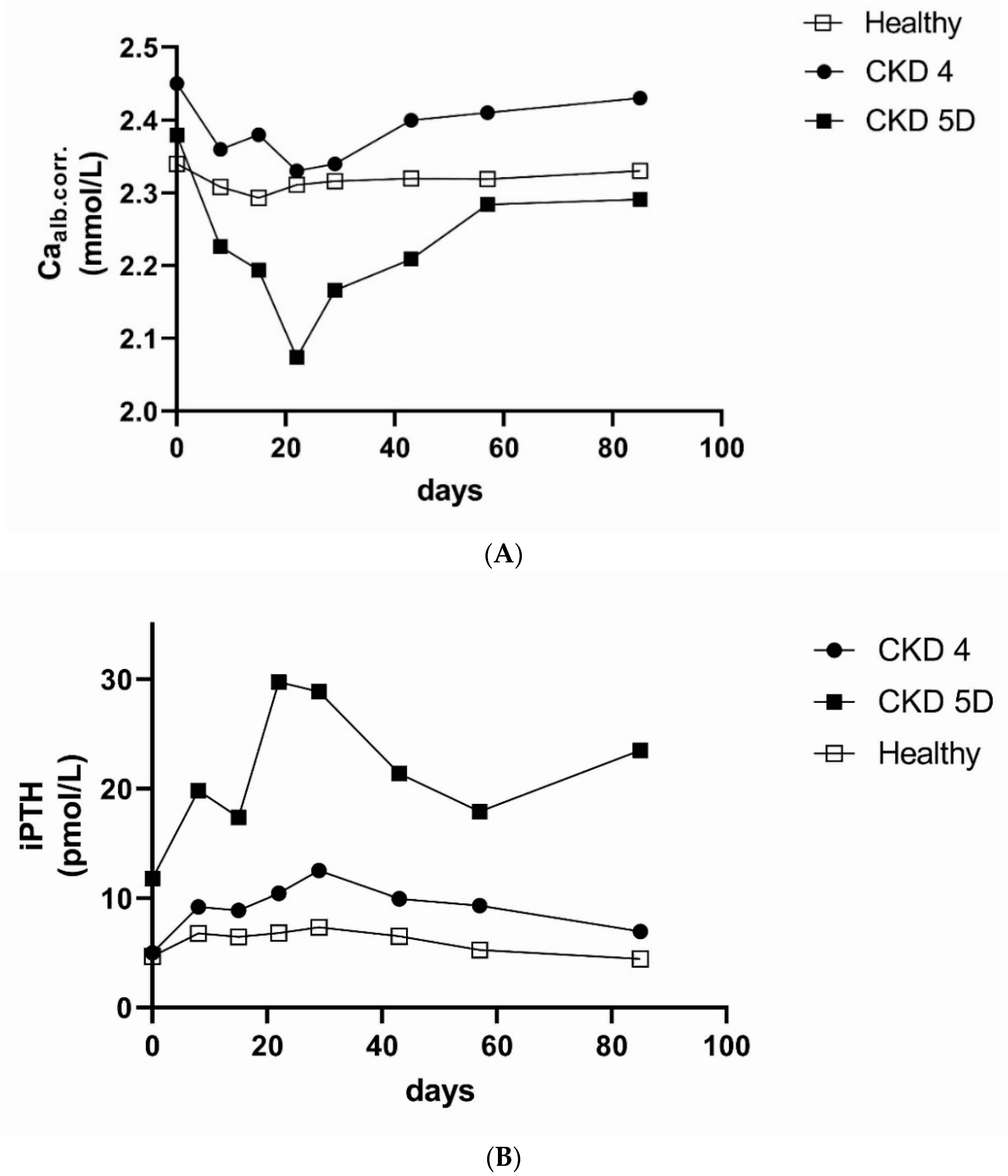
10. Anti-Sclerostin Therapy in CKD
11. Conclusions
Funding
Conflicts of Interest
References
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, Evaluation, and Classification of Renal Osteodystrophy: A Position Statement from Kidney Disease: Improving Global Outcomes (Kdigo). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef]
- Sprague, S.M.; Bellorin-Font, E.; Jorgetti, V.; Carvalho, A.B.; Malluche, H.H.; Ferreira, A.; D’Haese, P.C.; Drueke, T.B.; Du, H.; Manley, T.; et al. Diagnostic Accuracy of Bone Turnover Markers and Bone Histology in Patients with Ckd Treated by Dialysis. Am. J. Kidney Dis. 2015, 67. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; Verhulst, A.; Cavalier, E.; Delanaye, P.; Behets, G.J.; Meijers, B.; Kuypers, D.; D’Haese, P.C.; Evenepoel, P. Clinical Inference of Serum and Bone Sclerostin Levels in Patients with End-Stage Kidney Disease. J. Clin. Med. 2019, 8, 2027. [Google Scholar] [CrossRef]
- Mause, S.F.; Deck, A.; Hennies, M.; Kaesler, N.; Evenepoel, P.; Boisvert, W.A.; Janssen, U.; Brandenburg, V.M. Validation of Commercially Available Elisas for the Detection of Circulating Sclerostin in Hemodialysis Patients. Discoveries 2016, 4, e55. [Google Scholar] [CrossRef] [PubMed]
- Moyses, M.R.; Jamal, S.A.; Graciolli, F.G.; dos Reis, L.M.; Elias, R.M. Can We Compare Serum Sclerostin Results Obtained with Different Assays in Hemodialysis Patients? Int. Urol. Nephrol. 2015, 47, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in Renal Osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877–882. [Google Scholar] [CrossRef]
- De Oliveira, R.A.; Barreto, F.C.; Mendes, M.; dos Reis, L.M.; Castro, J.H.; Britto, Z.M.; Marques, I.D.; Carvalho, A.B.; Moyses, R.M.; Jorgetti, V. Peritoneal Dialysis Per Se Is a Risk Factor for Sclerostin-Associated Adynamic Bone Disease. Kidney Int. 2015, 87, 1039–1045. [Google Scholar] [CrossRef]
- Boltenstal, H.; Qureshi, A.R.; Behets, G.J.; Lindholm, B.; Stenvinkel, P.; D’Haese, P.C.; Haarhaus, M. Association of Serum Sclerostin with Bone Sclerostin in Chronic Kidney Disease Is Lost in Glucocorticoid Treated Patients. Calcif. Tissue Int. 2019, 104, 214–223. [Google Scholar] [CrossRef]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The Relation between Renal Function and Serum Sclerostin in Adult Patients with Ckd. Clin. J. Am. Soc. Nephrol. 2013, 8. [Google Scholar] [CrossRef]
- Cejka, D.; Jager-Lansky, A.; Kieweg, H.; Weber, M.; Bieglmayer, C.; Haider, D.G.; Diarra, D.; Patsch, J.; Kainberger, F.; Bohle, B.; et al. Sclerostin Serum Levels Correlate Positively with Bone Mineral Density and Microarchitecture in Haemodialysis Patients. Nephrol. Dial. Transplant. 2011, 27. [Google Scholar] [CrossRef]
- Lima, F.; Mawad, H.; El-Husseini, A.A.; Davenport, D.L.; Malluche, H.H. Serum Bone Markers in Rod Patients across the Spectrum of Decreases in Gfr: Activin a Increases before All Other Markers. Clin. Nephrol. 2019, 91, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Marculescu, R.; Kozakowski, N.; Plischke, M.; Reiter, T.; Gessl, A.; Haas, M. Renal Elimination of Sclerostin Increases with Declining Kidney Function. J. Clin. Endocrinol. Metab. 2013, 99. [Google Scholar] [CrossRef] [PubMed]
- Graciolli, F.G.; Neves, K.R.; Barreto, F.; Barreto, D.V.; Dos Reis, L.M.; Canziani, M.E.; Sabbagh, Y.; Carvalho, A.B.; Jorgetti, V.; Elias, R.M.; et al. The Complexity of Chronic Kidney Disease-Mineral and Bone Disorder across Stages of Chronic Kidney Disease. Kidney Int. 2017, 91, 1436–1446. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Graciolli, F.G.; O’Brien, S.; Tang, W.; dos Reis, L.M.; Ryan, S.; Phillips, L.; Boulanger, J.; Song, W.; Bracken, C.; et al. Repression of Osteocyte Wnt/Beta-Catenin Signaling Is an Early Event in the Progression of Renal Osteodystrophy. J. Bone Miner. Res. 2012, 27, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.J.C.L.N.; Marques, I.D.B.; Graciolli, F.G.; Fukuhara, L.; dos Reis, L.M.; Custódio, M.; Jorgetti, V.; Elias, R.M.; David-Neto, E.; Moysés, R.M. Comparison of Serum Levels with Bone Content and Gene Expression Indicate a Contradictory Effect of Kidney Transplantation on Sclerostin. Kidney Int. 2019, 96, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Nordholm, A.; Mace, M.L.; Gravesen, E.; Hofman-Bang, J.; Morevati, M.; Olgaard, K.; Lewin, E. Klotho and Activin a in Kidney Injury: Plasma Klotho Is Maintained in Unilateral Obstruction Despite No Upregulation of Klotho Biosynthesis in the Contralateral Kidney. Am. J. Physiol. Renal Physiol. 2018, 314, F753–F762. [Google Scholar] [CrossRef]
- Fayed, A.; Abdulazim, D.O.; Amin, M.; Elhadidy, S.; Samir, H.H.; Salem, M.M.; ElAzim, I.M.A.; el Hawary, K.E.S.; el Din, U.A.S.; Group Vascular Calcification. Serum Sclerostin in Acute Kidney Injury Patients. Nefrologia 2021, in press. [Google Scholar] [CrossRef]
- Gaudio, A.; Pennisi, P.; Bratengeier, C.; Torrisi, V.; Lindner, B.; Mangiafico, R.A.; Pulvirenti, I.; Hawa, G.; Tringali, G.; Fiore, C.E. Increased Sclerostin Serum Levels Associated with Bone Formation and Resorption Markers in Patients with Immobilization-Induced Bone Loss. J. Clin. Endocrinol. Metab. 2010, 95, 2248–2253. [Google Scholar] [CrossRef]
- Zhu, D.; Mackenzie, N.C.; Millan, J.L.; Farquharson, C.; MacRae, V.E. The Appearance and Modulation of Osteocyte Marker Expression During Calcification of Vascular Smooth Muscle Cells. PLoS ONE 2011, 6, e19595. [Google Scholar] [CrossRef] [PubMed]
- Bisson, S.K.; Ung, R.V.; Picard, S.; Valade, D.; Agharazii, M.; Lariviere, R.; Mac-Way, F. High Calcium, Phosphate and Calcitriol Supplementation Leads to an Osteocyte-Like Phenotype in Calcified Vessels and Bone Mineralisation Defect in Uremic Rats. J. Bone Miner. Metab. 2019, 37, 212–223. [Google Scholar] [CrossRef]
- Rukov, J.L.; Gravesen, E.; Mace, M.L.; Hofman-Bang, J.; Vinther, J.; Andersen, C.B.; Lewin, E.; Olgaard, K. Effect of Chronic Uremia on the Transcriptional Profile of the Calcified Aorta Analyzed by Rna Sequencing. Am. J. Physiol. Renal Physiol. 2016, 310, F477–F491. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, M.; Li, M.; Cui, L. Radial Artery Sclerostin Expression in Chronic Kidney Disease Stage 5 Predialysis Patients: A Cross-Sectional Observational Study. Int. Urol. Nephrol. 2017, 49, 1433–1437. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, M.; Xing, C. Relationship between Serum Sclerostin, Vascular Sclerostin Expression and Vascular Calcification Assessed by Different Methods in Esrd Patients Eligible for Renal Transplantation: A Cross-Sectional Study. Int. Urol. Nephrol. 2019, 51, 311–323. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Olauson, H.; Witasp, A.; Haarhaus, M.; Brandenburg, V.; Wernerson, A.; Lindholm, B.; Soderberg, M.; Wennberg, L.; Nordfors, L.; et al. Increased Circulating Sclerostin Levels in End-Stage Renal Disease Predict Biopsy-Verified Vascular Medial Calcification and Coronary Artery Calcification. Kidney Int. 2015, 88, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Verhulst, A.; Babler, A.; D’Haese, P.C.; Evenepoel, P.; Kaesler, N. Sclerostin in Chronic Kidney Disease-Mineral Bone Disorder Think First before You Block It! Nephrol. Dial. Transplant. 2019, 34, 408–414. [Google Scholar] [CrossRef]
- Mace, M.L.; Gravesen, E.; Nordholm, A.; Egstrand, S.; Morevati, M.; Nielsen, C.; Kjaer, A.; Behets, G.; D’Haese, P.; Olgaard, K.; et al. Chronic Kidney Disease-Induced Vascular Calcification Impairs Bone Metabolism. J. Bone Miner. Res. 2021, 36, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Brunkow, M.E.; Gardner, J.C.; Van Ness, J.; Paeper, B.W.; Kovacevich, B.R.; Proll, S.; Skonier, J.E.; Zhao, L.; Sabo, P.J.; Fu, Y.; et al. Bone Dysplasia Sclerosteosis Results from Loss of the Sost Gene Product, a Novel Cystine Knot-Containing Protein. Am. J. Hum. Genet. 2001, 68, 577–589. [Google Scholar] [CrossRef]
- Balemans, W.; Ebeling, M.; Patel, N.; Van, H.E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; van Den, E.J.; Willems, P.; et al. Increased Bone Density in Sclerosteosis Is Due to the Deficiency of a Novel Secreted Protein (Sost). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef]
- Balemans, W.; Patel, N.; Ebeling, M.; Van Hul, E.; Wuyts, W.; Lacza, C.; Dioszegi, M.; Dikkers, F.G.; Hildering, P.; Willems, P.J.; et al. Identification of a 52 Kb Deletion Downstream of the Sost Gene in Patients with Van Buchem Disease. J. Med. Genet. 2002, 39, 91–97. [Google Scholar] [CrossRef]
- Hamersma, H.; Gardner, J.; Beighton, P. The Natural History of Sclerosteosis. Clin. Genet. 2003, 63, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ominsky, M.S.; Warmington, K.S.; Morony, S.; Gong, J.; Cao, J.; Gao, Y.; Shalhoub, V.; Tipton, B.; Haldankar, R.; et al. Sclerostin Antibody Treatment Increases Bone Formation, Bone Mass, and Bone Strength in a Rat Model of Postmenopausal Osteoporosis*. J. Bone Miner. Res. 2009, 24, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Ominsky, M.S.; Vlasseros, F.; Jolette, J.; Smith, S.Y.; Stouch, B.; Doellgast, G.; Gong, J.; Gao, Y.; Cao, J.; Graham, K.; et al. Two Doses of Sclerostin Antibody in Cynomolgus Monkeys Increases Bone Formation, Bone Mineral Density, and Bone Strength. J. Bone Miner. Res. 2010, 25, 948–959. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Niu, Q.T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Turk, J.R.; Deaton, A.M.; Yin, J.; Stolina, M.; Felx, M.; Boyd, G.; Bienvenu, J.G.; Varela, A.; Guillot, M.; Holdsworth, G.; et al. Nonclinical Cardiovascular Safety Evaluation of Romosozumab, an Inhibitor of Sclerostin for the Treatment of Osteoporosis in Postmenopausal Women at High Risk of Fracture. Regul. Toxicol. Pharmacol. 2020, 115, 104697. [Google Scholar] [CrossRef]
- Kaesler, N.; Verhulst, A.; de Mare, A.; Deck, A.; Behets, G.J.; Hyusein, A.; Evenepoel, P.; Floege, J.; Marx, N.; Babler, A.; et al. Sclerostin Deficiency Modifies the Development of Ckd-Mbd in Mice. Bone 2018, 107, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bovijn, J.; Krebs, K.; Chen, C.Y.; Boxall, R.; Censin, J.C.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Laber, S.; Millwood, I.Y.; et al. Evaluating the Cardiovascular Safety of Sclerostin Inhibition Using Evidence from Meta-Analysis of Clinical Trials and Human Genetics. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Holdsworth, G.; Staley, J.R.; Hall, P.; van Koeverden, I.; Vangjeli, C.; Okoye, R.; Boyce, R.W.; Turk, J.R.; Armstrong, M.; Wolfreys, A.; et al. Sclerostin Downregulation Globally by Naturally Occurring Genetic Variants, or Locally in Atherosclerotic Plaques, Does Not Associate with Cardiovascular Events in Humans. J. Bone Miner. Res. 2021, 36. [Google Scholar] [CrossRef]
- Desjardins, L.; Liabeuf, S.; Oliveira, R.B.; Louvet, L.; Kamel, S.; Lemke, H.D.; Vanholder, R.; Choukroun, G.; Massy, Z.A.; Group European Uremic Toxin Work. Uremic Toxicity and Sclerostin in Chronic Kidney Disease Patients. Nephrol. Ther. 2014, 10, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.G.; Liou, H.H.; Lee, C.J.; Chen, Y.C.; Ho, G.J.; Lee, M.C. Serum Sclerostin as an Independent Marker of Peripheral Arterial Stiffness in Renal Transplantation Recipients: A Cross-Sectional Study. Medicine 2016, 95, e3300. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhu, M.; Yan, J.; Fang, Y.; Lu, R.; Zhang, W.; Zhang, Q.; Lu, J.; Qi, C.; Shao, X.; et al. Serum Sclerostin Level Might Be a Potential Biomarker for Arterial Stiffness in Prevalent Hemodialysis Patients. Biomark. Med. 2016, 10, 689–699. [Google Scholar] [CrossRef]
- Stavrinou, E.; Sarafidis, P.A.; Koumaras, C.; Loutradis, C.; Giamalis, P.; Tziomalos, K.; Karagiannis, A.; Papagianni, A. Increased Sclerostin, but Not Dickkopf-1 Protein, Is Associated with Elevated Pulse Wave Velocity in Hemodialysis Subjects. Kidney Blood Press. Res. 2019, 44, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Hou, J.S.; Wang, C.H.; Lin, Y.L.; Lai, Y.H.; Kuo, C.H.; Liou, H.H.; Tsai, J.P.; Hsu, B.G. Serum Sclerostin but Not Dkk-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients. Int. J. Environ. Res. Public Health 2020, 17, 1230. [Google Scholar] [CrossRef] [PubMed]
- Gelir, G.K.; Sengul, S.; Nergizoglu, G.; Erturk, S.; Duman, N.; Kutlay, S. Is Sclerostin Level Associated with Cardiovascular Diseases in Hemodialysis Patients? Blood Purif. 2018, 46, 118–125. [Google Scholar]
- Petrovic, M.; Baralic, M.; Brkovic, V.; Arsenovic, A.; Stojanov, V.; Lalic, N.; Stanisavljevic, D.; Jankovic, A.; Radivojevic, N.; Pejanovic, S.; et al. Significance of Acpwv for Survival of Hemodialysis Patients. Medicina 2020, 56, 435. [Google Scholar] [CrossRef] [PubMed]
- Thambiah, S.; Roplekar, R.; Manghat, P.; Fogelman, I.; Fraser, W.D.; Goldsmith, D.; Hampson, G. Circulating Sclerostin and Dickkopf-1 (Dkk1) in Predialysis Chronic Kidney Disease (Ckd): Relationship with Bone Density and Arterial Stiffness. Calcif. Tissue Int. 2012, 90, 473–480. [Google Scholar] [CrossRef]
- Chen, A.; Sun, Y.; Cui, J.; Zhao, B.; Wang, H.; Chen, X.; Mao, Y. Associations of Sclerostin with Carotid Artery Atherosclerosis and All-Cause Mortality in Chinese Patients Undergoing Maintenance Hemodialysis. BMC Nephrol. 2018, 19, 264. [Google Scholar] [CrossRef]
- Kalousova, M.; Dusilova-Sulkova, S.; Kubena, A.A.; Zakiyanov, O.; Tesar, V.; Zima, T. Sclerostin Levels Predict Cardiovascular Mortality in Long-Term Hemodialysis Patients: A Prospective Observational Cohort Study. Physiol. Res. 2019, 68, 547–558. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Wang, J.; Cui, L.; Jiang, Z.; Ding, J.; Li, M.; Zhou, H. Association of Sclerostin with Cardiovascular Events and Mortality in Dialysis Patients. Ren. Fail. 2020, 42, 282–288. [Google Scholar] [CrossRef]
- Zeng, S.; Slowinski, T.; Pommer, W.; Hasan, A.A.; Gaballa, M.M.S.; Lu, Y.; Kramer, B.K.; Hocher, B. Sclerostin Is an Independent Risk Factor for All-Cause Mortality in Kidney Transplant Recipients. Clin. Exp. Nephrol. 2020, 24, 1177–1183. [Google Scholar] [CrossRef]
- Stavrinou, E.; Sarafidis, P.A.; Loutradis, C.; Memmos, E.; Faitatzidou, D.; Giamalis, P.; Koumaras, C.; Karagiannis, A.; Papagianni, A. Associations of Serum Sclerostin and Dickkopf-Related Protein-1 Proteins with Future Cardiovascular Events and Mortality in Haemodialysis Patients: A Prospective Cohort Study. Clin. Kidney J. 2021, 14, 1165–1172. [Google Scholar] [CrossRef]
- Goncalves, F.L.; Elias, R.M.; dos Reis, L.M.; Graciolli, F.G.; Zampieri, F.G.; Oliveira, R.B.; Jorgetti, V.; Moyses, R.M. Serum Sclerostin Is an Independent Predictor of Mortality in Hemodialysis Patients. BMC Nephrol. 2014, 15, 190. [Google Scholar] [CrossRef]
- Gong, L.; Zheng, D.; Yuan, J.; Cao, L.; Ni, Z.; Fang, W. Elevated Levels of Serum Sclerostin Are Linked to Adverse Cardiovascular Outcomes in Peritoneal Dialysis Patients. Int. Urol. Nephrol. 2018, 50, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Viaene, L.; Behets, G.J.; Claes, K.; Meijers, B.; Blocki, F.; Brandenburg, V.; Evenepoel, P.; D’Haese, P.C. Sclerostin: Another Bone-Related Protein Related to All-Cause Mortality in Haemodialysis? Nephrol. Dial. Transplant. 2013, 28. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Unal, H.U.; Oguz, Y.; Sari, S.; et al. Serum Sclerostin and Adverse Outcomes in Nondialyzed Chronic Kidney Disease Patients. J. Clin. Endocrinol. Metab. 2014, 99, E1854–E1861. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Evenepoel, P.; Vervloet, M.G.; Wanner, C.; Ketteler, M.; Marx, N.; Floege, J.; Dekker, F.W.; Brandenburg, V.M.; Necosad Study Group. High Levels of Circulating Sclerostin Are Associated with Better Cardiovascular Survival in Incident Dialysis Patients: Results from the Necosad Study. Nephrol. Dial. Transplant. 2015, 30, 288–293. [Google Scholar] [CrossRef]
- Jean, G.; Chazot, C.; Bresson, E.; Zaoui, E.; Cavalier, E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron 2016, 132, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lips, L.; van Zuijdewijn, C.L.M.d.; Wee, P.M.T.; Bots, M.L.; Blankestijn, P.J.; van den Dorpel, M.A.; Fouque, D.; de Jongh, R.; Pelletier, S.; Vervloet, M.G.; et al. Serum Sclerostin: Relation with Mortality and Impact of Hemodiafiltration. Nephrol. Dial. Transplant. 2017, 32, 1217–1223. [Google Scholar] [CrossRef]
- Sato, M.; Hanafusa, N.; Kawaguchi, H.; Tsuchiya, K.; Nitta, K. A Prospective Cohort Study Showing No Association between Serum Sclerostin Level and Mortality in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, B.; Yu, X.; Wang, N.; Xu, X.; Zeng, M.; Zhang, B.; Mao, H.; Xing, C. Association of Serum Sclerostin Level, Coronary Artery Calcification, and Patient Outcomes in Maintenance Dialysis Patients. Blood Purif. 2021, 1–10. [Google Scholar] [CrossRef]
- Jorgensen, H.S.; Winther, S.; Dupont, L.; Bottcher, M.; Rejnmark, L.; Hauge, E.M.; Svensson, M.; Ivarsen, P. Sclerostin Is Not Associated with Cardiovascular Event or Fracture in Kidney Transplantation Candidates. Clin. Nephrol. 2018, 90, 18–26. [Google Scholar] [CrossRef]
- Kirkpantur, A.; Balci, M.; Turkvatan, A.; Afsar, B. Serum Sclerostin Levels, Arteriovenous Fistula Calcification and 2-Years All-Cause Mortality in Prevalent Hemodialysis Patients. Nefrologia 2016, 36, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, E.; Okuno, S.; Ichii, M.; Norimine, K.; Yamakawa, T.; Shoji, S.; Nishizawa, Y.; Inaba, M. Relationship between Serum Sclerostin, Bone Metabolism Markers, and Bone Mineral Density in Maintenance Hemodialysis Patients. J. Clin. Endocrinol. Metab. 2014, 99, 4315–4320. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.H.; Lin, W.H.; Chao, J.Y.; Wu, A.B.; Tseng, C.C.; Chang, Y.T.; Liou, H.H.; Wang, M.C. Serum Sclerostin Levels Are Positively Related to Bone Mineral Density in Peritoneal Dialysis Patients: A Cross-Sectional Study. BMC Nephrol. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.Y.; Chen, N.C.; Hsu, C.Y.; Huang, C.W.; Lee, P.T.; Chou, K.J.; Fang, H.C.; Chen, C.L. Evaluation of the Association of Wnt Signaling with Coronary Artery Calcification in Patients on Dialysis with Severe Secondary Hyperparathyroidism. BMC Nephrol. 2019, 20, 345. [Google Scholar] [CrossRef]
- Elsalam, M.A.; El-Abden, M.Z.; Mahmoud, E.; Zahab, Z.A.; Ahmed, H. Correlation between Serum Sclerostin Level and Bone Density Status in Children on Regular Hemodialysis. Saudi J. Kidney Dis. Transpl. 2019, 30, 1022–1031. [Google Scholar] [CrossRef]
- Szulc, P.; Boutroy, S.; Vilayphiou, N.; Schoppet, M.; Rauner, M.; Chapurlat, R.; Hamann, C.; Hofbauer, L.C. Correlates of Bone Microarchitectural Parameters and Serum Sclerostin Levels in Men—The Strambo Study. J. Bone Miner. Res. 2013, 28. [Google Scholar] [CrossRef]
- Atteritano, M.; Di Mauro, E.; Canale, V.; Bruzzese, A.M.; Ricciardi, C.A.; Cernaro, V.; Lacquaniti, A.; Buemi, M.; Santoro, D. Higher Serum Sclerostin Levels and Insufficiency of Vitamin D Are Strongly Associated with Vertebral Fractures in Hemodialysis Patients: A Case Control Study. Osteoporos. Int. 2017, 28, 577–584. [Google Scholar] [CrossRef]
- Malluche, H.H.; Davenport, D.L.; Cantor, T.; Monier-Faugere, M.C. Bone Mineral Density and Serum Biochemical Predictors of Bone Loss in Patients with Ckd on Dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1254–1262. [Google Scholar] [CrossRef]
- Malluche, H.H.; Monier-Faugere, M.C.; Blomquist, G.; Davenport, D.L. Two-Year Cortical and Trabecular Bone Loss in Ckd-5d: Biochemical and Clinical Predictors. Osteoporos. Int. 2018, 29, 125–134. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.L.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A Phase Iii Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men with Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef]
- Lv, F.; Cai, X.; Yang, W.; Gao, L.; Chen, L.; Wu, J.; Ji, L. Denosumab or Romosozumab Therapy and Risk of Cardiovascular Events in Patients with Primary Osteoporosis: Systematic Review and Meta-Analysis. Bone 2020, 130, 115121. [Google Scholar] [CrossRef]
- Li, L.; Gong, M.; Bao, D.; Sun, J.; Xiang, Z. Denosumab and Romosozumab Do Not Increase the Risk of Cardiovascular Events in Patients with Primary Osteoporosis: A Reanalysis of the Meta-Analysis. Bone 2020, 134, 115270. [Google Scholar] [CrossRef]
- Cummings, S.R.; McCulloch, C. Explanations for the Difference in Rates of Cardiovascular Events in a Trial of Alendronate and Romosozumab. Osteoporos. Int. 2020, 31, 1019–1021. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Hofbauer, L.C.; Forfar, J.C. Cardiovascular Safety and Sclerostin Inhibition. J. Clin. Endocrinol. Metab. 2021, 106, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Keller, J.J.; Lin, H.C. Bisphosphonates Reduced the Risk of Acute Myocardial Infarction: A 2-Year Follow-up Study. Osteoporos. Int. 2013, 24, 271–277. [Google Scholar] [CrossRef]
- Kim, D.H.; Rogers, J.R.; Fulchino, L.A.; Kim, C.A.; Solomon, D.H.; Kim, S.C. Bisphosphonates and Risk of Cardiovascular Events: A Meta-Analysis. PLoS ONE 2015, 10, e0122646. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, G.; Bartstra, J.W.; Weijmans, M.; de Jong, P.A.; Mali, W.P.; Verhaar, H.J.; Visseren, F.L.J.; Spiering, W. Bisphosphonates for Cardiovascular Risk Reduction: A Systematic Review and Meta-Analysis. Atherosclerosis 2016, 252, 106–115. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.Y.; Eastell, R.; Allen, I.E. Association between Drug Treatments for Patients with Osteoporosis and Overall Mortality Rates: A Meta-Analysis. JAMA Intern. Med. 2019, 179, 1491–1500. [Google Scholar] [CrossRef]
- Vestergaard Kvist, A.; Faruque, J.; Vallejo-Yague, E.; Weiler, S.; Winter, E.M.; Burden, A.M. Cardiovascular Safety Profile of Romosozumab: A Pharmacovigilance Analysis of the Us Food and Drug Administration Adverse Event Reporting System (Faers). J. Clin. Med. 2021, 10, 1660. [Google Scholar] [CrossRef]
- FDA. January 16, 2019: Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee Meeting Announcement; FDA: Silver Spring, MD, USA, 2019.
- Cejka, D.; Parada-Rodriguez, D.; Pichler, S.; Marculescu, R.; Kramer, I.; Kneissel, M.; Gross, T.; Reisinger, A.; Pahr, D.; Monier-Faugere, M.C.; et al. Only Minor Differences in Renal Osteodystrophy Features between Wild-Type and Sclerostin Knockout Mice with Chronic Kidney Disease. Kidney Int. 2016, 90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moe, S.M.; Chen, N.X.; Newman, C.L.; Organ, J.M.; Kneissel, M.; Kramer, I.; Gattone, V.H., 2nd; Allen, M.R. Anti-Sclerostin Antibody Treatment in a Rat Model of Progressive Renal Osteodystrophy. J. Bone Miner. Res. 2014, 30. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.; Adachi, J.; Albergari, B.; Cheung, A.M.; Chines, A.; Gielen, E.; Langdahl, B.; Miyauchi, A.; Oates, M.; Reid, I.; et al. Efficacy and Safety of Romosozumab among Postmenopausal Women with Osteoporosis and Mild-to-Moderate Chronic Kidney Disease. Ann. Rheum. Dis. 2020, 79, 185. [Google Scholar] [CrossRef]
- ClinicalTrials. Study of Romosozumab (Amg 785) Administered to Healthy Participants and Patients with Stage 4 Renal Impairment or Stage 5 Renal Impairment Requiring Hemodialysis. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01833754?term=romosozumab&cond=kidney&draw=2&rank=1 (accessed on 25 June 2021).
- Amgentrials. A Phase 1, Open-Label, Single-Dose Study of Romosozumab (Amg 785) Administered Subcutaneously to Healthy Subjects and Subjects with Stage 4 Renal Impairment or Stage 5 Renal Impairment Requiring Hemodialysis. Available online: https://www.amgentrials.com/study/?id=20110227 (accessed on 25 June 2021).
- Ferrari, S.L. Romosozumab to Rebuild the Foundations of Bone Strength. Nat. Rev. Rheumatol. 2018, 14, 128. [Google Scholar] [CrossRef]
- Block, G.A.; Bone, H.G.; Fang, L.; Lee, E.; Padhi, D. A Single-Dose Study of Denosumab in Patients with Various Degrees of Renal Impairment. J. Bone Miner. Res. 2012, 27, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Inaba, M.; Yamada, S.; Emoto, M.; Ohno, Y.; Tsujimoto, Y. Efficacy of Romosozumab in Patients with Osteoporosis on Maintenance Hemodialysis in Japan; an Observational Study. J. Bone Miner. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejka, D. Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites 2021, 11, 770. https://doi.org/10.3390/metabo11110770
Cejka D. Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites. 2021; 11(11):770. https://doi.org/10.3390/metabo11110770
Chicago/Turabian StyleCejka, Daniel. 2021. "Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease" Metabolites 11, no. 11: 770. https://doi.org/10.3390/metabo11110770
APA StyleCejka, D. (2021). Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites, 11(11), 770. https://doi.org/10.3390/metabo11110770





