Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease
Abstract
:1. Osteoporosis in CKD
2. Sclerostin as Marker of Bone Turnover in CKD
3. Serum Sclerostin Is Increased in CKD
4. Serum Sclerostin in CKD: Bone or Vessels?
5. Sclerostin and Cardiovascular Events
6. Sclerostin and Patient Outcome in CKD
7. Sclerostin, BMD and Fractures in CKD
8. Anti-Sclerostin Therapy
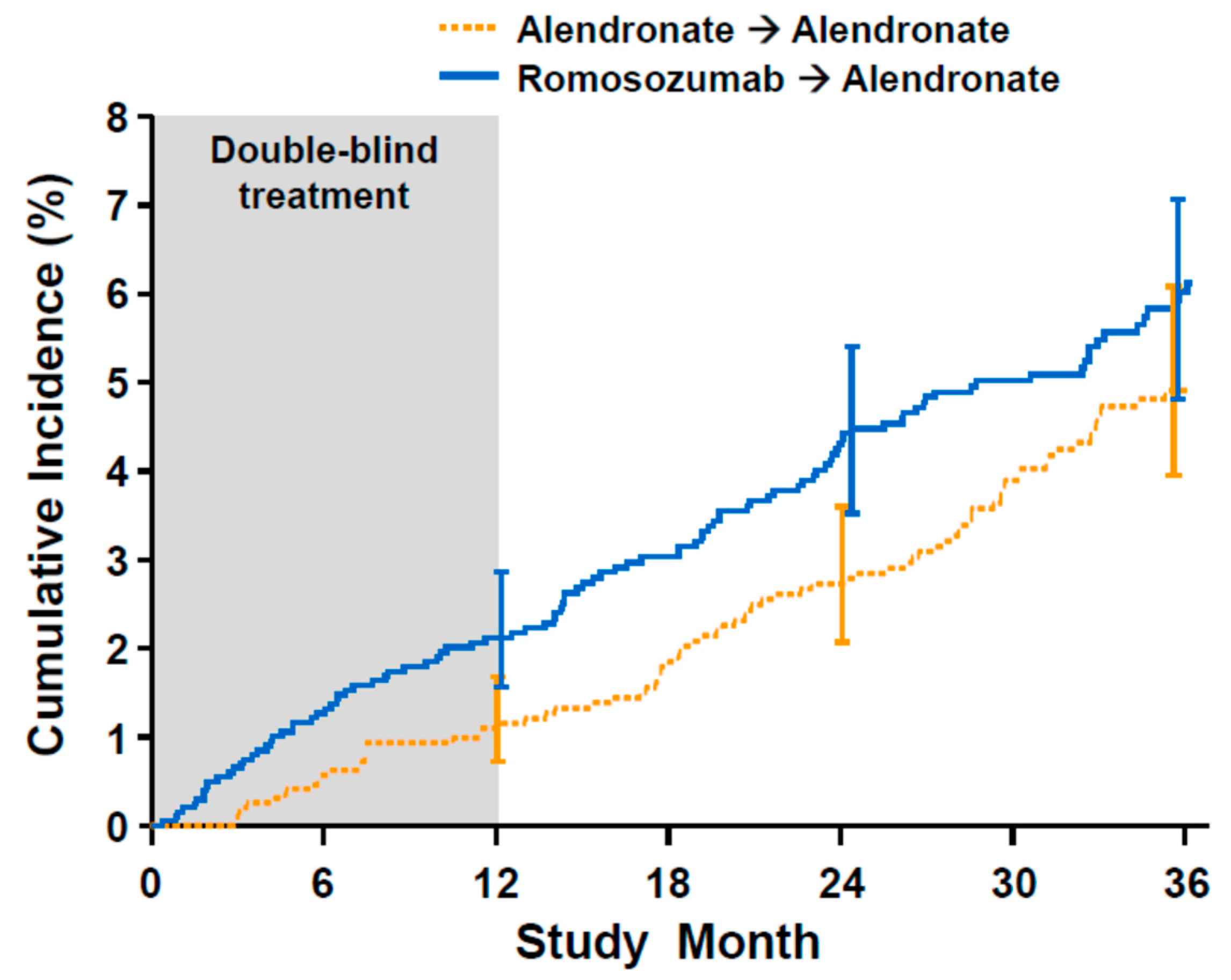
9. Romosozumab and Cardiovascular Events
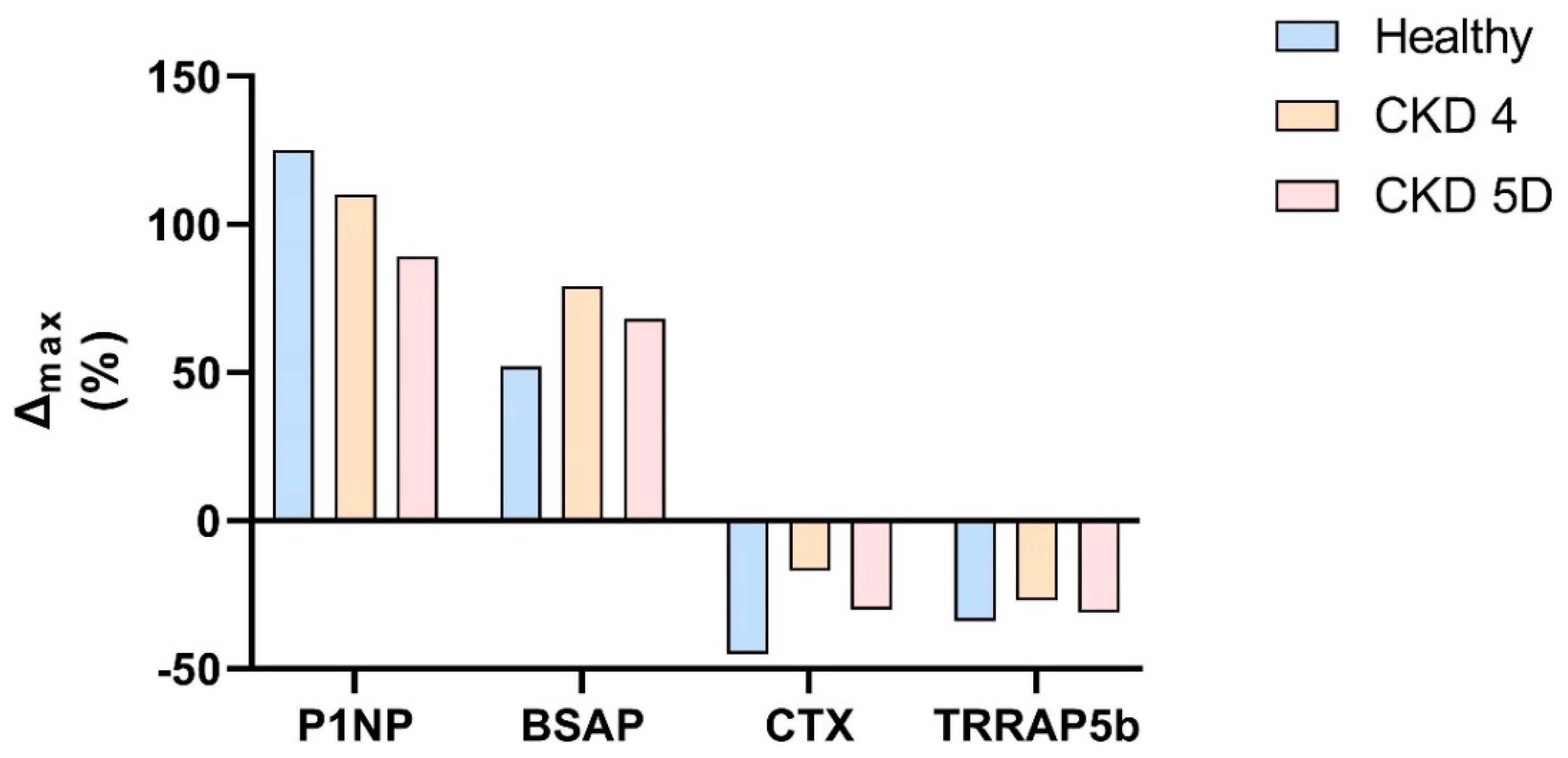
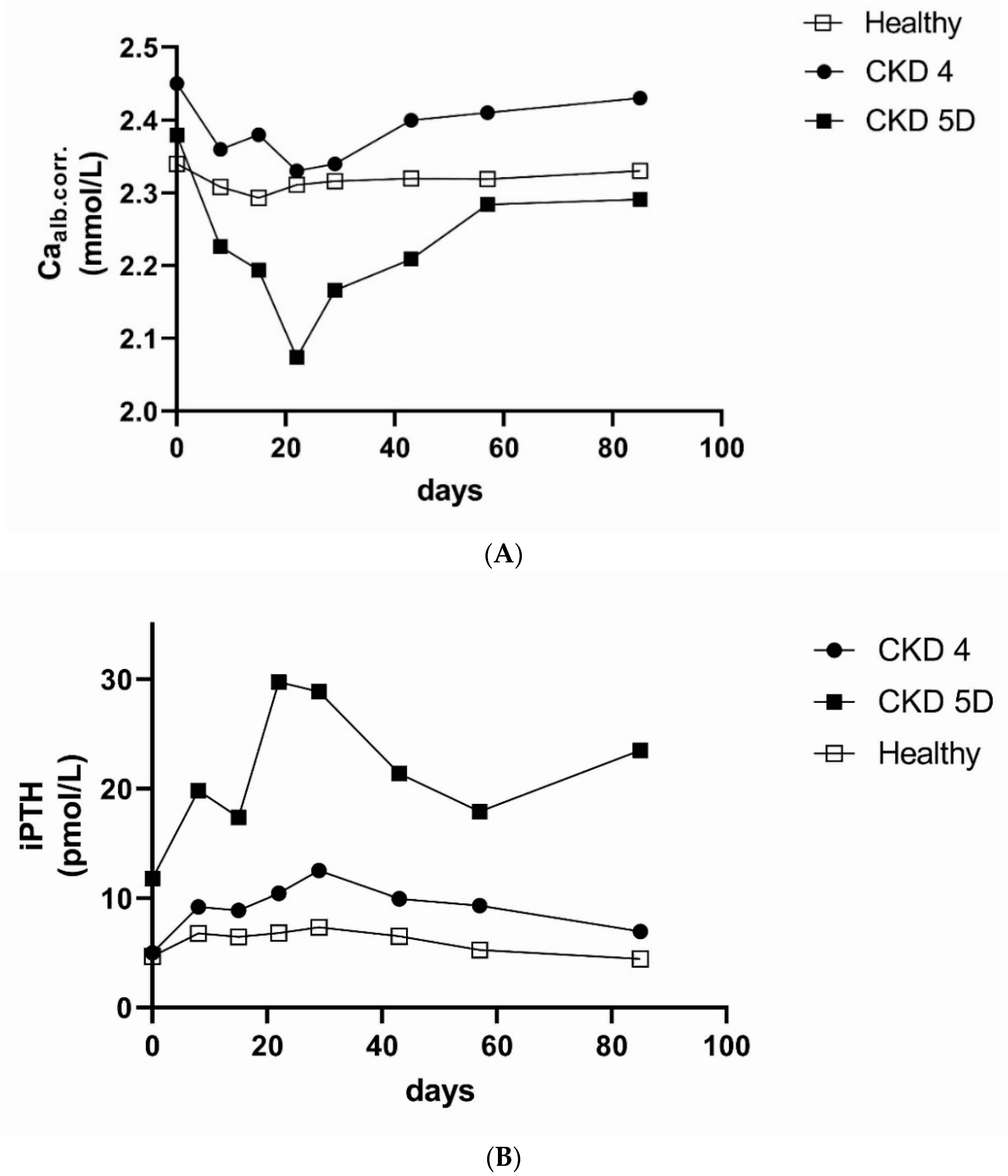
10. Anti-Sclerostin Therapy in CKD
11. Conclusions
Funding
Conflicts of Interest
References
- Moe, S.; Drüeke, T.; Cunningham, J.; Goodman, W.; Martin, K.; Olgaard, K.; Ott, S.; Sprague, S.; Lameire, N.; Eknoyan, G. Definition, Evaluation, and Classification of Renal Osteodystrophy: A Position Statement from Kidney Disease: Improving Global Outcomes (Kdigo). Kidney Int. 2006, 69, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Sprague, S.M.; Bellorin-Font, E.; Jorgetti, V.; Carvalho, A.B.; Malluche, H.H.; Ferreira, A.; D’Haese, P.C.; Drueke, T.B.; Du, H.; Manley, T.; et al. Diagnostic Accuracy of Bone Turnover Markers and Bone Histology in Patients with Ckd Treated by Dialysis. Am. J. Kidney Dis. 2015, 67. [Google Scholar] [CrossRef] [PubMed]
- De Maré, A.; Verhulst, A.; Cavalier, E.; Delanaye, P.; Behets, G.J.; Meijers, B.; Kuypers, D.; D’Haese, P.C.; Evenepoel, P. Clinical Inference of Serum and Bone Sclerostin Levels in Patients with End-Stage Kidney Disease. J. Clin. Med. 2019, 8, 2027. [Google Scholar] [CrossRef] [Green Version]
- Mause, S.F.; Deck, A.; Hennies, M.; Kaesler, N.; Evenepoel, P.; Boisvert, W.A.; Janssen, U.; Brandenburg, V.M. Validation of Commercially Available Elisas for the Detection of Circulating Sclerostin in Hemodialysis Patients. Discoveries 2016, 4, e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyses, M.R.; Jamal, S.A.; Graciolli, F.G.; dos Reis, L.M.; Elias, R.M. Can We Compare Serum Sclerostin Results Obtained with Different Assays in Hemodialysis Patients? Int. Urol. Nephrol. 2015, 47, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in Renal Osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877–882. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, R.A.; Barreto, F.C.; Mendes, M.; dos Reis, L.M.; Castro, J.H.; Britto, Z.M.; Marques, I.D.; Carvalho, A.B.; Moyses, R.M.; Jorgetti, V. Peritoneal Dialysis Per Se Is a Risk Factor for Sclerostin-Associated Adynamic Bone Disease. Kidney Int. 2015, 87, 1039–1045. [Google Scholar] [CrossRef] [Green Version]
- Boltenstal, H.; Qureshi, A.R.; Behets, G.J.; Lindholm, B.; Stenvinkel, P.; D’Haese, P.C.; Haarhaus, M. Association of Serum Sclerostin with Bone Sclerostin in Chronic Kidney Disease Is Lost in Glucocorticoid Treated Patients. Calcif. Tissue Int. 2019, 104, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, S.; Dubourg, L.; Carlier, M.C.; Hadj-Aissa, A.; Fouque, D. The Relation between Renal Function and Serum Sclerostin in Adult Patients with Ckd. Clin. J. Am. Soc. Nephrol. 2013, 8. [Google Scholar] [CrossRef]
- Cejka, D.; Jager-Lansky, A.; Kieweg, H.; Weber, M.; Bieglmayer, C.; Haider, D.G.; Diarra, D.; Patsch, J.; Kainberger, F.; Bohle, B.; et al. Sclerostin Serum Levels Correlate Positively with Bone Mineral Density and Microarchitecture in Haemodialysis Patients. Nephrol. Dial. Transplant. 2011, 27. [Google Scholar] [CrossRef]
- Lima, F.; Mawad, H.; El-Husseini, A.A.; Davenport, D.L.; Malluche, H.H. Serum Bone Markers in Rod Patients across the Spectrum of Decreases in Gfr: Activin a Increases before All Other Markers. Clin. Nephrol. 2019, 91, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Marculescu, R.; Kozakowski, N.; Plischke, M.; Reiter, T.; Gessl, A.; Haas, M. Renal Elimination of Sclerostin Increases with Declining Kidney Function. J. Clin. Endocrinol. Metab. 2013, 99. [Google Scholar] [CrossRef] [PubMed]
- Graciolli, F.G.; Neves, K.R.; Barreto, F.; Barreto, D.V.; Dos Reis, L.M.; Canziani, M.E.; Sabbagh, Y.; Carvalho, A.B.; Jorgetti, V.; Elias, R.M.; et al. The Complexity of Chronic Kidney Disease-Mineral and Bone Disorder across Stages of Chronic Kidney Disease. Kidney Int. 2017, 91, 1436–1446. [Google Scholar] [CrossRef]
- Sabbagh, Y.; Graciolli, F.G.; O’Brien, S.; Tang, W.; dos Reis, L.M.; Ryan, S.; Phillips, L.; Boulanger, J.; Song, W.; Bracken, C.; et al. Repression of Osteocyte Wnt/Beta-Catenin Signaling Is an Early Event in the Progression of Renal Osteodystrophy. J. Bone Miner. Res. 2012, 27, 1757–1772. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.J.C.L.N.; Marques, I.D.B.; Graciolli, F.G.; Fukuhara, L.; dos Reis, L.M.; Custódio, M.; Jorgetti, V.; Elias, R.M.; David-Neto, E.; Moysés, R.M. Comparison of Serum Levels with Bone Content and Gene Expression Indicate a Contradictory Effect of Kidney Transplantation on Sclerostin. Kidney Int. 2019, 96, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Nordholm, A.; Mace, M.L.; Gravesen, E.; Hofman-Bang, J.; Morevati, M.; Olgaard, K.; Lewin, E. Klotho and Activin a in Kidney Injury: Plasma Klotho Is Maintained in Unilateral Obstruction Despite No Upregulation of Klotho Biosynthesis in the Contralateral Kidney. Am. J. Physiol. Renal Physiol. 2018, 314, F753–F762. [Google Scholar] [CrossRef] [Green Version]
- Fayed, A.; Abdulazim, D.O.; Amin, M.; Elhadidy, S.; Samir, H.H.; Salem, M.M.; ElAzim, I.M.A.; el Hawary, K.E.S.; el Din, U.A.S.; Group Vascular Calcification. Serum Sclerostin in Acute Kidney Injury Patients. Nefrologia 2021, in press. [Google Scholar] [CrossRef]
- Gaudio, A.; Pennisi, P.; Bratengeier, C.; Torrisi, V.; Lindner, B.; Mangiafico, R.A.; Pulvirenti, I.; Hawa, G.; Tringali, G.; Fiore, C.E. Increased Sclerostin Serum Levels Associated with Bone Formation and Resorption Markers in Patients with Immobilization-Induced Bone Loss. J. Clin. Endocrinol. Metab. 2010, 95, 2248–2253. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Mackenzie, N.C.; Millan, J.L.; Farquharson, C.; MacRae, V.E. The Appearance and Modulation of Osteocyte Marker Expression During Calcification of Vascular Smooth Muscle Cells. PLoS ONE 2011, 6, e19595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisson, S.K.; Ung, R.V.; Picard, S.; Valade, D.; Agharazii, M.; Lariviere, R.; Mac-Way, F. High Calcium, Phosphate and Calcitriol Supplementation Leads to an Osteocyte-Like Phenotype in Calcified Vessels and Bone Mineralisation Defect in Uremic Rats. J. Bone Miner. Metab. 2019, 37, 212–223. [Google Scholar] [CrossRef]
- Rukov, J.L.; Gravesen, E.; Mace, M.L.; Hofman-Bang, J.; Vinther, J.; Andersen, C.B.; Lewin, E.; Olgaard, K. Effect of Chronic Uremia on the Transcriptional Profile of the Calcified Aorta Analyzed by Rna Sequencing. Am. J. Physiol. Renal Physiol. 2016, 310, F477–F491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yang, M.; Li, M.; Cui, L. Radial Artery Sclerostin Expression in Chronic Kidney Disease Stage 5 Predialysis Patients: A Cross-Sectional Observational Study. Int. Urol. Nephrol. 2017, 49, 1433–1437. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, M.; Xing, C. Relationship between Serum Sclerostin, Vascular Sclerostin Expression and Vascular Calcification Assessed by Different Methods in Esrd Patients Eligible for Renal Transplantation: A Cross-Sectional Study. Int. Urol. Nephrol. 2019, 51, 311–323. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Olauson, H.; Witasp, A.; Haarhaus, M.; Brandenburg, V.; Wernerson, A.; Lindholm, B.; Soderberg, M.; Wennberg, L.; Nordfors, L.; et al. Increased Circulating Sclerostin Levels in End-Stage Renal Disease Predict Biopsy-Verified Vascular Medial Calcification and Coronary Artery Calcification. Kidney Int. 2015, 88, 1356–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; Verhulst, A.; Babler, A.; D’Haese, P.C.; Evenepoel, P.; Kaesler, N. Sclerostin in Chronic Kidney Disease-Mineral Bone Disorder Think First before You Block It! Nephrol. Dial. Transplant. 2019, 34, 408–414. [Google Scholar] [CrossRef]
- Mace, M.L.; Gravesen, E.; Nordholm, A.; Egstrand, S.; Morevati, M.; Nielsen, C.; Kjaer, A.; Behets, G.; D’Haese, P.; Olgaard, K.; et al. Chronic Kidney Disease-Induced Vascular Calcification Impairs Bone Metabolism. J. Bone Miner. Res. 2021, 36, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Brunkow, M.E.; Gardner, J.C.; Van Ness, J.; Paeper, B.W.; Kovacevich, B.R.; Proll, S.; Skonier, J.E.; Zhao, L.; Sabo, P.J.; Fu, Y.; et al. Bone Dysplasia Sclerosteosis Results from Loss of the Sost Gene Product, a Novel Cystine Knot-Containing Protein. Am. J. Hum. Genet. 2001, 68, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Balemans, W.; Ebeling, M.; Patel, N.; Van, H.E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; van Den, E.J.; Willems, P.; et al. Increased Bone Density in Sclerosteosis Is Due to the Deficiency of a Novel Secreted Protein (Sost). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Balemans, W.; Patel, N.; Ebeling, M.; Van Hul, E.; Wuyts, W.; Lacza, C.; Dioszegi, M.; Dikkers, F.G.; Hildering, P.; Willems, P.J.; et al. Identification of a 52 Kb Deletion Downstream of the Sost Gene in Patients with Van Buchem Disease. J. Med. Genet. 2002, 39, 91–97. [Google Scholar] [CrossRef]
- Hamersma, H.; Gardner, J.; Beighton, P. The Natural History of Sclerosteosis. Clin. Genet. 2003, 63, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ominsky, M.S.; Warmington, K.S.; Morony, S.; Gong, J.; Cao, J.; Gao, Y.; Shalhoub, V.; Tipton, B.; Haldankar, R.; et al. Sclerostin Antibody Treatment Increases Bone Formation, Bone Mass, and Bone Strength in a Rat Model of Postmenopausal Osteoporosis*. J. Bone Miner. Res. 2009, 24, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Ominsky, M.S.; Vlasseros, F.; Jolette, J.; Smith, S.Y.; Stouch, B.; Doellgast, G.; Gong, J.; Gao, Y.; Cao, J.; Graham, K.; et al. Two Doses of Sclerostin Antibody in Cynomolgus Monkeys Increases Bone Formation, Bone Mineral Density, and Bone Strength. J. Bone Miner. Res. 2010, 25, 948–959. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Niu, Q.T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Turk, J.R.; Deaton, A.M.; Yin, J.; Stolina, M.; Felx, M.; Boyd, G.; Bienvenu, J.G.; Varela, A.; Guillot, M.; Holdsworth, G.; et al. Nonclinical Cardiovascular Safety Evaluation of Romosozumab, an Inhibitor of Sclerostin for the Treatment of Osteoporosis in Postmenopausal Women at High Risk of Fracture. Regul. Toxicol. Pharmacol. 2020, 115, 104697. [Google Scholar] [CrossRef]
- Kaesler, N.; Verhulst, A.; de Mare, A.; Deck, A.; Behets, G.J.; Hyusein, A.; Evenepoel, P.; Floege, J.; Marx, N.; Babler, A.; et al. Sclerostin Deficiency Modifies the Development of Ckd-Mbd in Mice. Bone 2018, 107, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bovijn, J.; Krebs, K.; Chen, C.Y.; Boxall, R.; Censin, J.C.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Laber, S.; Millwood, I.Y.; et al. Evaluating the Cardiovascular Safety of Sclerostin Inhibition Using Evidence from Meta-Analysis of Clinical Trials and Human Genetics. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Holdsworth, G.; Staley, J.R.; Hall, P.; van Koeverden, I.; Vangjeli, C.; Okoye, R.; Boyce, R.W.; Turk, J.R.; Armstrong, M.; Wolfreys, A.; et al. Sclerostin Downregulation Globally by Naturally Occurring Genetic Variants, or Locally in Atherosclerotic Plaques, Does Not Associate with Cardiovascular Events in Humans. J. Bone Miner. Res. 2021, 36. [Google Scholar] [CrossRef]
- Desjardins, L.; Liabeuf, S.; Oliveira, R.B.; Louvet, L.; Kamel, S.; Lemke, H.D.; Vanholder, R.; Choukroun, G.; Massy, Z.A.; Group European Uremic Toxin Work. Uremic Toxicity and Sclerostin in Chronic Kidney Disease Patients. Nephrol. Ther. 2014, 10, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.G.; Liou, H.H.; Lee, C.J.; Chen, Y.C.; Ho, G.J.; Lee, M.C. Serum Sclerostin as an Independent Marker of Peripheral Arterial Stiffness in Renal Transplantation Recipients: A Cross-Sectional Study. Medicine 2016, 95, e3300. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhu, M.; Yan, J.; Fang, Y.; Lu, R.; Zhang, W.; Zhang, Q.; Lu, J.; Qi, C.; Shao, X.; et al. Serum Sclerostin Level Might Be a Potential Biomarker for Arterial Stiffness in Prevalent Hemodialysis Patients. Biomark. Med. 2016, 10, 689–699. [Google Scholar] [CrossRef]
- Stavrinou, E.; Sarafidis, P.A.; Koumaras, C.; Loutradis, C.; Giamalis, P.; Tziomalos, K.; Karagiannis, A.; Papagianni, A. Increased Sclerostin, but Not Dickkopf-1 Protein, Is Associated with Elevated Pulse Wave Velocity in Hemodialysis Subjects. Kidney Blood Press. Res. 2019, 44, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Hou, J.S.; Wang, C.H.; Lin, Y.L.; Lai, Y.H.; Kuo, C.H.; Liou, H.H.; Tsai, J.P.; Hsu, B.G. Serum Sclerostin but Not Dkk-1 Correlated with Central Arterial Stiffness in End Stage Renal Disease Patients. Int. J. Environ. Res. Public Health 2020, 17, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelir, G.K.; Sengul, S.; Nergizoglu, G.; Erturk, S.; Duman, N.; Kutlay, S. Is Sclerostin Level Associated with Cardiovascular Diseases in Hemodialysis Patients? Blood Purif. 2018, 46, 118–125. [Google Scholar]
- Petrovic, M.; Baralic, M.; Brkovic, V.; Arsenovic, A.; Stojanov, V.; Lalic, N.; Stanisavljevic, D.; Jankovic, A.; Radivojevic, N.; Pejanovic, S.; et al. Significance of Acpwv for Survival of Hemodialysis Patients. Medicina 2020, 56, 435. [Google Scholar] [CrossRef] [PubMed]
- Thambiah, S.; Roplekar, R.; Manghat, P.; Fogelman, I.; Fraser, W.D.; Goldsmith, D.; Hampson, G. Circulating Sclerostin and Dickkopf-1 (Dkk1) in Predialysis Chronic Kidney Disease (Ckd): Relationship with Bone Density and Arterial Stiffness. Calcif. Tissue Int. 2012, 90, 473–480. [Google Scholar] [CrossRef]
- Chen, A.; Sun, Y.; Cui, J.; Zhao, B.; Wang, H.; Chen, X.; Mao, Y. Associations of Sclerostin with Carotid Artery Atherosclerosis and All-Cause Mortality in Chinese Patients Undergoing Maintenance Hemodialysis. BMC Nephrol. 2018, 19, 264. [Google Scholar] [CrossRef] [Green Version]
- Kalousova, M.; Dusilova-Sulkova, S.; Kubena, A.A.; Zakiyanov, O.; Tesar, V.; Zima, T. Sclerostin Levels Predict Cardiovascular Mortality in Long-Term Hemodialysis Patients: A Prospective Observational Cohort Study. Physiol. Res. 2019, 68, 547–558. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Wang, J.; Cui, L.; Jiang, Z.; Ding, J.; Li, M.; Zhou, H. Association of Sclerostin with Cardiovascular Events and Mortality in Dialysis Patients. Ren. Fail. 2020, 42, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Slowinski, T.; Pommer, W.; Hasan, A.A.; Gaballa, M.M.S.; Lu, Y.; Kramer, B.K.; Hocher, B. Sclerostin Is an Independent Risk Factor for All-Cause Mortality in Kidney Transplant Recipients. Clin. Exp. Nephrol. 2020, 24, 1177–1183. [Google Scholar] [CrossRef]
- Stavrinou, E.; Sarafidis, P.A.; Loutradis, C.; Memmos, E.; Faitatzidou, D.; Giamalis, P.; Koumaras, C.; Karagiannis, A.; Papagianni, A. Associations of Serum Sclerostin and Dickkopf-Related Protein-1 Proteins with Future Cardiovascular Events and Mortality in Haemodialysis Patients: A Prospective Cohort Study. Clin. Kidney J. 2021, 14, 1165–1172. [Google Scholar] [CrossRef]
- Goncalves, F.L.; Elias, R.M.; dos Reis, L.M.; Graciolli, F.G.; Zampieri, F.G.; Oliveira, R.B.; Jorgetti, V.; Moyses, R.M. Serum Sclerostin Is an Independent Predictor of Mortality in Hemodialysis Patients. BMC Nephrol. 2014, 15, 190. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Zheng, D.; Yuan, J.; Cao, L.; Ni, Z.; Fang, W. Elevated Levels of Serum Sclerostin Are Linked to Adverse Cardiovascular Outcomes in Peritoneal Dialysis Patients. Int. Urol. Nephrol. 2018, 50, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Viaene, L.; Behets, G.J.; Claes, K.; Meijers, B.; Blocki, F.; Brandenburg, V.; Evenepoel, P.; D’Haese, P.C. Sclerostin: Another Bone-Related Protein Related to All-Cause Mortality in Haemodialysis? Nephrol. Dial. Transplant. 2013, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanbay, M.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Gok, M.; Cetinkaya, H.; Karaman, M.; Unal, H.U.; Oguz, Y.; Sari, S.; et al. Serum Sclerostin and Adverse Outcomes in Nondialyzed Chronic Kidney Disease Patients. J. Clin. Endocrinol. Metab. 2014, 99, E1854–E1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drechsler, C.; Evenepoel, P.; Vervloet, M.G.; Wanner, C.; Ketteler, M.; Marx, N.; Floege, J.; Dekker, F.W.; Brandenburg, V.M.; Necosad Study Group. High Levels of Circulating Sclerostin Are Associated with Better Cardiovascular Survival in Incident Dialysis Patients: Results from the Necosad Study. Nephrol. Dial. Transplant. 2015, 30, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Jean, G.; Chazot, C.; Bresson, E.; Zaoui, E.; Cavalier, E. High Serum Sclerostin Levels Are Associated with a Better Outcome in Haemodialysis Patients. Nephron 2016, 132, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lips, L.; van Zuijdewijn, C.L.M.d.; Wee, P.M.T.; Bots, M.L.; Blankestijn, P.J.; van den Dorpel, M.A.; Fouque, D.; de Jongh, R.; Pelletier, S.; Vervloet, M.G.; et al. Serum Sclerostin: Relation with Mortality and Impact of Hemodiafiltration. Nephrol. Dial. Transplant. 2017, 32, 1217–1223. [Google Scholar] [CrossRef]
- Sato, M.; Hanafusa, N.; Kawaguchi, H.; Tsuchiya, K.; Nitta, K. A Prospective Cohort Study Showing No Association between Serum Sclerostin Level and Mortality in Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, B.; Yu, X.; Wang, N.; Xu, X.; Zeng, M.; Zhang, B.; Mao, H.; Xing, C. Association of Serum Sclerostin Level, Coronary Artery Calcification, and Patient Outcomes in Maintenance Dialysis Patients. Blood Purif. 2021, 1–10. [Google Scholar] [CrossRef]
- Jorgensen, H.S.; Winther, S.; Dupont, L.; Bottcher, M.; Rejnmark, L.; Hauge, E.M.; Svensson, M.; Ivarsen, P. Sclerostin Is Not Associated with Cardiovascular Event or Fracture in Kidney Transplantation Candidates. Clin. Nephrol. 2018, 90, 18–26. [Google Scholar] [CrossRef]
- Kirkpantur, A.; Balci, M.; Turkvatan, A.; Afsar, B. Serum Sclerostin Levels, Arteriovenous Fistula Calcification and 2-Years All-Cause Mortality in Prevalent Hemodialysis Patients. Nefrologia 2016, 36, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimura, E.; Okuno, S.; Ichii, M.; Norimine, K.; Yamakawa, T.; Shoji, S.; Nishizawa, Y.; Inaba, M. Relationship between Serum Sclerostin, Bone Metabolism Markers, and Bone Mineral Density in Maintenance Hemodialysis Patients. J. Clin. Endocrinol. Metab. 2014, 99, 4315–4320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.H.; Lin, W.H.; Chao, J.Y.; Wu, A.B.; Tseng, C.C.; Chang, Y.T.; Liou, H.H.; Wang, M.C. Serum Sclerostin Levels Are Positively Related to Bone Mineral Density in Peritoneal Dialysis Patients: A Cross-Sectional Study. BMC Nephrol. 2019, 20, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, T.Y.; Chen, N.C.; Hsu, C.Y.; Huang, C.W.; Lee, P.T.; Chou, K.J.; Fang, H.C.; Chen, C.L. Evaluation of the Association of Wnt Signaling with Coronary Artery Calcification in Patients on Dialysis with Severe Secondary Hyperparathyroidism. BMC Nephrol. 2019, 20, 345. [Google Scholar] [CrossRef] [Green Version]
- Elsalam, M.A.; El-Abden, M.Z.; Mahmoud, E.; Zahab, Z.A.; Ahmed, H. Correlation between Serum Sclerostin Level and Bone Density Status in Children on Regular Hemodialysis. Saudi J. Kidney Dis. Transpl. 2019, 30, 1022–1031. [Google Scholar] [CrossRef]
- Szulc, P.; Boutroy, S.; Vilayphiou, N.; Schoppet, M.; Rauner, M.; Chapurlat, R.; Hamann, C.; Hofbauer, L.C. Correlates of Bone Microarchitectural Parameters and Serum Sclerostin Levels in Men—The Strambo Study. J. Bone Miner. Res. 2013, 28. [Google Scholar] [CrossRef]
- Atteritano, M.; Di Mauro, E.; Canale, V.; Bruzzese, A.M.; Ricciardi, C.A.; Cernaro, V.; Lacquaniti, A.; Buemi, M.; Santoro, D. Higher Serum Sclerostin Levels and Insufficiency of Vitamin D Are Strongly Associated with Vertebral Fractures in Hemodialysis Patients: A Case Control Study. Osteoporos. Int. 2017, 28, 577–584. [Google Scholar] [CrossRef]
- Malluche, H.H.; Davenport, D.L.; Cantor, T.; Monier-Faugere, M.C. Bone Mineral Density and Serum Biochemical Predictors of Bone Loss in Patients with Ckd on Dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Malluche, H.H.; Monier-Faugere, M.C.; Blomquist, G.; Davenport, D.L. Two-Year Cortical and Trabecular Bone Loss in Ckd-5d: Biochemical and Clinical Predictors. Osteoporos. Int. 2018, 29, 125–134. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.L.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A Phase Iii Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men with Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef] [Green Version]
- Lv, F.; Cai, X.; Yang, W.; Gao, L.; Chen, L.; Wu, J.; Ji, L. Denosumab or Romosozumab Therapy and Risk of Cardiovascular Events in Patients with Primary Osteoporosis: Systematic Review and Meta-Analysis. Bone 2020, 130, 115121. [Google Scholar] [CrossRef]
- Li, L.; Gong, M.; Bao, D.; Sun, J.; Xiang, Z. Denosumab and Romosozumab Do Not Increase the Risk of Cardiovascular Events in Patients with Primary Osteoporosis: A Reanalysis of the Meta-Analysis. Bone 2020, 134, 115270. [Google Scholar] [CrossRef]
- Cummings, S.R.; McCulloch, C. Explanations for the Difference in Rates of Cardiovascular Events in a Trial of Alendronate and Romosozumab. Osteoporos. Int. 2020, 31, 1019–1021. [Google Scholar] [CrossRef] [Green Version]
- Langdahl, B.L.; Hofbauer, L.C.; Forfar, J.C. Cardiovascular Safety and Sclerostin Inhibition. J. Clin. Endocrinol. Metab. 2021, 106, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Keller, J.J.; Lin, H.C. Bisphosphonates Reduced the Risk of Acute Myocardial Infarction: A 2-Year Follow-up Study. Osteoporos. Int. 2013, 24, 271–277. [Google Scholar] [CrossRef]
- Kim, D.H.; Rogers, J.R.; Fulchino, L.A.; Kim, C.A.; Solomon, D.H.; Kim, S.C. Bisphosphonates and Risk of Cardiovascular Events: A Meta-Analysis. PLoS ONE 2015, 10, e0122646. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, G.; Bartstra, J.W.; Weijmans, M.; de Jong, P.A.; Mali, W.P.; Verhaar, H.J.; Visseren, F.L.J.; Spiering, W. Bisphosphonates for Cardiovascular Risk Reduction: A Systematic Review and Meta-Analysis. Atherosclerosis 2016, 252, 106–115. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.Y.; Eastell, R.; Allen, I.E. Association between Drug Treatments for Patients with Osteoporosis and Overall Mortality Rates: A Meta-Analysis. JAMA Intern. Med. 2019, 179, 1491–1500. [Google Scholar] [CrossRef]
- Vestergaard Kvist, A.; Faruque, J.; Vallejo-Yague, E.; Weiler, S.; Winter, E.M.; Burden, A.M. Cardiovascular Safety Profile of Romosozumab: A Pharmacovigilance Analysis of the Us Food and Drug Administration Adverse Event Reporting System (Faers). J. Clin. Med. 2021, 10, 1660. [Google Scholar] [CrossRef]
- FDA. January 16, 2019: Meeting of the Bone, Reproductive and Urologic Drugs Advisory Committee Meeting Announcement; FDA: Silver Spring, MD, USA, 2019.
- Cejka, D.; Parada-Rodriguez, D.; Pichler, S.; Marculescu, R.; Kramer, I.; Kneissel, M.; Gross, T.; Reisinger, A.; Pahr, D.; Monier-Faugere, M.C.; et al. Only Minor Differences in Renal Osteodystrophy Features between Wild-Type and Sclerostin Knockout Mice with Chronic Kidney Disease. Kidney Int. 2016, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moe, S.M.; Chen, N.X.; Newman, C.L.; Organ, J.M.; Kneissel, M.; Kramer, I.; Gattone, V.H., 2nd; Allen, M.R. Anti-Sclerostin Antibody Treatment in a Rat Model of Progressive Renal Osteodystrophy. J. Bone Miner. Res. 2014, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, P.; Adachi, J.; Albergari, B.; Cheung, A.M.; Chines, A.; Gielen, E.; Langdahl, B.; Miyauchi, A.; Oates, M.; Reid, I.; et al. Efficacy and Safety of Romosozumab among Postmenopausal Women with Osteoporosis and Mild-to-Moderate Chronic Kidney Disease. Ann. Rheum. Dis. 2020, 79, 185. [Google Scholar] [CrossRef]
- ClinicalTrials. Study of Romosozumab (Amg 785) Administered to Healthy Participants and Patients with Stage 4 Renal Impairment or Stage 5 Renal Impairment Requiring Hemodialysis. Available online: https://www.clinicaltrials.gov/ct2/show/results/NCT01833754?term=romosozumab&cond=kidney&draw=2&rank=1 (accessed on 25 June 2021).
- Amgentrials. A Phase 1, Open-Label, Single-Dose Study of Romosozumab (Amg 785) Administered Subcutaneously to Healthy Subjects and Subjects with Stage 4 Renal Impairment or Stage 5 Renal Impairment Requiring Hemodialysis. Available online: https://www.amgentrials.com/study/?id=20110227 (accessed on 25 June 2021).
- Ferrari, S.L. Romosozumab to Rebuild the Foundations of Bone Strength. Nat. Rev. Rheumatol. 2018, 14, 128. [Google Scholar] [CrossRef]
- Block, G.A.; Bone, H.G.; Fang, L.; Lee, E.; Padhi, D. A Single-Dose Study of Denosumab in Patients with Various Degrees of Renal Impairment. J. Bone Miner. Res. 2012, 27, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Inaba, M.; Yamada, S.; Emoto, M.; Ohno, Y.; Tsujimoto, Y. Efficacy of Romosozumab in Patients with Osteoporosis on Maintenance Hemodialysis in Japan; an Observational Study. J. Bone Miner. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejka, D. Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites 2021, 11, 770. https://doi.org/10.3390/metabo11110770
Cejka D. Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites. 2021; 11(11):770. https://doi.org/10.3390/metabo11110770
Chicago/Turabian StyleCejka, Daniel. 2021. "Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease" Metabolites 11, no. 11: 770. https://doi.org/10.3390/metabo11110770
APA StyleCejka, D. (2021). Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites, 11(11), 770. https://doi.org/10.3390/metabo11110770





