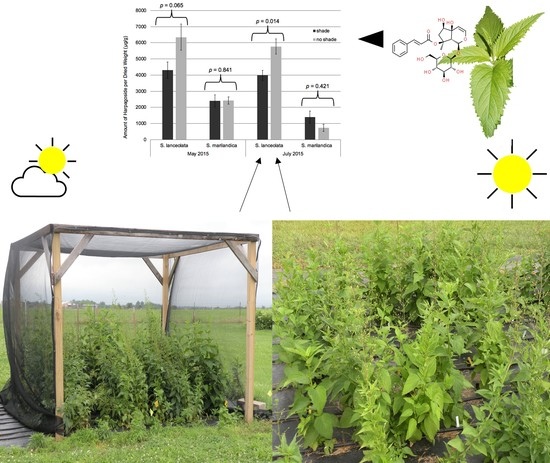
Changes in the Harpagide, Harpagoside, and Verbascoside Content of Field Grown Scrophularia lanceolata and Scrophularia marilandica in Response to Season and Shade
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheunert, A.; Heubl, G. Phylogenetic Relationships among New World Scrophularia L. (Scrophulariaceae): New insights inferred from DNA sequence data. Plant Syst. Evol. 2011, 291, 69–89. [Google Scholar] [CrossRef]
- de Galindez, J.S.; Lanza, A.M.D.; Fernandez, L.M. Biologically Active Substances from the Genus Scrophularia. Pharm. Biol. 2002, 40, 45–59. [Google Scholar] [CrossRef]
- Garcia, D.; Fernandez, A.; Saenz, T.; Ahumada, C. Anti-inflammatory Effects of Different Extracts and Harpagoside Isolated from Scrophularia frutescens L. Farmaco 1996, 51, 443–446. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological Activities of Iridoids: Recent Developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Herrick, J.W. Iroquois Medical Botany. Ph.D. Thesis, State University of New York, Albany, NY, USA, 1997. [Google Scholar]
- Hough, F.B. The medicinal qualities of Scrophularia marilandica. N. Engl. J. Med. 1849, 40, 462–464. [Google Scholar] [CrossRef]
- Moerman, D. Native American Ethnobotany; Timber Press: Portland, OR, USA, 1998. [Google Scholar]
- Brownstein, K.J.; Thomas, A.L.; Rottinghaus, G.E.; Lynch, B.A.; Gang, D.R.; Folk, W.R. Harpagide and related iridoid glycosides in vegetative tissues of cultivated Scrophularia lanceolata and Scrophularia marilandica. Acta Hortic. 2016, 1125, 83–90. [Google Scholar] [CrossRef]
- Stewart, K.M.; Cole, D. The commercial harvest of devil’s claw (Harpagophytum spp.) in southern Africa: The devil’s in the details. J. Ethnopharmacol. 2005, 100, 225–236. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Oltean, H.; van Tulder, M.W.; Berman, B.M.; Bombardier, C.; Robbins, C.B. Herbal medicine for low back pain: A Cochrane review. Spine 2016, 41, 116–133. [Google Scholar] [CrossRef]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.M.; Gericke, N. Devil’s claw: A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Oltean, H.; Robbins, C.; van Tulder, M.W.; Berman, B.M.; Bombardier, C.; Gagnier, J.J. Herbal medicine for low-back pain. Cochrane Database Syst. Rev. 2014, 12, CD004504. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, G.; Cui, J.; Ndam, T.; Bekemeier, M.; Song, H.; Li, R.; Siedhoff, H.R.; Yang, B.; Appenteng, M.K.; Greenlief, C.M.; et al. Harpagophytum procumbens extract ameliorates allodynia and modulates oxidative and antioxidant stress pathways in a rat model of spinal cord injury. Neuromol. Med. 2020, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Sanders, J.; Von Willert, D. Devil’s claw (Harpagophytum procumbens) from Southern Africa: Sustainable use by cultivation combined with controlled harvesting in semi-wild populations. Frontis 2006, 17, 181–202. [Google Scholar]
- Brownstein, K.J.; Gargouri, M.; Folk, W.R.; Gang, D.R. Iridoid and phenylethanoid/phenylpropanoid metabolite profiles of Scrophularia and Verbascum species used medicinally in North America. Metabolomics 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdelouahab, N.; Heard, C. Effect of the major glycosides of Harpagophytum procumbens (devil’s claw) on epidermal cyclooxygenase-2 (COX-2) in vitro. J. Nat. Prod. 2008, 71, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Anauate, M.C.; Torres, L.M.; de Mello, S.B.V. Effect of isolated fractions of Harpagophytum procumbens DC (devil’s claw) on COX-1, COX-2 activity and nitric oxide production on whole-blood assay. Phytother. Res. 2010, 24, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Ivanovska, N.; Alipieva, K.; Dimitrova, P.; Verpoorte, R. Harpagoside: From Kalahari Desert to pharmacy shelf. Phytochemistry 2013, 92, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gyurkovska, V.; Alipieva, K.; Maciuk, A.; Dimitrova, P.; Ivanovska, N.; Haas, C.; Bley, T.; Georgiev, M. Anti-inflammatory activity of devil’s claw in vitro systems and their active constituents. Food Chem. 2011, 125, 171–178. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef] [Green Version]
- de Galindez, J.S.; Matellano, L.F.; Lanza, A.M.D.; Castillo, L.V. Seasonal variation in the harpagoside content of Scrophularia scorodonia L. Z. Naturforsch. C J. Biosci. 2000, 55, 1035–1037. [Google Scholar]
- Jeong, B.R.; Sivanesan, I. Direct adventitious shoot regeneration, in vitro flowering, fruiting, secondary metabolite content and antioxidant activity of Scrophularia takesimensis Nakai. Plant Cell Tissue Organ Cult. 2015, 123, 607–618. [Google Scholar] [CrossRef]
- Li, H.W.; Liu, P.; Zhang, H.Q.; Feng, W.M.; Yan, H.; Guo, S.; Qian, D.W.; Duan, J.A. Determination of bioactive compounds in the nonmedicinal parts of Scrophularia ningpoensis using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry and chemometric analysis. J. Sep. Sci. 2020, 43, 4191–4201. [Google Scholar] [CrossRef]
- Liang, Z.S.; An, Y.Y.; Liu, H.Y. High temperature stress decreases root iridoid glycosides biosynthesis of Scrophularia ningpoensis during florescence. J. Med. Plant Res. 2014, 8, 392–394. [Google Scholar]
- Mncwangi, N.P.; Viljoen, A.M.; Zhao, J.; Vermaak, I.; Chen, W.; Khan, I. What the devil is in your phytomedicine? Exploring species substitution in Harpagophytum through chemometric modeling of 1 H-NMR and UHPLC-MS datasets. Phytochemistry 2014, 106, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Du, F.; Liu, H.Y.; Liang, Z.S. Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J. Med. Plant Res. 2010, 24, 2691–2699. [Google Scholar]
- Xie, G.; Jiang, Y.; Huang, M.; Zhu, Y.; Wu, G.; Qin, M. Dynamic analysis of secondary metabolites in various parts of Scrophularia ningpoensis by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 186, 113307. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Zhao, Y.; Chen, B.; Fu, C. Harpagoside variation is positively correlated with temperature in Scrophularia ningpoensis Hemsl. J. Agric. Food Chem. 2011, 59, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- National Oceanic and Atmospheric Administration (NOAA). North American Climate Extremes Monitoring. Available online: https://www.ncdc.noaa.gov/extremes/nacem/methodology (accessed on 21 May 2020).
- Georgiev, M.I.; Alipieva, K.I.; Denev, P. Antioxidant activity and bioactive constituents of the aerial parts of Harpagophytum procumbens plants. Biotechnol. Biotechnol. Equip. 2010, 24, 438–443. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Falahi, H.; Sharifi, M.; Maivan, H.Z.; Chashmi, N.A. Phenylethanoid glycosides accumulation in roots of Scrophularia striata as a response to water stress. Environ. Exp. Bot. 2018, 147, 13–21. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Londono, P.T.; Papagiannopoulos, M.; Gobbo-Neto, L.; Muller, C. Variation in flavonoid pattern in leaves and flowers of Primula veris of different origin and impact of UV-B. Biochem. Syst. Ecol. 2014, 53, 81–88. [Google Scholar]
- Alipieva, K.I.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside: A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Gowan, E.; Lewis, B.A.; Turgeon, R. Phloem transport of antirrhinoside, an iridoid glycoside, in Asarina scandens (Scrophulariaceae). J. Chem. Ecol. 1995, 21, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Pungitore, C.R.; Ayub, M.J.; Garcia, M.; Borkowski, E.J.; Sosa, M.E.; Ciuffo, G.; Giordano, O.S.; Tonn, C.E. Iridoids as allelochemicals and DNA polymerase inhibitors. J. Nat. Prod. 2004, 67, 357–361. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brownstein, K.J.; Thomas, A.L.; Nguyen, H.T.T.; Gang, D.R.; Folk, W.R. Changes in the Harpagide, Harpagoside, and Verbascoside Content of Field Grown Scrophularia lanceolata and Scrophularia marilandica in Response to Season and Shade. Metabolites 2021, 11, 464. https://doi.org/10.3390/metabo11070464
Brownstein KJ, Thomas AL, Nguyen HTT, Gang DR, Folk WR. Changes in the Harpagide, Harpagoside, and Verbascoside Content of Field Grown Scrophularia lanceolata and Scrophularia marilandica in Response to Season and Shade. Metabolites. 2021; 11(7):464. https://doi.org/10.3390/metabo11070464
Chicago/Turabian StyleBrownstein, Korey J., Andrew L. Thomas, Hien T. T. Nguyen, David R. Gang, and William R. Folk. 2021. "Changes in the Harpagide, Harpagoside, and Verbascoside Content of Field Grown Scrophularia lanceolata and Scrophularia marilandica in Response to Season and Shade" Metabolites 11, no. 7: 464. https://doi.org/10.3390/metabo11070464
APA StyleBrownstein, K. J., Thomas, A. L., Nguyen, H. T. T., Gang, D. R., & Folk, W. R. (2021). Changes in the Harpagide, Harpagoside, and Verbascoside Content of Field Grown Scrophularia lanceolata and Scrophularia marilandica in Response to Season and Shade. Metabolites, 11(7), 464. https://doi.org/10.3390/metabo11070464