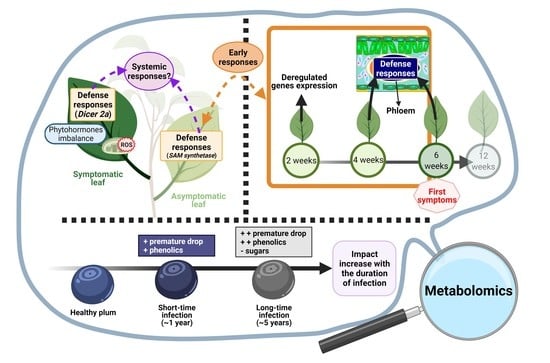
Deciphering Prunus Responses to PPV Infection: A Way toward the Use of Metabolomics Approach for the Diagnostic of Sharka Disease
Abstract
1. Introduction
2. How Does the Plum Pox Virus Affect the Susceptible Prunus Leaves?
2.1. The Chloroplast: The Organelle Most Affected by PPV Infection
2.2. Major Changes in the Antioxidant System as a Result of PPV Infection in Susceptible Varities
2.3. Imbalance at the Phytohormonal Level
2.4. Defense Responses in Planta at the Transcriptomic Level in Susceptible Varieties
2.5. Alterations at the Proteomic Level
2.6. Translatomic Analysis: An Original Approach to Study the Impact of PPV Infection
3. How Does the Plum Pox Virus Affect the Resistant Prunus Leaves?
3.1. Major Changes in the Antioxidant System as Result of PPV Infection in Resistant Varities
3.2. Phytohormonal Level: Transfer of Resistance to PPV by Grafting “Garrigues” Almonds to Peaches
3.3. Defense Responses in Planta at the Transcriptomic Level in Resistant Varities
3.3.1. Overview: Transcriptome Modifications in Resistant Plants
3.3.2. Regulation of Genes Coding for MATH Domain in PPVres Locus
3.3.3. eiFiso4G Transcription Initiation Factor: A Key Role in Virus Replication
3.4. Case Study: ‘Jojo’ Variety, An Example of the Hypersensitive Response
4. How Does the Plum Pox Virus Affect the Susceptible Prunus Fruit?
4.1. Yield Attributes and Physical Properties of Fruit
4.2. Primary Compounds
4.2.1. Soluble Solids Content (SSC)
4.2.2. Sugars: Content and Composition
4.2.3. Organic Acids: Content and Composition
4.2.4. Quality Fruit Indexes
4.3. Secondary Compounds: The Case of Phenolics
4.3.1. Hydroxycinnamic Acids: Content and Composition
4.3.2. Anthocyanins: Content and Composition
4.3.3. Flavonols: Content and Composition
5. Metabolomics: An Insightful Tool to Study Prunus—PPV Interactions?
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García, J.A.; Glasa, M.; Cambra, M.; Candresse, T. Plum pox virus and sharka: A model potyvirus and a major disease. Mol. Plant Pathol. 2014, 15, 226–241. [Google Scholar] [CrossRef]
- Rodamilans, B.; Valli, A.; García, J.A. Molecular Plant-Plum Pox Virus Interactions. Mol. Plant Microbe Interact. 2020, 33, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Rimbaud, L.; Dallot, S.; Gottwald, T.; Decroocq, V.; Jacquot, E.; Soubeyrand, S.; Thébaud, G. Sharka Epidemiology and Worldwide Management Strategies: Learning Lessons to Optimize Disease Control in Perennial Plants. Annu. Rev. Phytopathol. 2015, 53, 357–378. [Google Scholar] [CrossRef]
- Ilardi, V.; Tavazza, M. Biotechnological strategies and tools for Plum pox virus resistance: Trans-, intra-, cis-genesis, and beyond. Front. Plant Sci. 2015, 6, 379. [Google Scholar] [CrossRef]
- Rimbaud, L.; Dallot, S.; Bruchou, C.; Thoyer, S.; Jacquot, E.; Soubeyrand, S.; Thébaud, G. Improving Management Strategies of Plant Diseases Using Sequential Sensitivity Analyses. Phytopathology 2019, 109, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- FAO. ISPM 27 Diagnostic Protocols for Regulated Pests, DP 2: Plum pox virus; International Plant Protection Convention: Roma, Italy, 2012; pp. 1–16. [Google Scholar]
- Rimbaud, L.; Dallot, S.; Delaunay, A.; Borron, S.; Soubeyrand, S.; Thébaud, G.; Jacquot, E. Assessing the mismatch between incubation and latent periods for vector-borne diseases: The case of sharka. Phytopathology 2015, 105, 1408–1416. [Google Scholar] [CrossRef][Green Version]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an Emerging Tool for the Study of Plant–Pathogen Interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.A.; Rubio, M.; Olmos, E.; Ros-Barcelo, A.; Martinez-Gomez, P. Oxidative stress induced by long-term plum pox virus infection in peach (Prunus persica). Physiol. Plant. 2004, 122, 486–495. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Díaz-Vivancos, P.; Rubio, M.; Fernández-García, N.; Hernández, J.A. Chloroplast protection in plum pox virus-infected peach plants by L-2-oxo-4-thiazolidine-carboxylic acid treatments: Effect in the proteome. Plant. Cell Environ. 2013, 36, 640–654. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Hernández, J.A.; Diaz-Vivancos, P. Sharka: How do plants respond to Plum pox virus infection? J. Exp. Bot. 2015, 66, 25–35. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Diaz-Vivancos, P.; Rubio, M.; Olmos, E.; Ros-Barcelo, A.; Martinez-Gomez, P. Long-term plum pox virus infection produces an oxidative stress in a susceptible apricot, Prunus armeniaca, cultivar but not in a resistant cultivar. Physiol. Plant. 2006, 126, 140–152. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Piqueras, A.; Hernández, J.A. Implication of peroxidase activity in development of healthy and PPV-infected micropropagated GF305 peach plants. Plant Growth Regul. 2011, 65, 359–367. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Rubio, M.; Mesonero, V.; Periago, P.; Ros Barcelo, A.; Martinez-Gomez, P.; Hernandez, J. The apoplastic antioxidant system in Prunus: Response to long-term plum pox virus infection. J. Exp. Bot. 2006, 57, 3813–3824. [Google Scholar] [CrossRef]
- Hernández, J.; Díaz-Vivancos, P.; Rubio, M.; Olmos, E.; Clemente, M.; Ros-Barceló, A.; Martínez-Gómez, P. Plum pox virus (PPV) infection produces an imbalance on the antioxidative systems in Prunus species. Acta Phytopathol. Entomol. Hung. 2007, 42, 209–221. [Google Scholar] [CrossRef]
- Hernández, J.A.; Talavera, J.M.; Martínez-Gómez, P.; Dicenta, F.; Sevilla, F. Response of antioxidative enzymes to plum pox virus in two apricot cultivars. Physiol. Plant. 2001, 111, 313–321. [Google Scholar] [CrossRef]
- Dehkordi, A.; Rubio, M.; Babaeian, N.; Albacete, A.; Martínez-Gómez, P. Phytohormone Signaling of the Resistance to Plum pox virus (PPV, Sharka Disease) Induced by Almond (Prunus dulcis (Miller) Webb) Grafting to Peach (P. persica L. Batsch). Viruses 2018, 10, 238. [Google Scholar] [CrossRef]
- Rubio, M.; Rodríguez-Moreno, L.; Ballester, A.R.; de Moura, M.C.; Bonghi, C.; Candresse, T.; Martínez-Gómez, P. Analysis of gene expression changes in peach leaves in response to Plum pox virus infection using RNA-Seq. Mol. Plant Pathol. 2015, 16, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Moreno, M.J.; Díaz-Vivancos, P.; Piqueras, A.; Hernández, J.A. Plant growth stimulation in Prunus species plantlets by BTH or OTC treatments under in vitro conditions. J. Plant Physiol. 2012, 169, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Chapman, P.; Chen, L.; Stobbs, L.W.; Brown, D.C.W.; Brandle, J.E. A comparative survey, by expressed sequence tag analysis, of genes expressed in peach leaves infected with Plum pox virus (PPV) and free from PPV. Can. J. Plant Pathol. 2005, 27, 410–419. [Google Scholar] [CrossRef]
- Rubio, M.; Ballester, A.R.; Olivares, P.M.; De Moura, M.C.; Dicenta, F.; Martínez-Gómez, P. Gene expression analysis of plum pox virus (Sharka) susceptibility/resistance in apricot (prunus armeniaca L.). PLoS ONE 2015, 10, e0144670. [Google Scholar] [CrossRef] [PubMed]
- Zuriaga, E.; Romero, C.; Blanca, J.M.; Badenes, M.L. Resistance to Plum Pox Virus (PPV) in apricot (Prunus armeniaca L.) is associated with down-regulation of two MATHd genes. BMC Plant Biol. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Collum, T.D.; Stone, A.L.; Sherman, D.J.; Rogers, E.E.; Dardick, C.; Culver, J.N. Translatome Profiling of Plum Pox Virus–Infected Leaves in European Plum Reveals Temporal and Spatial Coordination of Defense Responses in Phloem Tissues. Mol. Plant Microbe Interact. 2020, 33, 66–77. [Google Scholar] [CrossRef]
- King, H.A.; Gerber, A.P. Translatome profiling: Methods for genome-scale analysis of mRNA translation. Brief. Funct. Genom. 2014, 15, 22–31. [Google Scholar] [CrossRef]
- Collum, T.D.; Lutton, E.; Raines, C.D.; Dardick, C.; Culver, J.N. Identification of phloem-associated translatome alterations during leaf development in Prunus domestica L. Hortic. Res. 2019, 6, 16. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Cantabella, D.; Hernández, J.A.; Diaz-Vivancos, P. The effect of mandelonitrile, a recently described salicylic acid precursor, on peach plant response against abiotic and biotic stresses. Plant Biol. 2018, 20, 986–994. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N. Roles of plant hormones in the regulation of host–virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Rubio, J.; Sánchez, E.; Tricon, D.; Montes, C.; Eyquard, J.-P.; Chague, A.; Aguirre, C.; Prieto, H.; Decroocq, V. Silencing of one copy of the translation initiation factor eIFiso4G in Japanese plum (Prunus salicina) impacts susceptibility to Plum pox virus (PPV) and small RNA production. BMC Plant Biol. 2019, 19, 440. [Google Scholar] [CrossRef]
- Decroocq, V.; Sicard, O.; Alamillo, J.M.; Lansac, M.; Eyquard, J.P.; García, J.A.; Candresse, T.; Le Gall, O.; Revers, F. Multiple Resistance Traits Control Plum pox virus Infection in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2006, 19, 541–549. [Google Scholar] [CrossRef]
- Wang, X.; Kohalmi, S.E.; Svircev, A.; Wang, A.; Sanfaçon, H.; Tian, L. Silencing of the Host Factor eIF(iso)4E Gene Confers Plum Pox Virus Resistance in Plum. PLoS ONE 2013, 8, e50627. [Google Scholar] [CrossRef] [PubMed]
- Rodamilans, B.; San León, D.; Mühlberger, L.; Candresse, T.; Neumüller, M.; Oliveros, J.C.; García, J.A. Transcriptomic analysis of Prunus domestica undergoing hypersensitive response to Plum Pox Virus infection. PLoS ONE 2014, 9, e100477. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.; Michalczuk, L.; Neumüller, M. Hypersensitive reaction of plum (Prunus domestica) in response to Plum pox virus infection: Changes in gene expression and identification of potential molecular markers. Sci. Hortic. 2019, 247, 430–435. [Google Scholar] [CrossRef]
- Milosevic, T.M.; Glisic, I.P.; Milosevic, N.T.; Glisic, I.S. Plum pox virus as a stress factor in the vegetative growth, fruit growth and yield of plum (Prunus domestica) cv. ‘Cacanska Rodna’. Eur. J. Plant Pathol. 2010, 126, 73–79. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Stampar, F.; Virscek Marn, M. Effect of Plum pox virus on Chemical Composition and Fruit Quality of Plum. J. Agric. Food Chem. 2015, 63, 51–60. [Google Scholar] [CrossRef]
- Usenik, V.; Marn, M.V. Sugars and organic acids in plum fruit affected by Plum pox virus. J. Sci. Food Agric. 2017, 97, 2154–2158. [Google Scholar] [CrossRef]
- Usenik, V.; Franci, S.; Damijana, K. How does sharka affect the phenolics of plum fruit (Prunus domestica L.)? Hortic. Sci. 2017, 44, 64–72. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J.; Jevremović, D. Impact of Sharka disease on tree growth, productivity and fruit quality of apricot (Prunus armeniaca L.). Sci. Hortic. 2019, 244, 270–276. [Google Scholar] [CrossRef]
- Samara, R.; Hunter, D.M.; Stobbs, L.W.; Greig, N.; Lowery, D.T.; Delury, N.C. Impact of Plum pox virus (PPV-D) infection on peach tree growth, productivity and bud cold hardiness. Can. J. Plant Pathol. 2017, 39, 218–228. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, X.; Yang, S.; Cui, H.; Wang, G. Metabolomic Signature Between Metabolically Healthy Overweight/Obese and Metabolically Unhealthy Overweight/Obese: A Systematic Review. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 991–1010. [Google Scholar] [CrossRef]
- Asteggiano, A.; Franceschi, P.; Zorzi, M.; Aigotti, R.; Dal Bello, F.; Baldassarre, F.; Lops, F.; Carlucci, A.; Medana, C.; Ciccarella, G. HPLC-HRMS Global Metabolomics Approach for the Diagnosis of “Olive Quick Decline Syndrome” Markers in Olive Trees Leaves. Metabolites 2021, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Galeano Garcia, P.; Neves dos Santos, F.; Zanotta, S.; Eberlin, M.; Carazzone, C. Metabolomics of Solanum lycopersicum Infected with Phytophthora infestans Leads to Early Detection of Late Blight in Asymptomatic Plants. Molecules 2018, 23, 3330. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Goldansaz, S.A.; Jaine, J. Translational Metabolomics: Current Challenges and Future Opportunities. Metabolites 2019, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, L.; Jäntti, S.; Suvitaival, T.; Theilade, S.; Risz, C.; Kostiainen, R.; Rossing, P.; Orešič, M.; Hyötyläinen, T. Targeted Clinical Metabolite Profiling Platform for the Stratification of Diabetic Patients. Metabolites 2019, 9, 184. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, C.; Bascou, B.; Calvayrac, C.; Bertrand, C. Deciphering Prunus Responses to PPV Infection: A Way toward the Use of Metabolomics Approach for the Diagnostic of Sharka Disease. Metabolites 2021, 11, 465. https://doi.org/10.3390/metabo11070465
Espinoza C, Bascou B, Calvayrac C, Bertrand C. Deciphering Prunus Responses to PPV Infection: A Way toward the Use of Metabolomics Approach for the Diagnostic of Sharka Disease. Metabolites. 2021; 11(7):465. https://doi.org/10.3390/metabo11070465
Chicago/Turabian StyleEspinoza, Christian, Benoît Bascou, Christophe Calvayrac, and Cédric Bertrand. 2021. "Deciphering Prunus Responses to PPV Infection: A Way toward the Use of Metabolomics Approach for the Diagnostic of Sharka Disease" Metabolites 11, no. 7: 465. https://doi.org/10.3390/metabo11070465
APA StyleEspinoza, C., Bascou, B., Calvayrac, C., & Bertrand, C. (2021). Deciphering Prunus Responses to PPV Infection: A Way toward the Use of Metabolomics Approach for the Diagnostic of Sharka Disease. Metabolites, 11(7), 465. https://doi.org/10.3390/metabo11070465