Metabolomic Studies for the Evaluation of Toxicity Induced by Environmental Toxicants on Model Organisms
Abstract
:1. Introduction
| Year | Model Organisms | Toxicants | Biochemical Study | Main Findings | References |
|---|---|---|---|---|---|
| 2017 | C. elegans | Rare earth elements | Neurotoxicity | Rare earth elements are now widely used in daily life. Trichloride neodymium, praseodymium, and scandium induced loss of dendrite in dopaminergic and GABAergic neurons and downregulated dat-1::GFP and unc-47::GFP in C. elegans. | Xu et al. [29] |
| 2017 | D. rerio | Heavy metals | Swimming and AChE activity | Exposure of cadmium toward zebrafish strongly inhibited acetylcholinesterase (AChE) activity in the gill of zebrafish and decreased swimming behavior, which might be an evidence of neurotoxicity. | Pan et al. [30] |
| 2017 | D. rerio | Fine particulate matter | Multi-organ toxicity | This study evaluated toxicity of fine particulate matter (PM2.5) in a zebrafish. PM2.5 induced embryonic toxicity, hepatotoxicity, and neurotoxicity on model organisms. | Duan et al. [31] |
| 2018 | C. elegans | Phthalates | Multigenerational toxicity | Phthalates induced multigenerational toxicity regarding locomotive effects and total brood size, which might be related to disruption of vitellogenin and H3Kme2 demethylase. | Li et al. [32] |
| 2018 | E. fetida | Insecticides | Avoidance behavior and reproduction | Toxic effect of insecticides toward earthworms were studied. As a result, avoidance behavior was observed along with decrease of reproduction. | Ge et al. [33] |
| 2018 | L. fortunei | Herbicides | Biochemical responses | This study evaluated biochemical responses of the golden mussel Limnoperna fortunei upon exposure to glyphosate. Dietary exposure of glyphosate altered detoxification responses; however, it did not affect oxidative stress parameters. | Iummato et al. [34] |
| 2019 | D. rerio | Pharmaceuticals | Embryonic development and biochemical effects | Effects of environmental relevant levels of Paracetamol and Ciprofloxacin on zebrafish were evaluated. These pharmaceuticals affected developmental process, behaviors, epigenetics, and enzyme activities. | Nogueira et al. [35] |
| 2020 | C. elegans | Nanopolystyrene | Locomotion and sensory systems | Nanopolystyrene induced toxic effect on locomotion and sensory systems, specifically on development of dopaminergic neurons. | Wang et al. [36] |
| 2020 | D. rerio | Personal Care | Transcriptional, biochemical, and histological effects | Biochemical Effects of Benzotriazole UV stabilizer on zebrafish were evaluated. The compound altered level of antioxidant enzymes, expression of stress response gene, and induced damage on liver. | Hemalatha et al. [37] |
| 2020 | D. magna | Organometallic biocide | Biochemical Effects | Toxicity of Zinc pyrithione (ZnPT) in Daphnia manga was evaluated regarding biochemical effects. As a result, ZnPT induced oxidative and neurotoxic effects, which may be a potential threat to aquatic organisms. | Sousa et al. [38] |
| 2020 | D. magna | Drink water treatment residue | Physiological and biochemical responses | Drink water treatment residue (DWTR) is a byproduct produced during drinking water production. Adverse effects induced by DWTR was evaluated. The study evaluated effects of DWTR on the survival, growth, reproduction, body morphology, and oxidative stress. | Yuan et al. [39] |
| 2020 | E. eugeniae | Pesticides | Physiological behavior | Exposure of pesticides on earthworms induced decrease in reproductive activity, rupture of muscles and tissues, and increase in mortality rate. | Gowri et al. [40] |
| 2021 | A. parthenogenetica | PAHs | Lethal, behavioral, growth and developmental toxicities | Polycyclic aromatic hydrocarbons (PAHs) are one of widespread pollutants in aquatic environments. The effects of these compounds toward brine shrimp were evaluated. Survival, behavior, and growth were affected upon exposure to PAHs and body length could be used as an indicator for the evaluation of development. | Cong et al. [41] |
| 2021 | C. pyrenoidosa | Nanoplastics | Growth, photosynthesis, and oxidative stress | Toxic mechanisms of nanoplastics on microalgae (Chlorella pyrenoidosa) was investigated. By transcriptomic analysis, nanoplastics could be responsible for decreased gene expression of aminoacyl-tRNA synthetase. Algae have detoxification mechanisms by regulating intracellular osmotic pressure. | Yang et al. [42] |
2. Application of Metabolomics in the Study of Biological Responses to Environmental Toxicants
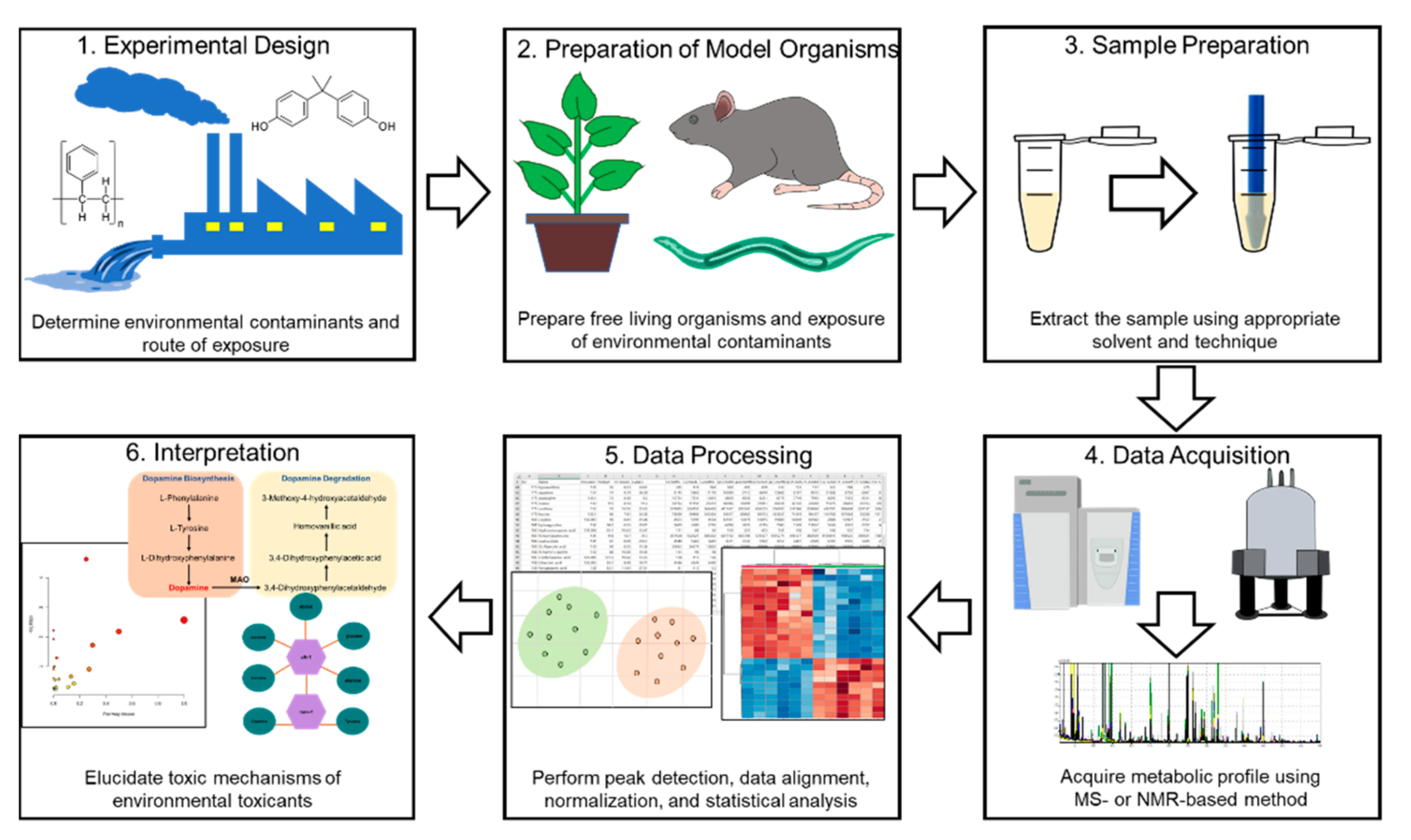
3. Metabolomics Workflow
3.1. Preparation of Model Organisms
3.2. Sample Treatment
3.3. High-Throughput Techniques for Metabolite Screening
3.4. Data Processing
3.5. Interpretation of Data
4. Exposure on Emerging Environmental Pollutant
4.1. Model Organisms for Environmental Metabolomics
4.2. Pharmaceuticals and Personal Care Products
4.3. Pesticides
4.4. Nanoparticles
4.5. Additives in Consumer Products
| Organisms | Environmental Toxicants | Experimental Conditions | Toxic Mechanisms | Biochemical Assays | References | |
|---|---|---|---|---|---|---|
| Pharmaceuticals and personal care products | Danio rerio | Clarithromycin Florfenicol Sulfamethazine | Adult fish (n = 30) 0.1 mg/L for 72 h Extraction with Bligh-Dyer LC-QToF-MS | Dysregulation of choline, guanosine, and ADP | Impaired swimming behavior | De Sotto et al. [146] |
| Triclsoan (1, 30 and 300 μg/L) Methyl triclosan (MTCS) (0.5, 10 and 400 μg/L) | 50 embryos (n = 6) for 96 h Extraction with acetonitrile: isopropanol: water (3:3:2) GC-MS | Dysregulation of energy metabolism, nitrogen metabolism, and fatty acid synthesis | Dysregulation of eight genes related to energy metabolism, nitrogen metabolism, and fatty acid synthesis | Fu et al. [147] | ||
| Gammarus pulex | Propranolol (100, 153 mg/L) Triclosan (0.1, 0.3 mg/L) Nimesulide (0.5, 1.4 mg/L) | Adult G. pulex (2, 6, 24 h) (approx. 100 specimens, n = 4) Extraction with 90% MeOH LC-Orbitrap-MS | Possible alterations of protein syntehsis and oxidative stress | Three pharmaceuticals affected 23 functional pathways | Sheikholeslami et al. [171] | |
| Mytilus galloprovincialis | Diclofenac (100 μg/L) | 3 mussels (n = 6) for 7 days Extraction with water: methanol: dichlromethane LC-Orbitrap-MS | Dysregulation of tyrosine and tryptophan metabolism | Potential risk of osmoregulation and reprodution | Bonnefille et al. [172] | |
| Sulfamethoxazole | 10 mussels exposed 4 days Extraction with metanol/water (1:2), clean up with SPE LC-QTrap-MS | Significant change in four amino acids, Benzoic acid, and Inosine | Perturbation of osmoregulation, energy metabolism, and organoleptic properties | Serra-Compte et al. [173] | ||
| Caenorhabditis elegans | Triclosan (0.1 and 1 mg/L) | Adult worms exposed 24 h Extraction with 80% MeOH GC-MS after silylation | Significantly affected amino aicds, tricyclic acid intermediates, carbohydrates and poly amines. | Decreased lifespan, reproduction, and locomotion. Increased oxidative stress | Kim et al. [150] | |
| Pesticides | Danio rerio | Dieldrin (16 or 163.5 ng/g) | Adult fish Extraction with acetone: hexane (5:2), reconstitution with ACN, clean up with SPE, GC-MS/MS | Dieldrin altered composition and function of intestinal microbiome | No change in body mass, growth rate or histopathology. | Hua et al. [174] |
| Isocarbophos (50 and 200 μg/L) | Adult fish exposed 4 days Extraction with 20% MeOH 1H-NMR analyasis | Significant alteration with energy related metabolism (lactate, alanine, and creatin) | Significant down-regulation of antioxidant enzyme activity. Accumulation of Isocarbophos in zebrafish | Jia et al. [175] | ||
| Oryza sativa | Butachlor (3.148 kg/a.i.ha) Chlorpyrifos (1.440 kg/a.i.ha) Tricyclazole (0.607 kg/a.i.ha) | 10 mg of dried leaves Extraction with methanol: chloroform: water (5:2:2) GC-MS after silylation | Signifcantly affected TCA cyle, amino acid, and fatty acid metabolism. | Fifferentially expressed genes starch-sucrose distribution, protein contents and photosynthesis | Liu et al. [156] | |
| Eisenia fetida | Sulfoxaflor (0/2 mg/kg) | Earthworms exposed 14 days Extractions with methanol: acetonitrile: water (2:2:1) LC-Orbitrap-MS | Sulfoxaflor altered carbohydrates, TCA cycle, pyrimidine purine, and some amino acids | Oxidative damage by sulfoxaflor was confirmed by SOD, CAT, GST, and MDA assay | Fang et al. [153] | |
| Imidacloprid Dinotefuran | Earthworms exposed 7, 14, 21, 28 days Extration with acetontirile: methanol:water (1:2:1) LC-QToF-MS | Disturbance of TCA/Urea cycle, energy production and oxidative stress. | Alteration of activity acetylcholineesterase, superoixde dismutase, and catalase | Zhang et al. [176] | ||
| Caenorhabditis elegans | Atrazine (4 mg/L) | 10,000 worms (n = 5) for 48 h Extractions with methanol: acetonitrile: water (2:2:1) LC-Orbitrap-MS | Perturbation of glycolysis, gluconeogenesis, and phosphatidylcholine metabolism | Increased oxidative stress and disrput ATP synthesis. Reduction of reproduction, locomotion and brood size | Yin et al. [177] | |
| Nanoparticles | Oreochromis mossambicus | 100 nm polystyrene (20 mg/L) | 20 fish for 7 days Extractions with methanol: acetonitrile: water (2:2:1) LC-QToF-MS | Disorder of energy, amino acid, and lipid metabolism. | Damage of feeding and sensing behavior and signaling disorder | Pang et al. [178] |
| Danio rerio | polypropylene fibers (10 and 100 μg/L) | Adult fish for 21 days Intestines were extracted with methanol: water (4:1) UPLC-MS | Up-regulation of glycerophospholipids metabolism and down-regulation of fatty acyls metabolism. | Intestinal damage, nutritional deficiency, and oxidative stress were induced by microplastic fibers | Zhao et al. [179] | |
| Cyprinus carpio | Silver nanoparticle (0.1, 0.5, 1 and 2 mg/L) | 10 adult fish for 24~96 h Fish gills were extracted with methanol: water (4:1) UPLC-QToF-MS | Inhibition of TCA cycle. Perturbation of lipid metabolism. | Induced epithelial hyperplasis of gill. Perturbation of genes in asparatate metabolism pathways | Xiang et al. [180] | |
| Poterioochromonas malhamensis | Silver nanoparticle (1 mg/L) | Algae for 2 and 24 h Extractions with methanol: water (4:1) LC-QqQ-MS | Perturbation of amino acids, nucleobases, sugars, and fatty acids metabolism. | Increased level of ROS and decrease of the photosynthetic efficiency | Liu et al. [181] | |
| Eisenia fetida | TiO2 nanoparticles (5, 50, 500 mg/kg) | Earthworms for 120 days Extractions with chloroform: water: methanol (2:2:5) GC-MS after silylation | Alteration of glutathione and starch, sucrose metabolism. | Decreased GSH/GSSG ratio. Slight increase in ROS level. Alteration of genes in TGF-beta singaling pathway | Zhu et al. [182] | |
| Enchytraeus crypticus | Silver nanoparticles (60–102 mg/kg) Silver ions (45–60 mg/kg) | Worms (n = 5) for 7 and 14 days Extraction with methanol LC-Orbitrap-MS | Alteration of phenylalanine, histidine, lipid, and energy metabolism. | Activation of cellular iron ion homeostasis, tyrosine catabolism, glycosylation, and stress response | Maria et al. [183] | |
| Consumer Products Additives | Drosophila melanogaster | BDE-47 (2, 10 or 50 μM) | 5 flies for 30 days Extraction with methanol LC-Orbitrap-MS Extraction with methanol: water (4:1) GC-MS after silylation | Perturbation of metabolites involved in tryptophan, phenlyalanine, and purine metabolism | Decreased ratio of SAM/SAH and GSH/GSSG. Imbalance of kynurenine metabolism and oxidative stress | Ji et al. [166] |
| Eisenia fetida | BDE-47 BDE-209 (10, 50, 100 and 200 mg/kg) | 3 earthworms (n = 6) for 14 days Extraction with water 1H-NMR analysis | Increase of lactate, glutamate, betaine, leucine and lysine. Decrease of fumarate and glycine. | Toxic effects by disturbing osmo regulation, energy metabolism, nerve activities, and TCA cycle | Liang et al. [184] | |
| Danio rerio | Bisphenol A (4.4, 8.8, 17.5 μM) | 20 zebrafish embryos (n = 6) Extraction with methanol: water: chloroform LC-QToF-MS | Perturbation of amino acids, prostaglandin, folate, ascorbate and nucleotide metabolic pathways. | Altered gene expression with estrogenic, CYP450 enzyme, tissue development and cell proliferation | Ortiz-Villanueva et al. [185] | |
| Rattus norvegicus | Bisphenol A Bisphenol S (50 μg/kg) | Rat plasma (n = 14) Extraction with methanol: water (4:1) LC-Orbitrap-MS | BPA exposure decreased citric acid, oxoglutaric acid, and malic acid. While BPS decreased poly unsaturated fatty acid. | Toxic effects of endocrine disruption, cytotoxicity, and genotoxicity | Mao et al. [186] | |
| Mytilus coruscus | Phthalates (0.04, 0.40, 1.00 mg/L) | 4 mussels for 7 days. Extraction with methanol LC-QToF-MS | Significant changes in amino acids, lipids, energy storage compounds, osmolytes and neurotransmittes. | Activation of antioxidant defense system | Gu et al. [187] |
5. Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Tan, Q.; Song, Q.; Li, J. An analysis of the plastic waste trade and management in Asia. Waste Manag. 2021, 119, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Blettler, M.C.; Wantzen, K.M. Threats underestimated in freshwater plastic pollution: Mini-review. Water Air Soil Pollut. 2019, 230, 1–11. [Google Scholar] [CrossRef]
- Mezzelani, M.; Nardi, A.; Bernardini, I.; Milan, M.; Peruzza, L.; d’Errico, G.; Fattorini, D.; Gorbi, S.; Patarnello, T.; Regoli, F. Environmental pharmaceuticals and climate change: The case study of carbamazepine in M. galloprovincialis under ocean acidification scenario. Environ. Int. 2021, 146, 106269. [Google Scholar] [CrossRef] [PubMed]
- Balmer, J.E.; Morris, A.D.; Hung, H.; Jantunen, L.; Vorkamp, K.; Rigét, F.; Evans, M.; Houde, M.; Muir, D.C. Levels and trends of current-use pesticides (CUPs) in the arctic: An updated review, 2010–2018. Emerg. Contam. 2019, 5, 70–88. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Shi, B.; Meng, J.; He, B.; Yang, H.; Yoon, S.J.; Kim, T.; Kwon, B.-O.; Khim, J.S.; et al. Anthropogenic impacts on the contamination of pharmaceuticals and personal care products (PPCPs) in the coastal environments of the Yellow and Bohai seas. Environ. Int. 2020, 135, 105306. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Green, P.G.; Voulvoulis, N.; Lester, J.N. Questioning the Excessive Use of Advanced Treatment to Remove Organic Micropollutants from Wastewater. Environ. Sci. Technol. 2007, 41, 5085–5089. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, Y.; Chen, Y.; Fang, C.; Chi, Y.; Zhu, H.; Lin, Y.; Ye, G.; Dong, S. New insights into the metabolism and toxicity of bisphenol A on marine fish under long-term exposure. Environ. Pollut. 2018, 242, 914–921. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lei, Z.; Chen, X.; Ye, G.; Zhang, J.; Shen, H.; Yan, C. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef]
- Cong, Y.; Jin, F.; Wang, J.; Mu, J. The embryotoxicity of ZnO nanoparticles to marine medaka, Oryzias melastigma. Aquat. Toxicol. 2017, 185, 11–18. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Q.; Zhu, B.; Lan, Y.; Duan, S. Long-term exposure to benzo [a] pyrene affects sexual differentiation and embryos toxicity in three generations of marine Medaka (Oryzias melastigma). Int. J. Environ. Res. Public Health 2020, 17, 970. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.H.; Sørensen, L.; Størseth, T.R.; Altin, D.; Gonzalez, S.V.; Skancke, J.; Rønsberg, M.U.; Nordtug, T. The use of PAH, metabolite and lipid profiling to assess exposure and effects of produced water discharges on pelagic copepods. Sci. Total Environ. 2020, 714, 136674. [Google Scholar] [CrossRef] [PubMed]
- Aru, V.; Balling Engelsen, S.; Savorani, F.; Culurgioni, J.; Sarais, G.; Atzori, G.; Cabiddu, S.; Marincola, F.C. The effect of season on the metabolic profile of the European clam Ruditapes decussatus as studied by 1H-NMR spectroscopy. Metabolites 2017, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Huang, X.; Sun, W.; Too, H.Z.; Laserna, A.K.C.; Li, S.F.Y. A global metabolomic insight into the oxidative stress and membrane damage of copper oxide nanoparticles and microparticles on microalga Chlorella vulgaris. Environ. Pollut. 2020, 258, 113647. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.; Hu, L.; Zhou, Y.; Min, X.; She, S.; Chen, S.; Huang, M. Current status, spatial features, health risks, and potential driving factors of soil heavy metal pollution in China at province level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, F.; Qiao, Z.; Yu, H.; Sun, S.; Li, X.; Zhang, J.; Jiang, X. Toxicity of thifluzamide in earthworm (Eisenia fetida). Ecotoxicol. Environ. Saf. 2020, 188, 109880. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, B.; Wu, R.; Li, W.; Wang, J.; Wang, J.; Du, Z.; Juhasz, A.; Zhu, L. Acute toxicity, oxidative stress and DNA damage of chlorpyrifos to earthworms (Eisenia fetida): The difference between artificial and natural soils. Chemosphere 2020, 255, 126982. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.-K.; Long, N.P.; Kwon, S.W.; Park, J.H. Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans. Environ. Pollut. 2019, 246, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Long, N.P.; Min, J.E.; Anh, N.H.; Kim, S.J.; Yoon, S.J.; Kwon, S.W. Comprehensive phenotyping and multi-omic profiling in the toxicity assessment of nanopolystyrene with different surface properties. J. Hazard. Mater. 2020, 399, 123005. [Google Scholar] [CrossRef]
- Geng, N.; Song, X.; Cao, R.; Luo, Y.; Mila, A.; Cai, Z.; Yu, K.; Gao, Y.; Ni, Y.; Zhang, H. The effect of toxic components on metabolomic response of male SD rats exposed to fine particulate matter. Environ. Pollut. 2021, 272, 115922. [Google Scholar] [CrossRef]
- Du, X.; Zeng, X.; Pan, K.; Zhang, J.; Song, L.; Zhou, J.; Chen, R.; Xie, Y.; Sun, Q.; Zhao, J. Metabolomics analysis of urine from healthy wild type mice exposed to ambient PM2.5. Sci. Total Environ. 2020, 714, 136790. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Sa, M.R.; Ji, J.; Vaniya, A.; Wancewicz, B.; Roberts, B.S.; Torbašinović, H.; Lee, T.; Mehta, S.S.; et al. Structure annotation of all mass spectra in untargeted metabolomics. Anal. Chem. 2019, 91, 2155–2162. [Google Scholar] [CrossRef]
- Long, N.P.; Nghi, T.D.; Kang, Y.P.; Anh, N.H.; Kim, H.M.; Park, S.K.; Kwon, S.W. Toward a standardized strategy of clinical metabolomics for the advancement of precision medicine. Metabolites 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labine, L.M.; Simpson, M.J. The use of nuclear magnetic resonance (NMR) and mass spectrometry (MS)–based metabolomics in environmental exposure assessment. Curr. Opin. Environ. Sci. Health 2020, 15, 7–15. [Google Scholar] [CrossRef]
- Gil-Solsona, R.; Álvarez-Muñoz, D.; Serra-Compte, A.; Rodríguez-Mozaz, S. (Xeno) Metabolomics for the evaluation of aquatic organism’s exposure to field contaminated water. Trends Environ. Anal. Chem. 2021, 31, e00132. [Google Scholar] [CrossRef]
- Kovacevic, V.; Simpson, M.J. Fundamentals of environmental metabolomics. In Environmental Metabolomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Liang, D.; Moutinho, J.L.; Golan, R.; Yu, T.; Ladva, C.N.; Niedzwiecki, M.; Walker, D.I.; Sarnat, S.E.; Chang, H.H.; Greenwald, R. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ. Int. 2018, 120, 145–154. [Google Scholar] [CrossRef]
- Matich, E.K.; Soria, N.G.C.; Aga, D.S.; Atilla-Gokcumen, G.E. Applications of metabolomics in assessing ecological effects of emerging contaminants and pollutants on plants. J. Hazard. Mater. 2019, 373, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, M.; Hu, J.; Li, Z.; Wu, T.; Bao, J.; Wu, S.; Lei, L.; He, D. Behavioral deficits and neural damage of Caenorhabditis elegans induced by three rare earth elements. Chemosphere 2017, 181, 55–62. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, X.; Ren, B.; Yang, H.; Ren, Z.; Wang, W. Toxic assessment of cadmium based on online swimming behavior and the continuous AChE activity in the gill of zebrafish (Danio rerio). Water Air Soil Pollut. 2017, 228, 1–9. [Google Scholar] [CrossRef]
- Duan, J.; Hu, H.; Zhang, Y.; Feng, L.; Shi, Y.; Miller, M.R.; Sun, Z. Multi-organ toxicity induced by fine particulate matter PM2. 5 in zebrafish (Danio rerio) model. Chemosphere 2017, 180, 24–32. [Google Scholar] [CrossRef]
- Li, S.-W.; How, C.M.; Liao, V.H.-C. Prolonged exposure of di (2-ethylhexyl) phthalate induces multigenerational toxic effects in Caenorhabditis elegans. Sci. Total Environ. 2018, 634, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Xiao, Y.; Chai, Y.; Yan, H.; Wu, R.; Xin, X.; Wang, D.; Yu, X. Sub-lethal effects of six neonicotinoids on avoidance behavior and reproduction of earthworms (Eisenia fetida). Ecotoxicol. Environ. Saf. 2018, 162, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Iummato, M.M.; Sabatini, S.E.; Cacciatore, L.C.; Cochón, A.C.; Cataldo, D.; de Molina, M.d.C.R.; Juárez, Á.B. Biochemical responses of the golden mussel Limnoperna fortunei under dietary glyphosate exposure. Ecotoxicol. Environ. Saf. 2018, 163, 69–75. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Pinto, G.; Correia, B.; Nunes, B. Embryonic development, locomotor behavior, biochemical, and epigenetic effects of the pharmaceutical drugs paracetamol and ciprofloxacin in larvae and embryos of Danio rerio when exposed to environmental realistic levels of both drugs. Environ. Toxicol. 2019, 34, 1177–1190. [Google Scholar] [CrossRef]
- Qu, M.; Wang, D. Toxicity comparison between pristine and sulfonate modified nanopolystyrene particles in affecting locomotion behavior, sensory perception, and neuronal development in Caenorhabditis elegans. Sci. Total Environ. 2020, 703, 134817. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, D.; Rangasamy, B.; Nataraj, B.; Maharajan, K.; Narayanasamy, A.; Ramesh, M. Transcriptional, biochemical and histological alterations in adult zebrafish (Danio rerio) exposed to benzotriazole ultraviolet stabilizer-328. Sci. Total Environ. 2020, 739, 139851. [Google Scholar] [CrossRef]
- Sousa, A.P.; Nunes, B. Standard and biochemical toxicological effects of zinc pyrithione in Daphnia magna and Daphnia longispina. Environ. Toxicol. Pharmacol. 2020, 80, 103402. [Google Scholar] [CrossRef]
- Yuan, N.; Pei, Y.; Bao, A.; Wang, C. The Physiological and Biochemical Responses of Daphnia magna to Dewatered Drinking Water Treatment Residue. Int. J. Environ. Res. Public Health 2020, 17, 5863. [Google Scholar] [CrossRef] [PubMed]
- Gowri, S.; Thangaraj, R. Studies on the toxic effects of agrochemical pesticide (Monocrotophos) on physiological and reproductive behavior of indigenous and exotic earthworm species. Int. J. Environ. Health Res. 2020, 30, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Wang, Y.; Zhang, M.; Jin, F.; Mu, J.; Li, Z.; Wang, J. Lethal, behavioral, growth and developmental toxicities of alkyl-PAHs and non-alkyl PAHs to early-life stage of brine shrimp, Artemia parthenogenetica. Ecotoxicol. Environ. Saf. 2021, 220, 112302. [Google Scholar] [CrossRef]
- Yang, W.; Gao, P.; Ma, G.; Huang, J.; Wu, Y.; Wan, L.; Ding, H.; Zhang, W. Transcriptome analysis of the toxic mechanism of nanoplastics on growth, photosynthesis and oxidative stress of microalga Chlorella pyrenoidosa during chronic exposure. Environ. Pollut. 2021, 284, 117413. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Carpena, M.; Garcia-Oliveira, P.; Pereira, A.; Prieto, M.; Simal-Gandara, J. Analytical metabolomics and applications in health, environmental and food science. Crit. Rev. Anal. Chem. 2020, 1–23. [Google Scholar] [CrossRef]
- Percival, B.C.; Grootveld, M.; Gibson, M.; Osman, Y.; Molinari, M.; Jafari, F.; Sahota, T.; Martin, M.; Casanova, F.; Mather, M.L. Low-field, benchtop NMR spectroscopy as a potential tool for point-of-care diagnostics of metabolic conditions: Validation, protocols and computational models. High-Throughput 2019, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Locci, E.; Bazzano, G.; Chighine, A.; Locco, F.; Ferraro, E.; Demontis, R.; d’Aloja, E. Forensic NMR metabolomics: One more arrow in the quiver. Metabolomics 2020, 16, 1–16. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.; Gibson, M.; Osman, Y.; Edgar, M.; Molinari, M.; Mather, M.L.; Casanova, F.; Wilson, P.B. Progress in low-field benchtop NMR spectroscopy in chemical and biochemical analysis. Anal. Chim. Acta 2019, 1067, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crook, A.A.; Powers, R. Quantitative NMR-Based Biomedical Metabolomics: Current Status and Applications. Molecules 2020, 25, 5128. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Simpson, M.J.; Soong, R. Environmental nuclear magnetic resonance spectroscopy: An overview and a primer. Anal. Chem. 2018, 90, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Augustijn, D.; de Groot, H.J.; Alia, A. HR-MAS NMR applications in plant metabolomics. Molecules 2021, 26, 931. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.P.; Alseekh, S.; Naake, T.; Fernie, A. Mass Spectrometry-Based Untargeted Plant Metabolomics. Curr. Protoc. Plant Biol. 2019, 4. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, K.; Futrelle, J.; Coburn, E.; Soule, M.C.K.; Kujawinski, E.B. Environmental metabolomics: Databases and tools for data analysis. Mar. Chem. 2015, 177, 366–373. [Google Scholar] [CrossRef]
- Kusano, M.; Yang, Z.; Okazaki, Y.; Nakabayashi, R.; Fukushima, A.; Saito, K. Using metabolomic approaches to explore chemical diversity in rice. Mol. Plant 2015, 8, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ramautar, R. CE-MS for metabolomics: Developments and applications in the period 2018–2020. Electrophoresis 2021, 42, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Stettin, D.; Poulin, R.X.; Pohnert, G. Metabolomics Benefits from Orbitrap GC–MS—Comparison of Low-and High-Resolution GC–MS. Metabolites 2020, 10, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, E.R.; Li, X.; Kfoury, N.; Morimoto, J.; Han, W.-Y.; Ahmed, S.; Cash, S.B.; Griffin, T.S.; Stepp, J.R.; Robbat, A., Jr.; et al. Interactive effects of drought severity and simulated herbivory on tea (Camellia sinensis) volatile and non-volatile metabolites. Environ. Exp. Bot. 2019, 157, 283–292. [Google Scholar] [CrossRef]
- Ren, J.-L.; Zhang, A.-H.; Kong, L.; Wang, X.-J. Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Adv. 2018, 8, 22335–22350. [Google Scholar] [CrossRef] [Green Version]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.; Dias, D.A. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics 2018, 14, 1–31. [Google Scholar] [CrossRef]
- Liebeke, M.; Puskás, E. Drying enhances signal intensities for global GC–MS metabolomics. Metabolites 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.A.; Ammar, N.M.; Serag, A.; Shaker, O.G.; El Gendy, A.N.; Abdel-Hamid, A.-H.Z. Metabolomics driven analysis of obesity-linked colorectal cancer patients via GC-MS and chemometrics: A pilot study. Microchem. J. 2020, 155, 104742. [Google Scholar] [CrossRef]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Chetwynd, A.J.; David, A. A review of nanoscale LC-ESI for metabolomics and its potential to enhance the metabolome coverage. Talanta 2018, 182, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.-F.; Lu, W.; Rabinowitz, J.D. Avoiding misannotation of in-source fragmentation products as cellular metabolites in liquid chromatography–mass spectrometry-based metabolomics. Anal. Chem. 2015, 87, 2273–2281. [Google Scholar] [CrossRef] [Green Version]
- Xian, F.; Hendrickson, C.L.; Marshall, A.G. High resolution mass spectrometry. Anal. Chem. 2012, 84, 708–719. [Google Scholar] [CrossRef]
- Kind, T.; Tsugawa, H.; Cajka, T.; Ma, Y.; Lai, Z.; Mehta, S.S.; Wohlgemuth, G.; Barupal, D.K.; Showalter, M.R.; Arita, M. Identification of small molecules using accurate mass MS/MS search. Mass Spectrom. Rev. 2018, 37, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion–switching, targeted mass spectrometry–based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Zhan, H.; Yu, X.; Li, J.; Wang, X.; Xie, Z. Detection of organosulfates and nitrooxy-organosulfates in Arctic and Antarctic atmospheric aerosols, using ultra-high resolution FT-ICR mass spectrometry. Sci. Total Environ. 2021, 767, 144339. [Google Scholar] [CrossRef]
- Naz, S.; Gallart-Ayala, H.; Reinke, S.N.; Mathon, C.; Blankley, R.; Chaleckis, R.; Wheelock, C.E. Development of a liquid chromatography–high resolution mass spectrometry metabolomics method with high specificity for metabolite identification using all ion fragmentation acquisition. Anal. Chem. 2017, 89, 7933–7942. [Google Scholar] [CrossRef] [Green Version]
- Cajka, T.; Smilowitz, J.T.; Fiehn, O. Validating quantitative untargeted lipidomics across nine liquid chromatography–high-resolution mass spectrometry platforms. Anal. Chem. 2017, 89, 12360–12368. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, D.; Barbas-Bernardos, C.; García, A.; Barbas, C. Quality assurance procedures for mass spectrometry untargeted metabolomics. a review. J. Pharm. Biomed. Anal. 2018, 147, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Bonvallot, N.; David, A.; Chalmel, F.; Chevrier, C.; Cordier, S.; Cravedi, J.-P.; Zalko, D. Metabolomics as a powerful tool to decipher the biological effects of environmental contaminants in humans. Curr. Opin. Toxicol. 2018, 8, 48–56. [Google Scholar] [CrossRef]
- Gehrke, S.; Reisz, J.A.; Nemkov, T.; Hansen, K.C.; D’Alessandro, A. Characterization of rapid extraction protocols for high-throughput metabolomics. Rapid Commun. Mass Spectrom. 2017, 31, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, G.; Scognamiglio, M.; Russo, C.; Fiorentino, A.; Lavorgna, M. Environmental Metabolomics: A Powerful Tool to Investigate Biochemical Responses to Drugs in Nontarget Organisms. In Fate and Effects of Anticancer Drugs in the Environment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 441–465. [Google Scholar]
- Lin, C.Y.; Wu, H.; Tjeerdema, R.S.; Viant, M.R. Evaluation of metabolite extraction strategies from tissue samples using NMR metabolomics. Metabolomics 2007, 3, 55–67. [Google Scholar] [CrossRef]
- Yan, S.-C.; Chen, Z.-F.; Zhang, H.; Chen, Y.; Qi, Z.; Liu, G.; Cai, Z. Evaluation and optimization of sample pretreatment for GC/MS-based metabolomics in embryonic zebrafish. Talanta 2020, 207, 120260. [Google Scholar] [CrossRef]
- Geier, F.M.; Want, E.J.; Leroi, A.M.; Bundy, J.G. Cross-platform comparison of Caenorhabditis elegans tissue extraction strategies for comprehensive metabolome coverage. Anal. Chem. 2011, 83, 3730–3736. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Hoene, M.; Li, J.; Li, Y.; Zhao, X.; Häring, H.-U.; Schleicher, E.D.; Weigert, C.; Xu, G.; Lehmann, R. Simultaneous extraction of metabolome and lipidome with methyl tert-butyl ether from a single small tissue sample for ultra-high performance liquid chromatography/mass spectrometry. J. Chromatogr. A 2013, 1298, 9–16. [Google Scholar] [CrossRef]
- Medina, J.; van der Velpen, V.; Teav, T.; Guitton, Y.; Gallart-Ayala, H.; Ivanisevic, J. Single-Step Extraction Coupled with Targeted HILIC-MS/MS Approach for Comprehensive Analysis of Human Plasma Lipidome and Polar Metabolome. Metabolites 2020, 10, 495. [Google Scholar] [CrossRef]
- Ibáñez, C.; Simó, C.; Palazoglu, M.; Cifuentes, A. GC-MS based metabolomics of colon cancer cells using different extraction solvents. Anal. Chim. Acta 2017, 986, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moros, G.; Chatziioannou, A.C.; Gika, H.G.; Raikos, N.; Theodoridis, G. Investigation of the derivatization conditions for GC–MS metabolomics of biological samples. Bioanalysis 2017, 9, 53–65. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Luo, Q.; Liu, Z.; Gong, L. Extensive evaluation of sample preparation workflow for gas chromatography-mass spectrometry-based plasma metabolomics and its application in rheumatoid arthritis. Anal. Chim. Acta 2020, 1131, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. New trends in solid-phase extraction. TrAC Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Tang, D.Q.; Zou, L.; Yin, X.X.; Ong, C.N. HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass Spectrom. Rev. 2016, 35, 574–600. [Google Scholar] [CrossRef]
- Spagou, K.; Tsoukali, H.; Raikos, N.; Gika, H.; Wilson, I.D.; Theodoridis, G. Hydrophilic interaction chromatography coupled to MS for metabonomic/metabolomic studies. J. Sep. Sci. 2010, 33, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. BioSyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contrepois, K.; Jiang, L.; Snyder, M. Optimized analytical procedures for the untargeted metabolomic profiling of human urine and plasma by combining hydrophilic interaction (HILIC) and reverse-phase liquid chromatography (RPLC)–mass spectrometry. Mol. Cell. Proteom. 2015, 14, 1684–1695. [Google Scholar] [CrossRef] [Green Version]
- Schwaiger, M.; Schoeny, H.; El Abiead, Y.; Hermann, G.; Rampler, E.; Koellensperger, G. Merging metabolomics and lipidomics into one analytical run. Analyst 2019, 144, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ezernieks, V.; Rochfort, S.; Cocks, B. Comparison of methylation methods for fatty acid analysis of milk fat. Food Chem. 2018, 261, 210–215. [Google Scholar] [CrossRef]
- Ooi, M.; Nishiumi, S.; Yoshie, T.; Shiomi, Y.; Kohashi, M.; Fukunaga, K.; Nakamura, S.; Matsumoto, T.; Hatano, N.; Shinohara, M. GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflamm. Res. 2011, 60, 831–840. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, K.; Li, X.; Tang, Z.; Xiang, L.; Zhao, H.; Fu, J.; Wang, L.; Zhu, N.; Cai, Z. Mass spectrometry-based metabolomics reveals occupational exposure to per-and polyfluoroalkyl substances relates to oxidative stress, fatty acid β-oxidation disorder, and kidney injury in a manufactory in China. Environ. Sci. Technol. 2019, 53, 9800–9809. [Google Scholar] [CrossRef]
- Lebedev, A.T.; Polyakova, O.V.; Mazur, D.M.; Artaev, V.B. The benefits of high resolution mass spectrometry in environmental analysis. Analyst 2013, 138, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Feith, A.; Teleki, A.; Graf, M.; Favilli, L.; Takors, R. HILIC-enabled 13C metabolomics strategies: Comparing quantitative precision and spectral accuracy of QTOF high-and QQQ low-resolution mass spectrometry. Metabolites 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Song, Z.; Hong, Y.; Yang, Z.; Song, Y.; Chen, Z.; Chen, Z.; Cai, Z. Large-scale targeted metabolomics method for metabolite profiling of human samples. Anal. Chim. Acta 2020, 1125, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Zheng, C.; D’Alessandro, A.; Nemkov, T. Untargeted and semi-targeted lipid analysis of biological samples using mass spectrometry-based metabolomics. In High-Throughput Metabolomics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–135. [Google Scholar]
- Yuan, P.; Dong, M.; Lei, H.; Xu, G.; Chen, G.; Song, Y.; Ma, J.; Cheng, L.; Zhang, L. Targeted metabolomics reveals that 2, 3, 7, 8-tetrachlorodibenzofuran exposure induces hepatic steatosis in male mice. Environ. Pollut. 2020, 259, 113820. [Google Scholar] [CrossRef]
- Domingo-Almenara, X.; Montenegro-Burke, J.R.; Ivanisevic, J.; Thomas, A.; Sidibé, J.; Teav, T.; Guijas, C.; Aisporna, A.E.; Rinehart, D.; Hoang, L. XCMS-MRM and METLIN-MRM: A cloud library and public resource for targeted analysis of small molecules. Nat. Methods 2018, 15, 681–684. [Google Scholar] [CrossRef]
- Guo, J.; Huan, T. Comparison of Full-Scan, Data-Dependent, and Data-Independent Acquisition Modes in Liquid Chromatography–Mass Spectrometry Based Untargeted Metabolomics. Anal. Chem. 2020, 92, 8072–8080. [Google Scholar] [CrossRef]
- Barbier Saint Hilaire, P.; Rousseau, K.; Seyer, A.; Dechaumet, S.; Damont, A.; Junot, C.; Fenaille, F. Comparative Evaluation of Data Dependent and Data Independent Acquisition Workflows Implemented on an Orbitrap Fusion for Untargeted Metabolomics. Metabolites 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmer, S.; Manier, S.K.; Fischmann, S.; Westphal, F.; Wagmann, L.; Meyer, M.R. Comparison of three untargeted data processing workflows for evaluating LC-HRMS metabolomics data. Metabolites 2020, 10, 378. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Guo, Y.; Cao, H.; Wang, Q.; Shui, W. Comprehensive evaluation of untargeted metabolomics data processing software in feature detection, quantification and discriminating marker selection. Anal. Chim. Acta 2018, 1029, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Beger, R.D.; Dunn, W.B.; Bandukwala, A.; Bethan, B.; Broadhurst, D.; Clish, C.B.; Dasari, S.; Derr, L.; Evans, A.; Fischer, S. Towards quality assurance and quality control in untargeted metabolomics studies. Metabolomics 2019, 15, 4. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.I.; Edison, A.; Guillou, C.; Viant, M.R.; Bearden, D.W.; Beger, R.D. Quality assurance and quality control processes: Summary of a metabolomics community questionnaire. Metabolomics 2017, 13, 50. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Katajamaa, M.; Orešič, M. Data processing for mass spectrometry-based metabolomics. J. Chromatogr. A 2007, 1158, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Karaman, I. Preprocessing and Pretreatment of Metabolomics Data for Statistical Analysis. In Metabolomics: From Fundamentals to Clinical Applications; Springer: Cham, Switzerland, 2017; pp. 145–161. [Google Scholar]
- Nam, S.L.; Mata, A.; Dias, R.P.; Harynuk, J.J. Towards Standardization of Data Normalization Strategies to Improve Urinary Metabolomics Studies by GC× GC-TOFMS. Metabolites 2020, 10, 376. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So you think you can PLS-DA? BMC Bioinform. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Vinaixa, M.; Samino, S.; Saez, I.; Duran, J.; Guinovart, J.J.; Yanes, O. A guideline to univariate statistical analysis for LC/MS-based untargeted metabolomics-derived data. Metabolites 2012, 2, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Capela, R.; Garric, J.; Castro, L.F.C.; Santos, M.M. Embryo bioassays with aquatic animals for toxicity testing and hazard assessment of emerging pollutants: A review. Sci. Total Environ. 2020, 705, 135740. [Google Scholar] [CrossRef] [PubMed]
- van de Merwe, J.P.; Neale, P.A.; Melvin, S.D.; Leusch, F.D. In vitro bioassays reveal that additives are significant contributors to the toxicity of commercial household pesticides. Aquat. Toxicol. 2018, 199, 263–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinlaan, M.; Kasemets, K.; Aruoja, V.; Blinova, I.; Bondarenko, O.; Lukjanova, A.; Khosrovyan, A.; Kurvet, I.; Pullerits, M.; Sihtmäe, M. Hazard evaluation of polystyrene nanoplastic with nine bioassays did not show particle-specific acute toxicity. Sci. Total Environ. 2020, 707, 136073. [Google Scholar] [CrossRef]
- Hashmi, M.Z.; Kumar, V.; Varma, A. Xenobiotics in the Soil Environment: Monitoring, Toxicity and Management; Springer: Berlin/Heidelberg, Germany, 2017; Volume 49. [Google Scholar]
- Abdelsalam, N.A.; Ramadan, A.T.; ElRakaiby, M.T.; Aziz, R.K. Toxicomicrobiomics: The Human Microbiome vs. Pharmaceutical, Dietary, and Environmental Xenobiotics. Front. Pharmacol. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Magalhães Filho, F.J.C.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ. 2020, 705, 135568. [Google Scholar] [CrossRef]
- Nieto-García, A.J.; Domínguez, I.; Romero-González, R.; Arrebola, F.J.; Vidal, J.L.M.; Frenich, A.G. Automated determination of xenobiotics (pesticides, PCBs, PAHs, and PBDEs) in sediment samples applying HS-SPME-GC-HRMS. J. AOAC Int. 2019, 102, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Kalavrouziotis, I.K.; Koukoulakis, P.H.; Vasquez, M. The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci. Total Environ. 2011, 409, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Thelusmond, J.-R.; Strathmann, T.J.; Cupples, A.M. Carbamazepine, triclocarban and triclosan biodegradation and the phylotypes and functional genes associated with xenobiotic degradation in four agricultural soils. Sci. Total Environ. 2019, 657, 1138–1149. [Google Scholar] [CrossRef]
- Byrns, G. The fate of xenobiotic organic compounds in wastewater treatment plants. Water Res. 2001, 35, 2523–2533. [Google Scholar] [CrossRef]
- Bond, T.; Ferrandiz-Mas, V.; Felipe-Sotelo, M.; Van Sebille, E. The occurrence and degradation of aquatic plastic litter based on polymer physicochemical properties: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 685–722. [Google Scholar] [CrossRef]
- Rodríguez, A.; Castrejón-Godínez, M.L.; Salazar-Bustamante, E.; Gama-Martínez, Y.; Sánchez-Salinas, E.; Mussali-Galante, P.; Tovar-Sánchez, E.; Ortiz-Hernández, M.L. Omics approaches to pesticide biodegradation. Curr. Microbiol. 2020, 77, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Dai, S.; Meng, K.; Wang, Y.; Ren, W.; Zhao, L.; Christie, P.; Teng, Y. Occurrence and risk assessment of potentially toxic elements and typical organic pollutants in contaminated rural soils. Sci. Total Environ. 2018, 630, 618–629. [Google Scholar] [CrossRef]
- Haque, E.; Ward, A.C. Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.M.; Keller, E.T. The use of mature zebrafish (Danio rerio) as a model for human aging and disease. In Conn’s Handbook of Models for Human Aging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 351–359. [Google Scholar] [CrossRef]
- Hollert, H.; Keiter, S.H. Danio rerio as a model in aquatic toxicology and sediment research. Environ. Sci. Pollut. Res. 2015, 22, 16243–16246. [Google Scholar] [CrossRef] [Green Version]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna model in the toxicity assessment of pharmaceuticals: A review. Sci. Total Environ. 2020, 763, 143038. [Google Scholar] [CrossRef]
- Blaser, R.; Chadwick, L.; McGinnis, G. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sancho, E.; Villarroel, M.; Fernández, C.; Andreu, E.; Ferrando, M. Short-term exposure to sublethal tebuconazole induces physiological impairment in male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2010, 73, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef]
- Ficociello, G.; Inverni, A.; Massimi, L.; Buccini, G.; Canepari, S.; Uccelletti, D. Assessment of the effects of atmospheric pollutants using the animal model Caenorhabditis elegans. Environ. Res. 2020, 191, 110209. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Chen, X.; Vollertsen, J.; Nielsen, J.L.; Dall, A.G.; Bester, K. Degradation of PPCPs in activated sludge from different WWTPs in Denmark. Ecotoxicology 2015, 24, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lemus, N.; López-Serna, R.; Pérez-Elvira, S.I.; Barrado, E. Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: A critical review. Anal. Chim. Acta 2019, 1083, 19–40. [Google Scholar] [CrossRef] [Green Version]
- Dumas, T.; Boccard, J.; Gomez, E.; Fenet, H.; Courant, F. Multifactorial analysis of environmental metabolomic data in ecotoxicology: Wild marine mussel exposed to wwtp effluent as a case study. Metabolites 2020, 10, 269. [Google Scholar] [CrossRef]
- Dumas, T.; Bonnefille, B.; Gomez, E.; Boccard, J.; Castro, N.A.; Fenet, H.; Courant, F. Metabolomics approach reveals disruption of metabolic pathways in the marine bivalve Mytilus galloprovincialis exposed to a WWTP effluent extract. Sci. Total Environ. 2020, 712, 136551. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.; Medriano, C.D.; Cho, Y.; Kim, H.; Chung, I.-Y.; Seok, K.-S.; Song, K.G.; Hong, S.W.; Park, Y.; Kim, S. Sub-lethal pharmaceutical hazard tracking in adult zebrafish using untargeted LC–MS environmental metabolomics. J. Hazard. Mater. 2017, 339, 63–72. [Google Scholar]
- Fu, J.; Tan, Y.X.R.; Gong, Z.; Bae, S. The toxic effect of triclosan and methyl-triclosan on biological pathways revealed by metabolomics and gene expression in zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 189, 110039. [Google Scholar] [CrossRef]
- Teplova, V.V.; Belosludtsev, K.N.; Kruglov, A.G. Mechanism of triclosan toxicity: Mitochondrial dysfunction including complex II inhibition, superoxide release and uncoupling of oxidative phosphorylation. Toxicol. Lett. 2017, 275, 108–117. [Google Scholar] [CrossRef]
- Gillis, J.D.; Price, G.W.; Prasher, S. Lethal and sub-lethal effects of triclosan toxicity to the earthworm Eisenia fetida assessed through GC–MS metabolomics. J. Hazard. Mater. 2017, 323, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Long, N.P.; Yoon, S.J.; Nguyen, H.T.; Kwon, S.W. Metabolomics and phenotype assessment reveal cellular toxicity of triclosan in Caenorhabditis elegans. Chemosphere 2019, 236, 124306. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, Y.; Yang, J.; Zhu, W.; Zhou, Z.; Zhang, R. Effects of Dufulin on Oxidative Stress and Metabolomic Profile of Tubifex. Metabolites 2021, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Zhang, Y.; You, X.; Sun, P.; Qiu, J.; Kong, F. Lethal toxicity and sublethal metabolic interference effects of sulfoxaflor on the earthworm (Eisenia fetida). J. Agric. Food Chem. 2018, 66, 11902–11908. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Elfikrie, N.; Ho, Y.B.; Zaidon, S.Z.; Juahir, H.; Tan, E.S.S. Occurrence of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers in Tengi River Basin, Malaysia. Sci. Total Environ. 2020, 712, 136540. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, L. Metabolomic and transcriptomic investigation of metabolic perturbations in Oryza sativa L. triggered by three pesticides. Environ. Sci. Technol. 2020, 54, 6115–6124. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Neto, J.A.B.; da Fonseca, E.M. Nanoplastics in aquatic systems-are they more hazardous than microplastics? Environ. Pollut. 2020, 115950. [Google Scholar] [CrossRef]
- Baudrimont, M.; Arini, A.; Guégan, C.; Venel, Z.; Gigault, J.; Pedrono, B.; Prunier, J.; Maurice, L.; Ter Halle, A.; Feurtet-Mazel, A. Ecotoxicity of polyethylene nanoplastics from the North Atlantic oceanic gyre on freshwater and marine organisms (microalgae and filter-feeding bivalves). Environ. Sci. Pollut. Res. 2020, 27, 3746–3755. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Mahana, A.; Guliy, O.I.; Mehta, S.K. Accumulation and cellular toxicity of engineered metallic nanoparticle in freshwater microalgae: Current status and future challenges. Ecotoxicol. Environ. Saf. 2021, 208, 111662. [Google Scholar] [CrossRef]
- Chen, F.; Xiao, Z.; Yue, L.; Wang, J.; Feng, Y.; Zhu, X.; Wang, Z.; Xing, B. Algae response to engineered nanoparticles: Current understanding, mechanisms and implications. Environ. Sci. Nano 2019, 6, 1026–1042. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Chen, D.; Xu, E.G.; Luo, X.; Zeng, J.; Huan, T.; Li, L.; Wang, Y. Toxicity Mechanisms of Polystyrene Microplastics in Marine Mussels Revealed by High-Coverage Quantitative Metabolomics Using Chemical Isotope Labeling Liquid Chromatography Mass Spectrometry. J. Hazard. Mater. 2021, 147, 126003. [Google Scholar] [CrossRef]
- Li, X.; Ban, Z.; Yu, F.; Hao, W.; Hu, X. Untargeted metabolic pathway analysis as an effective strategy to connect various nanoparticle properties to nanoparticle-induced ecotoxicity. Environ. Sci. Technol. 2020, 54, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Suh, S. Health risks of chemicals in consumer products: A review. Environ. Int. 2019, 123, 580–587. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.J.; Ball, A.S.; Clarke, B.O. Critical review of soil contamination by polybrominated diphenyl ethers (PBDEs) and novel brominated flame retardants (NBFRs); concentrations, sources and congener profiles. Environ. Pollut. 2017, 230, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Wei, J.; Luan, H.; Li, M.; Cai, Z. Study of metabolic disorders associated with BDE-47 exposure in Drosophila model by MS-based metabolomics. Ecotoxicol. Environ. Saf. 2019, 184, 109606. [Google Scholar] [CrossRef]
- Ji, F.; Sreenivasmurthy, S.G.; Wei, J.; Shao, X.; Luan, H.; Zhu, L.; Song, J.; Liu, L.; Li, M.; Cai, Z. Study of BDE-47 induced Parkinson’s disease-like metabolic changes in C57BL/6 mice by integrated metabolomic, lipidomic and proteomic analysis. J. Hazard. Mater. 2019, 378, 120738. [Google Scholar] [CrossRef]
- Jurek, A.; Leitner, E. Analytical determination of bisphenol A (BPA) and bisphenol analogues in paper products by GC-MS/MS. Food Addit. Contam. Part A 2017, 34, 1225–1238. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sendón, R.; van der Kellen, A.; Vaz, M.; Silva, A.S. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019, 91, 33–65. [Google Scholar] [CrossRef]
- Lee, S.-W.; Chatterjee, N.; Im, J.-E.; Yoon, D.; Kim, S.; Choi, J. Integrated approach of eco-epigenetics and eco-metabolomics on the stress response of bisphenol-A exposure in the aquatic midge Chironomus riparius. Ecotoxicol. Environ. Saf. 2018, 163, 111–116. [Google Scholar] [CrossRef]
- Sheikholeslami, M.N.; Gómez-Canela, C.; Barron, L.P.; Barata, C.; Vosough, M.; Tauler, R. Untargeted metabolomics changes on Gammarus pulex induced by propranolol, triclosan, and nimesulide pharmaceutical drugs. Chemosphere 2020, 260, 127479. [Google Scholar] [CrossRef] [PubMed]
- Bonnefille, B.; Gomez, E.; Alali, M.; Rosain, D.; Fenet, H.; Courant, F. Metabolomics assessment of the effects of diclofenac exposure on Mytilus galloprovincialis: Potential effects on osmoregulation and reproduction. Sci. Total Environ. 2018, 613, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Serra-Compte, A.; Álvarez-Muñoz, D.; Solé, M.; Cáceres, N.; Barceló, D.; Rodríguez-Mozaz, S. Comprehensive study of sulfamethoxazole effects in marine mussels: Bioconcentration, enzymatic activities and metabolomics. Environ. Res. 2019, 173, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Hua, Q.; Adamovsky, O.; Vespalcova, H.; Boyda, J.; Schmidt, J.T.; Kozuch, M.; Craft, S.L.; Ginn, P.E.; Smatana, S.; Budinska, E. Microbiome analysis and predicted relative metabolomic turnover suggest bacterial heme and selenium metabolism are altered in the gastrointestinal system of zebrafish (Danio rerio) exposed to the organochlorine dieldrin. Environ. Pollut. 2021, 268, 115715. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wang, Y.; Teng, M.; Wang, D.; Yan, J.; Miao, J.; Zhou, Z.; Zhu, W. Toxicity and metabolomics study of isocarbophos in adult zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 163, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Aspinall, J.V.; Lv, W.; Zheng, X.; Zhang, H.; Li, S.; Zhang, J.; Bai, N.; Zhang, Y.; Wang, X. Differences in kinetic metabolomics in Eisenia fetida under single and dual exposure of imidacloprid and dinotefuran at environmentally relevant concentrations. J. Hazard. Mater. 2021, 417, 126001. [Google Scholar] [CrossRef]
- Yin, J.; Hong, X.; Ma, L.; Liu, R.; Bu, Y. Non-targeted metabolomic profiling of atrazine in Caenorhabditis elegans using UHPLC-QE Orbitrap/MS. Ecotoxicol. Environ. Saf. 2020, 206, 111170. [Google Scholar] [CrossRef]
- Pang, M.; Wang, Y.; Tang, Y.; Dai, J.; Tong, J.; Jin, G. Transcriptome sequencing and metabolite analysis reveal the toxic effects of nanoplastics on tilapia after exposure to polystyrene. Environ. Pollut. 2021, 277, 116860. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, R.; Zhang, S.; Wang, G. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio). J. Hazard. Mater. 2021, 403, 123663. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.-Q.; Yan, H.; Luo, X.-W.; Kang, Y.-H.; Hu, J.-M.; Chen, L.-Q. Integration of transcriptomics and metabolomics reveals damage and recovery mechanisms of fish gills in response to nanosilver exposure. Aquat. Toxicol. 2021, 237, 105895. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Majumdar, S.; Li, W.; Keller, A.A.; Slaveykova, V.I. Metabolomics for early detection of stress in freshwater alga Poterioochromonas malhamensis exposed to silver nanoparticles. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Zhu, Y.; Wu, X.; Liu, Y.; Zhang, J.; Lin, D. Integration of transcriptomics and metabolomics reveals the responses of earthworms to the long-term exposure of TiO2 nanoparticles in soil. Sci. Total Environ. 2020, 719, 137492. [Google Scholar] [CrossRef]
- Maria, V.L.; Licha, D.; Scott-Fordsmand, J.J.; Huber, C.G.; Amorim, M.J. Multiomics assessment in Enchytraeus crypticus exposed to Ag nanomaterials (Ag NM300K) and ions (AgNO3)–Metabolomics, proteomics (& transcriptomics). Environ. Pollut. 2021, 286, 117571. [Google Scholar]
- Liang, R.; Chen, J.; Shi, Y.; Lu, Y.; Sarvajayakesavalu, S.; Xu, X.; Zheng, X.; Khan, K.; Su, C. Toxicological effects on earthworms (Eisenia fetida) exposed to sub-lethal concentrations of BDE-47 and BDE-209 from a metabolic point. Environ. Pollut. 2018, 240, 653–660. [Google Scholar] [CrossRef]
- Ortiz-Villanueva, E.; Navarro-Martín, L.; Jaumot, J.; Benavente, F.; Sanz-Nebot, V.; Piña, B.; Tauler, R. Metabolic disruption of zebrafish (Danio rerio) embryos by bisphenol A. An integrated metabolomic and transcriptomic approach. Environ. Pollut. 2017, 231, 22–36. [Google Scholar] [CrossRef]
- Mao, L.; Fang, S.; Zhao, M.; Liu, W.; Jin, H. Effects of Bisphenol A and Bisphenol S Exposure at Low Doses on the Metabolome of Adolescent Male Sprague–Dawley Rats. Chem. Res. Toxicol. 2021, 34, 1578–1587. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Wei, Q.; Wang, L.-Y.; Zhang, Z.-M.; Zhang, X.-Q.; Sun, A.-L.; Chen, J.; Shi, X.-Z. A comprehensive study of the effects of phthalates on marine mussels: Bioconcentration, enzymatic activities and metabolomics. Mar. Pollut. Bull. 2021, 168, 112393. [Google Scholar] [CrossRef] [PubMed]
- Morrison, N.; Bearden, D.; Bundy, J.G.; Collette, T.; Currie, F.; Davey, M.P.; Haigh, N.S.; Hancock, D.; Jones, O.A.; Rochfort, S. Standard reporting requirements for biological samples in metabolomics experiments: Environmental context. Metabolomics 2007, 3, 203–210. [Google Scholar] [CrossRef]
- Spurgeon, D.J.; Jones, O.A.; Dorne, J.-L.C.; Svendsen, C.; Swain, S.; Stürzenbaum, S.R. Systems toxicology approaches for understanding the joint effects of environmental chemical mixtures. Sci. Total Environ. 2010, 408, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
| Platform | Separation | Mobile Phase | Metabolic Scope | Limitation |
|---|---|---|---|---|
| RPLC-MS | C18 column | Water → ACN or MeOH | Polar and medium polar |
|
| HILIC-MS | Amide, Silica | ACN → Water | Very polar metabolite |
|
| IP-RPLC-MS | C18 | Water → ACN or MeOH with ion paring agent | Very polar metabolite |
|
| GC-MS | Polysiloxane | Helium or Nitrogen | Volatile metabolites |
|
| CE-MS | Fused-silica Capillary with polymer | Water, ACN, MeOH | Neutral, anionic, cationic metabolite |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Kang, J.S. Metabolomic Studies for the Evaluation of Toxicity Induced by Environmental Toxicants on Model Organisms. Metabolites 2021, 11, 485. https://doi.org/10.3390/metabo11080485
Kim HM, Kang JS. Metabolomic Studies for the Evaluation of Toxicity Induced by Environmental Toxicants on Model Organisms. Metabolites. 2021; 11(8):485. https://doi.org/10.3390/metabo11080485
Chicago/Turabian StyleKim, Hyung Min, and Jong Seong Kang. 2021. "Metabolomic Studies for the Evaluation of Toxicity Induced by Environmental Toxicants on Model Organisms" Metabolites 11, no. 8: 485. https://doi.org/10.3390/metabo11080485
APA StyleKim, H. M., & Kang, J. S. (2021). Metabolomic Studies for the Evaluation of Toxicity Induced by Environmental Toxicants on Model Organisms. Metabolites, 11(8), 485. https://doi.org/10.3390/metabo11080485






