Cardiovascular/Stroke Risk Stratification in Parkinson’s Disease Patients Using Atherosclerosis Pathway and Artificial Intelligence Paradigm: A Systematic Review
Abstract
:1. Introduction
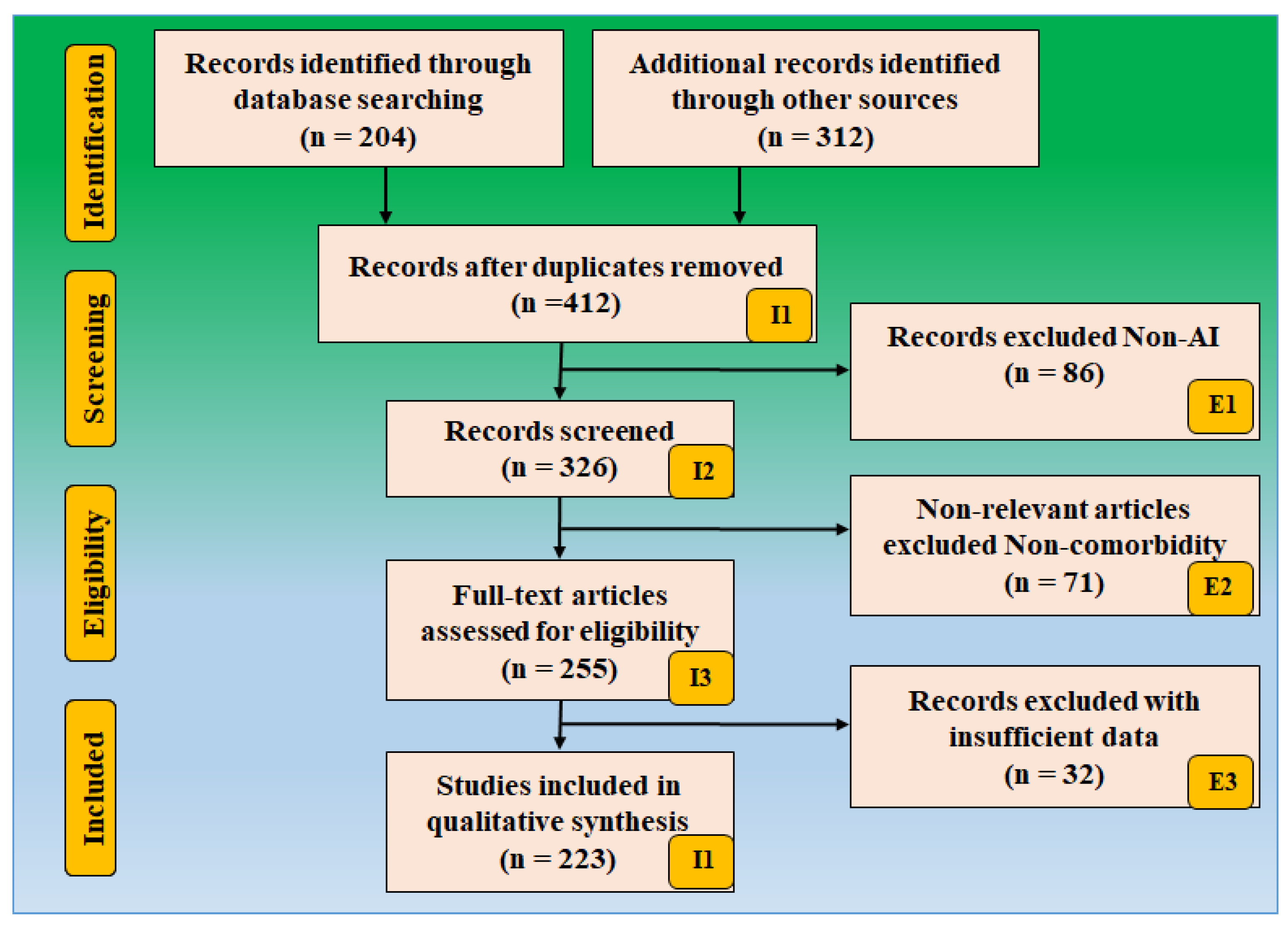
2. Methods
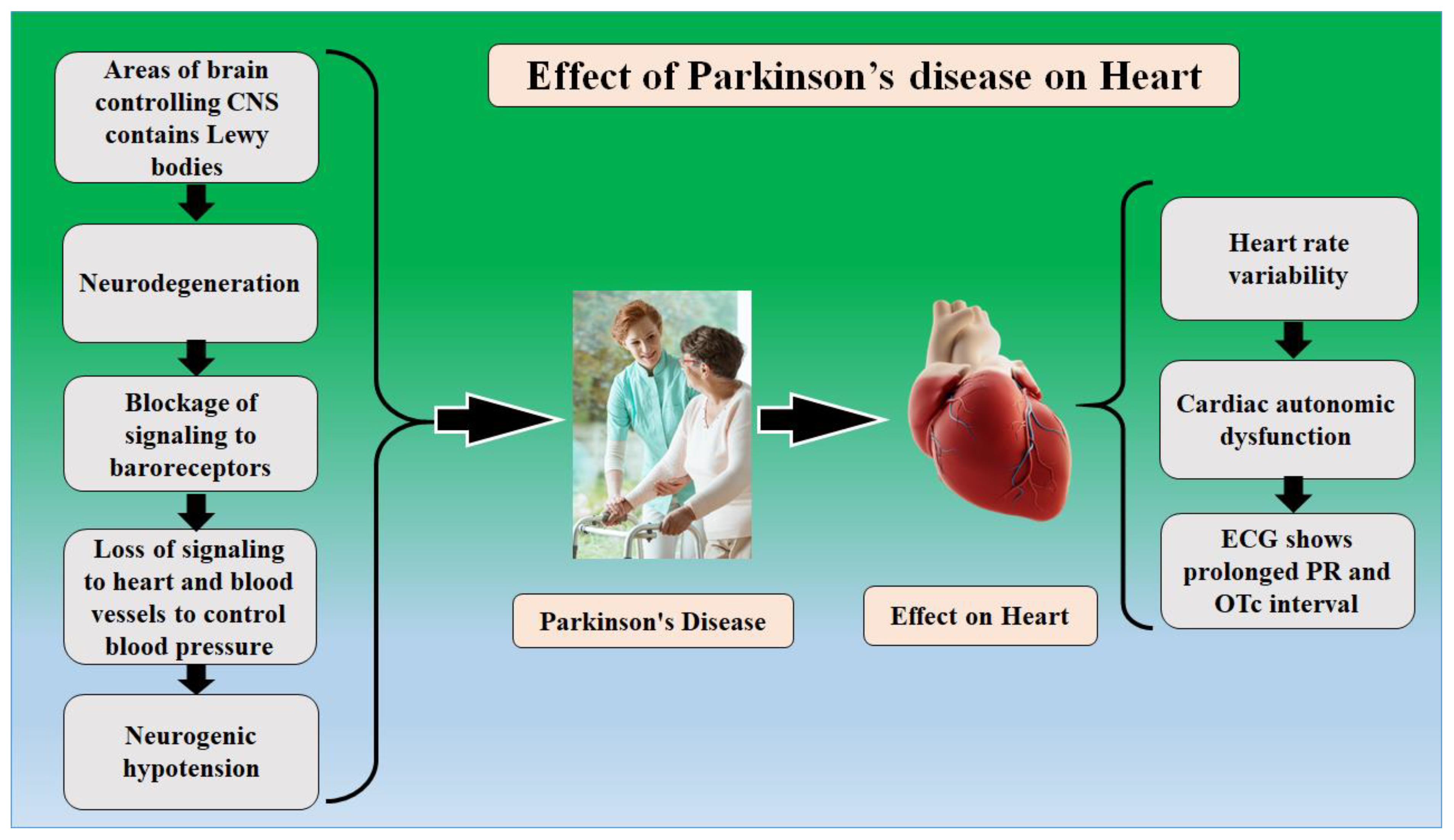
3. The Relationship between PD and Combined Heart and Brain Diseases
3.1. The Relationship between PD and Atherosclerosis Leading to CVD
3.2. The Relationship between Parkinson’s Disease with the Brain
3.3. The Relationship between PD and Combined CVD and Stroke
3.4. The Role of the Shared Gene in Parkinson’s with CVD and Stroke
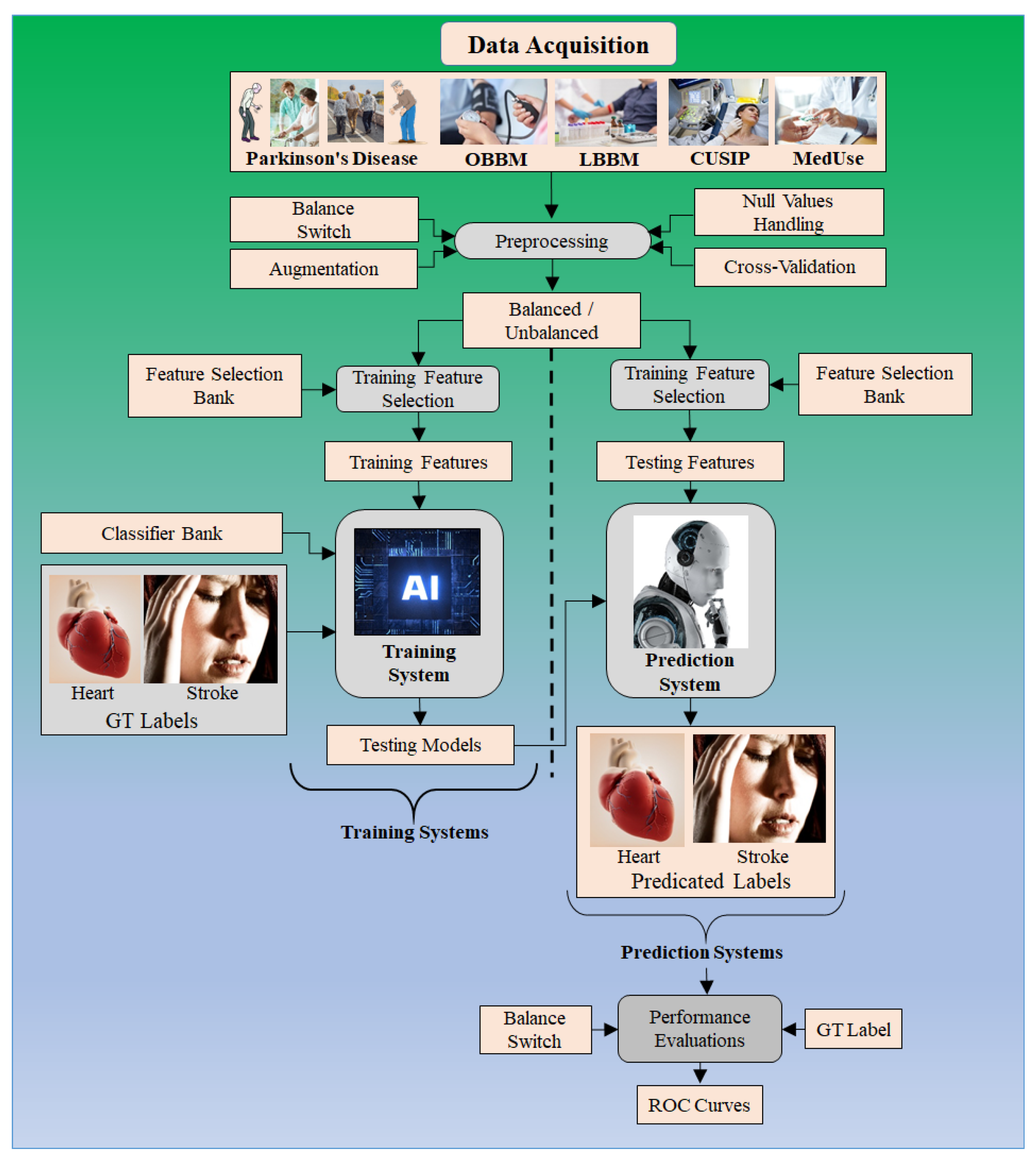
4. Machine Learning-Based System for CVD/Stroke Risk Assessment for PD Patients
5. Critical Discussions
5.1. Principal Findings
5.2. Benchmarking
5.3. A Special Note on PD-Stroke Hypothesis
5.4. A Special Note on PD-CVD Hypothesis
5.5. A Short on Contrast-Based Imaging for ORGAN
5.6. A Short Note on the Effect of COVID-19 Infection on PD
5.7. A Short Note on Bias in AI System
5.8. Strengths, Weakness, and Extensions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Glossary
| Acute Stroke | A stage of stroke that starts at the beginning of symptoms and lasts for a few hours after. |
| ANOVA | Is an analysis tool used in statistics that splits an observed aggregate variability found inside a dataset into two parts: systematic factors and random factors. |
| Arrhythmia | An abnormal heartbeat. |
| Arteriosclerosis | A disease process, commonly called “hardening of the arteries,” includes a variety of conditions that cause artery walls to thicken and lose elasticity. |
| Artificial Intelligence | Artificial intelligence (AI) is intelligence demonstrated by machines, as opposed to the natural intelligence displayed by animals, including humans. |
| Atherosclerosis | A disease in which plaque builds up inside your arteries. This narrows the arteries and blocks blood flow to the brain, which increases the risk of a stroke. |
| Autonomic nervous system | The part of the body’s complex system of nerves that controls the involuntary activity of some of the internal organs, such as breathing or heartbeat. |
| Basal ganglia | These are structures located deep in the brain that are responsible for normal movement, such as walking. The basal ganglia are made up of three main parts, the caudate nucleus, the putamen, and the globus pallidus. |
| Bradycardia | Abnormally slow heartbeat. |
| Bradykinesia | Slowing down of movement. It is a major symptom of Parkinson’s. |
| Cardiac arrest | The stopping of the heartbeat, usually because of interference with the electrical signal. |
| Cardiovascular | About the heart and blood vessels that make up the circulatory system. |
| Carotid artery | An artery located on either side of the neck supplies the front part of the brain with blood. |
| Cerebellum | Part of the brain is involved in the coordination of movements. |
| Cerebral cortex | The largest part of the brain is responsible for thought, reasoning, memory, sensation, and voluntary movement. |
| Cerebrovascular Disease | One or more diseases are caused by blood flow (circulation) problems, such as blood flow restriction or a blockage or clot, in vessels that supply blood to the brain. |
| Chorea | A type of abnormal movement or dyskinesia, characterized by continuing, rapid, dance-like movements. May result from high doses of levodopa and/or long-term levodopa treatment. |
| Cognitive Impairment | Difficulty with thinking abilities such as paying attention, memory, communication, and problem-solving. |
| Cogwheel rigidity | Stiffness in the muscles, with a jerky quality, when arms and legs are repeatedly moved. |
| Congestive heart failure | A condition in which the heart cannot pump all the blood returning to it, leading to a backup of blood in the vessels and an accumulation of fluid in the body’s tissues, including the lungs. |
| Deep Learning | Are a type of machine learning and artificial intelligence (AI) that imitates the way humans gain certain types of knowledge. |
| Dementia | The loss of some intellectual abilities is characterized by a loss of awareness and confusion. |
| Dopamine | A chemical produced by the brain; assists in the effective transmission of messages from one nerve cell to the next. People with Parkinson’s have decreased amounts of the chemical in the basal ganglia and substantia nigra, two structures located deep in the brain. Dopamine coordinates the actions of movement, balance, and walking. |
| DVT (Deep Vein Thrombosis) | A blood clot that forms in a vein deep in the body. It can cause a potentially life-threatening complication if the clot detaches and moves to the lungs resulting in a blockage known as a pulmonary embolism (PE). |
| Dysarthria | Difficulty saying words clearly due to problems with muscle strength and coordination. |
| Dysarthria | Speech difficulties due to impairment of the muscles associated with speech. |
| Dyskinesia | Abnormal muscle movements. These may appear as a side effect of long-term drug treatment in Parkinson’s and may worsen in response to stress. |
| Dysphagia | Difficulty with swallowing. |
| Edema | Swelling is caused by fluid accumulation in body tissues. |
| Embolic Stroke | A stroke is caused by an embolus (a free-floating mass traveling through the bloodstream). The embolus may be a blood clot (thrombus), a ball of fat, a bubble of air or other gas (gas embolism), or foreign material. |
| Hemorrhagic Stroke | Sudden bleeding into or around the brain. It is also called a brain hemorrhage or brain bleed. |
| Heredity | The genetic transmission of a particular quality or trait from parent to child. |
| High-density lipoprotein (HDL) | Also known as “good cholesterol.” HDL helps move the “bad cholesterol” from the arteries back to the liver; thus, it can break down and leave the body. |
| Hypertrophy | Enlargement of tissues or organs because of increased workload. |
| Hypoxia | A state of decreased oxygen delivery to a cell thus that the oxygen falls below normal levels. |
| Intracerebral Hemorrhage (ICH) | A type of stroke occurs when a vessel within the brain leaks blood into the brain. |
| Ischemic Stroke | Damage to the brain is caused by a lack of blood flow, usually from a clot. |
| Levodopa | A drug containing a form of the important brain chemical dopamine commonly used to treat symptoms of Parkinson’s disease. In combination with carbidopa, it is called Sinemet; combined with benserazide, it is called Prolopa. |
| Lewy body | Brain cells have abnormally pigmented spheres inside them. They are found in the damaged parts of the brain in people with Parkinson’s disease. |
| Low-density lipoprotein (LDL) | Also known as the “bad cholesterol”; a compound that carries most of the total cholesterol in the blood and deposits the excess along the inside of arterial walls. |
| Machine learning | Machine learning is a method of data analysis that automates analytical model building. |
| Myocardial infarction | A heart attack. The damage or death of an area of the heart muscle (myocardium) resulting from a blocked blood supply to the area. The affected tissue dies, injuring the heart. Symptoms include prolonged, intensive chest pain, and a decrease in blood pressure that often causes shock. |
| Navi byes | Naive Bayes classifiers are a family of simple “probabilistic classifiers” based on applying Bayes’ theorem with strong (naive) independence assumptions between the features. |
| Principal Component Analysis | Is the process of computing the principal components and using them to perform a change of basis on the data, sometimes using only the first few principal components and ignoring the rest. |
| Pulmonary Embolism (PE) | A blockage of an artery in the lungs by a substance that has traveled from elsewhere in the body through the bloodstream. Severe cases can lead to passing out, abnormally low blood pressure, and sudden death. |
| Random forests | Is an ensemble learning method for classification, regression, and other tasks that operates by constructing a multitude of decision trees at training time? |
| Resting tremor | Shaking occurs in a relaxed and supported limb. |
| Rigidity | Muscular stiffness is common in people with Parkinson’s disease. It is characterized by a resistance to movement in the limbs. |
| Stenosis | Narrowing of an artery due to the buildup of plaque within the artery. |
| Stroke | Occurs when the blood supply to part of the brain is suddenly interrupted or when a blood vessel in the brain bursts, spilling blood into the spaces surrounding brain cells. There are two types of stroke: ischemic (clot) or hemorrhagic (bleeding). |
| Support Vector Machine | Supervised learning models with associated learning algorithms that analyze data for classification and regression analysis. |
| Thrombosis | The formation of a blood clot in one of the brain arteries of the head or neck that stays attached to the artery wall until it grows large enough to block blood flow. |
| Abbreviations | |
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| ANOVA | Analysis of variance |
| ASCVD | Atherosclerotic cardiovascular disease |
| ANS | Autonomic Nervous System |
| AUC | Area-under-the-curve |
| AI | Artificial Intelligence |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| CAS | Coronary artery syndrome |
| CHD | Coronary Heart Disease |
| CKD | Chronic kidney disease |
| CT | Computed Tomography |
| CUSIP | Carotid ultrasound image phenotype |
| CV | Cross-validation |
| CVD | Cardiovascular disease |
| CVE | Cardiovascular events |
| DA | Endogenous Dopamine |
| DL | Deep learning |
| DM | Diabetes mellitus |
| EEGS | Event-equivalent gold standard |
| EMG | Electromyography |
| FH | Family history |
| FoG | Freezing of Gait |
| GT | Ground truth |
| HTN | Hypertension |
| HDL | Hybrid deep learning |
| ICAM | Intercellular Adhesion Molecule |
| VCAM | vascular cell adhesion molecule |
| LBBM | Laboratory-based biomarker |
| MedUSE | Medication use |
| ML | Machine learning |
| MRI | Magnetic Resonance Imaging |
| MIBG | Iodine-123 meta-iodobenzylguanidine |
| NPV | Negative predictive value |
| NB | Naive byes |
| NO | Nitric Oxide |
| nOH | Neurogenic orthostatic hypotension |
| Non-ML | Non-machine learning |
| OBBM | Office-based biomarker |
| OH | orthostatic hypotension |
| OxLDL | Oxidation of low-density lipoprotein |
| QTc | chaotic heartbeat |
| PD | Parkinson Disease |
| PE | Performance evaluation matrices |
| PPV | Positive predictive value |
| PCA | Principal Component Analysis |
| PTC | Plaque tissue characterization |
| RA | Rheumatoid arthritis |
| PR | Period measured in milliseconds |
| RF | Random forest |
| ROS | Reactive Oxides Stress |
| RoB | Risk of bias |
| ROC | Receiver operating-characteristics |
| SCORE | Systematic coronary risk evaluation |
| SMOTE | Synthetic minority over-sampling technique |
| SVM | Support vector machine |
| TPA | Total plaque area |
| US | Ultrasound |
| DNA | Deoxyribonucleic acid |
References
- Bhat, S.; Acharya, U.R.; Hagiwara, Y.; Dadmehr, N.; Adeli, H. Parkinson’s disease: Cause factors, measurable indicators, and early diagnosis. Comput. Biol. Med. 2018, 102, 234–241. [Google Scholar] [CrossRef]
- Cilia, R.; Bonvegna, S.; Straccia, G.; Andreasi, N.G.; Elia, A.E.; Romito, L.M.; Devigili, G.; Cereda, E.; Eleopra, R.J.M.D. Effects of COVID-19 on Parkinson’s disease clinical features: A community-based case-control study. J. Mov. Disord. 2020, 35, 1287–1292. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [Green Version]
- Simunovic, F.; Yi, M.; Wang, Y.; Macey, L.; Brown, L.T.; Krichevsky, A.M.; Andersen, S.L.; Stephens, R.M.; Benes, F.M.; Sonntag, K.C. Gene expression profiling of substantia nigra dopamine neurons: Further insights into Parkinson’s disease pathology. Brain Res. 2009, 132, 1795–1809. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, A.; Li, Y.; Hayashi, H.; Kondo, N. Dementia risks identified by vocal features via telephone conversations: A novel machine learning prediction model. PLoS ONE 2021, 16, e0253988. [Google Scholar] [CrossRef]
- Findley, L.J. The economic impact of Parkinson’s disease. Parkinsonism Relat. Disord. 2007, 13, S8–S12. [Google Scholar] [CrossRef]
- Sulzer, D.; Antonini, A.; Leta, V.; Nordvig, A.; Smeyne, R.J.; Goldman, J.E.; Al-Dalahmah, O.; Zecca, L.; Sette, A.; Bubacco, L. COVID-19 and possible links with Parkinson’s disease and parkinsonism: From bench to bedside. NPJ Parkinson’s Dis. 2020, 6, 18. [Google Scholar] [CrossRef]
- Kaiyrzhanov, R.; Rizig, M.; Aitkulova, A.; Zharkinbekova, N.; Shashkin, C.; Kaishibayeva, G.; Karimova, A.; Khaibullin, T.; Sadykova, D.; Ganieva, M. Parkinson’s disease in Central asian and Transcaucasian countries: A review of epidemiology, genetics, clinical characteristics, and access to care. Parkinson’s Dis. 2019, 2019, 2905739. [Google Scholar] [CrossRef]
- Murray, N.M.; Unberath, M.; Hager, G.D.; Hui, F.K. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: A systematic review. J. Neurointerv. Surg. 2020, 12, 156–164. [Google Scholar] [CrossRef]
- Strong, K.; Mathers, C.; Bonita, R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007, 6, 182–187. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, L.; Zhang, Y.; Xie, A. Association Between Stroke and Parkinson’s Disease: A Meta-analysis. J. Mol. Neurosci. 2020, 70, 1169–1176. [Google Scholar] [CrossRef]
- Cahill, J.; Zhang, J.H. Subarachnoid hemorrhage: Is it time for a new direction? Stroke 2009, 40, S86–S87. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.L.; Srikanth, V.K.; Thrift, A.G. The large and growing burden of stroke. Curr. Drug Targets CNS Neurol. Disord. 2007, 8, 786–793. [Google Scholar] [CrossRef]
- Mende, K. Die In Vivo-Wirkung von N-Methyl-Norsalsolinol auf das Dopaminerge und das Serotonerge System der Ratte. Ph.D. Thesis, University of Lübeck, Lübeck, Germany, 2008. [Google Scholar]
- Sun, Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, A.; Bower, J.H.; Peterson, B.J.; Maraganore, D.M.; McDonnell, S.K.; Ahlskog, J.E.; Schaid, D.J.; Rocca, W.A. Survival study of Parkinson disease in Olmsted county, Minnesota. Arch. Neurol. 2003, 60, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Pohar, S.L.; Jones, C.A. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch. Gerontol. Geriatr. 2009, 49, 317–321. [Google Scholar] [CrossRef]
- Becker, C.; Jick, S.S.; Meier, C.R. Risk of stroke in patients with idiopathic Parkinson disease. Parkinsonism Relat. Disord. 2010, 16, 31–35. [Google Scholar] [CrossRef]
- Driver, J.; Kurth, T.; Buring, J.; Gaziano, J.; Logroscino, G. Parkinson disease and risk of mortality: A prospective comorbidity-matched cohort study. J. Neurol. 2008, 70, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Van, W.B.; Vanholder, R.; Verbeke, F.; Lameire, N. Is peritoneal dialysis associated with increased cardiovascular morbidity and mortality? Perit. Dial. Int. 2006, 26, 429–434. [Google Scholar]
- Nam, G.E.; Kim, S.M.; Han, K.; Kim, N.H.; Chung, H.S.; Kim, J.W.; Han, B.; Cho, S.J.; Yu, J.H.; Park, Y.G. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. 2018, 15, e1002640. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Hu, G.; Kivipelto, M.; Laatikainen, T.; Antikainen, R.; Fratiglioni, L.; Jousilahti, P.; Tuomilehto, J. Association of blood pressure and hypertension with the risk of Parkinson disease: The National FINRISK Study. Hypertension 2011, 57, 1094–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bots, M.L.; Grobbee, D.E.; Hofman, A.; Witteman, J.C. Common carotid intima-media thickness and risk of acute myocardial infarction: The role of lumen diameter. Stroke 2005, 36, 762–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleyasin, H.; Rousseaux, M.W.; Phillips, M.; Kim, R.H.; Bland, R.J.; Callaghan, S.; Slack, R.S.; During, M.J.; Mak, T.W.; Park, D.S. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc. Natl. Acad. Sci. USA 2007, 104, 18748–18753. [Google Scholar] [CrossRef] [Green Version]
- Kurl, S.; Laukkanen, J.A.; Rauramaa, R.; Lakka, T.A.; Sivenius, J.; Salonen, J.T. Cardiorespiratory fitness and the risk for stroke in men. Arch. Intern. Med. 2003, 163, 1682–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, B.-k.; Han, M.-K.; Hong, J.-H.; Yum, K.S.; Lee, D.-I. Deep Learning for Prediction of Mechanism in Acute Ischemic Stroke Using Brain MRI. Res. Sq. 2021, preprint. [Google Scholar] [CrossRef]
- Malek, N.; Lawton, M.A.; Swallow, D.M.; Grosset, K.A.; Marrinan, S.L.; Bajaj, N.; Barker, R.A.; Burn, D.J.; Hardy, J.; Morris, H.R. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov. Disord. 2016, 31, 1518–1526. [Google Scholar] [CrossRef] [Green Version]
- Lucatelli, P.; Raz, E.; Saba, L.; Argiolas, G.M.; Montisci, R.; Wintermark, M.; King, K.S.; Molinari, F.; Ikeda, N.; Siotto, P. Relationship between leukoaraiosis, carotid intima-media thickness and intima-media thickness variability: Preliminary results. Eur. Radiol. 2016, 26, 4423–4431. [Google Scholar] [CrossRef]
- Lucatelli, P.; Montisci, R.; Sanfilippo, R.; Sacconi, B.; Suri, J.S.; Catalano, C.; Saba, L. Is there an association between leukoaraiosis volume and diabetes? J. Neuroradiol. 2016, 43, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Jamthikar, A.; Gupta, D.; Saba, L.; Khanna, N.N.; Araki, T.; Viskovic, K.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M.; et al. Cardiovascular/stroke risk predictive calculators: A comparison between statistical and machine learning models. Cardiovasc. Diagn. Ther. 2020, 10, 919. [Google Scholar] [CrossRef]
- Liang, H.-W.; Huang, Y.-P.; Pan, S.-L. Parkinson disease and risk of acute myocardial infarction: A population-based, propensity score–matched, longitudinal follow-up study. Am. Heart J. 2015, 169, 508–514. [Google Scholar] [CrossRef]
- Ako, J.; Sudhir, K.; Farouque, H.O.; Honda, Y.; Fitzgerald, P.J. Transient left ventricular dysfunction under severe stress: Brain-heart relationship revisited. Am. J. Med. 2006, 119, 10–17. [Google Scholar] [CrossRef]
- Orayj, K.; Lacey, A.; Akbari, A.; Smith, M.; Pickrell, O.; Lane, E. Association between levodopa and ischemic heart disease. Int. J. Popul. Data Sci. 2019, 4, 3. [Google Scholar] [CrossRef]
- Omichi, C.; Momose, Y.; Kitahara, S. Congenital long QT syndrome presenting with a history of epilepsy: Misdiagnosis or relationship between channelopathies of the heart and brain? Epilepsia 2010, 51, 289–292. [Google Scholar] [CrossRef]
- Bartko, D.; Dukat, A.; Janco, S.; Porubec, V.; Traubner, P. The heart and the brain. Aspects of their interrelations. Vnitr. Lek. 1996, 42, 482–489. [Google Scholar] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Ser, T.; Hachinski, V.; Merskey, H.; Munoz, D.G. Clinical and pathologic features of two groups of patients with dementia with Lewy bodies: Effect of coexisting Alzheimer-type lesion load. Alzheimer Dis. Assoc. Disord. 2001, 15, 31–44. [Google Scholar] [PubMed]
- Gupta, V.; Lipsitz, L.A. Orthostatic hypotension in the elderly: Diagnosis and treatment. Am. J. Med. 2007, 120, 841–847. [Google Scholar] [CrossRef]
- Thames, M.; Kontos, H. Mechanisms of baroreceptor-induced changes in heart rate. Am. J. Physiol. Leg. Content 1970, 218, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Senard, J.; Rai, S.; Lapeyre-Mestre, M.; Brefel, C.; Rascol, O.; Rascol, A.; Montastruc, J. Prevalence of orthostatic hypotension in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 63, 584–589. [Google Scholar] [CrossRef]
- Palma, J.A.; Kaufmann, H. Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov. Disord. Clin. Pract. 2017, 4, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Low, P.A.; Singer, W. Management of neurogenic orthostatic hypotension: An update. Lancet Neurol. 2008, 7, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Min, L.C.; Suri, J.S. Heart rate variability. In Advances in Cardiac Signal Processing; Springer: Berlin/Heidelberg, Germany, 2007; pp. 121–165. [Google Scholar]
- Metzler, M.; Duerr, S.; Granata, R.; Krismer, F.; Robertson, D.; Wenning, G.K. Neurogenic orthostatic hypotension: Pathophysiology, evaluation, and management. J. Neurol. 2013, 260, 2212–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.T.; Levin, N.W.; Chertow, G.M.; Larive, B.; Schulman, G.; Kotanko, P. Determinants of cardiac autonomic dysfunction in ESRD. Clin. J. Am. Soc. Nephrol. 2010, 5, 1821–1827. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S. Dysautonomia in Parkinson’s disease: Neurocardiological abnormalities. Compr. Physiol. 2014, 4, 805. [Google Scholar] [CrossRef]
- Mallet, N.; Pogosyan, A.; Márton, L.F.; Bolam, J.P.; Brown, P.; Magill, P.J. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J. Neurosci. 2008, 28, 14245–14258. [Google Scholar] [CrossRef] [Green Version]
- Khanna, N.N.; Jamthikar, A.D.; Gupta, D.; Nicolaides, A.; Araki, T.; Saba, L.; Cuadrado-Godia, E.; Sharma, A.; Omerzu, T.; Suri, H.S.; et al. Performance evaluation of 10-year ultrasound image-based stroke/cardiovascular (CV) risk calculator by comparing against ten conventional CV risk calculators: A diabetic study. Comput. Biol. Med. 2019, 105, 125–143. [Google Scholar] [CrossRef]
- Zhang, W.; Nan, S.L.; Bai, W.K.; Hu, B. Low-frequency ultrasound combined with microbubbles improves gene transfection in prostate cancer cells in vitro and in vivo. J. Asia-Pac. J. Clin. Oncol. 2022, 18, 93–98. [Google Scholar] [CrossRef]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Araki, T.; Saba, L.; Nicolaides, A.; Sharma, A.; Omerzu, T.; Suri, H.S.; Gupta, A.; et al. A Special Report on Changing Trends in Preventive Stroke/Cardiovascular Risk Assessment Via B-Mode Ultrasonography. Curr. Atheroscler. Rep. 2019, 21, 25. [Google Scholar] [CrossRef]
- Guo, Y. A New Paradigm of “Real-Time” Stroke Risk Prediction and Integrated Care Management in the Digital Health Era: Innovations Using Machine Learning and Artificial Intelligence Approaches. J. Thromb. Haemost. 2022, 122, 5–7. [Google Scholar] [CrossRef]
- Viskovic, K.; Mavrogeni, S.; Laird, J.R.; Sattar, N.; Johri, A.M.; Pareek, G. Artificial intelligence framework for predictive cardiovascular and stroke risk assessment models: A narrative review of integrated approaches using carotid ultrasound. Comput. Biol. Med. 2020, 126, 104043. [Google Scholar]
- Mu, D.; Bai, J.; Chen, W.; Yu, H.; Liang, J.; Yin, K.; Li, H.; Qing, Z.; He, K.; Yang, H.-Y. Calcium scoring at coronary CT angiography using deep learning. J. Radiol. 2022, 302, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Tandel, G.S.; Biswas, M.; Kakde, O.G.; Tiwari, A.; Suri, H.S.; Turk, M.; Laird, J.R.; Asare, C.K.; Ankrah, A.A.; Khanna, N.J.C. A review on a deep learning perspective in brain cancer classification. Cancers 2019, 11, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, M.; Kuppili, V.; Saba, L.; Edla, D.R.; Suri, H.S.; Cuadrado-Godia, E.; Laird, J.R.; Marinhoe, R.T.; Sanches, J.M.; Nicolaides, A.J.F.B. State-of-the-art review on deep learning in medical imaging. Front. Biosci. 2019, 24, 392–426. [Google Scholar]
- Saba, L.; Biswas, M.; Kuppili, V.; Godia, E.C.; Suri, H.S.; Edla, D.R.; Omerzu, T.; Laird, J.R.; Khanna, N.N.; Mavrogeni, S. The present and future of deep learning in radiology. Eur. J. Radiol. 2019, 114, 14–24. [Google Scholar] [CrossRef]
- Kuppili, V.; Biswas, M.; Sreekumar, A.; Suri, H.S.; Saba, L.; Edla, D.R.; Marinhoe, R.T.; Sanches, J.M.; Suri, J.S. Extreme learning machine framework for risk stratification of fatty liver disease using ultrasound tissue characterization. J. Med. Syst. 2017, 41, 1–20. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Kumar, N.; Abedin, M.M.; Islam, M.S.; Suri, H.S.; El-Baz, A.; Suri, J.S. Comparative approaches for classification of diabetes mellitus data: Machine learning paradigm. Comput. Methods Programs Biomed. 2017, 152, 23–34. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Rahman, M.J.; Al-MehediHasan, M.; Suri, H.S.; Abedin, M.M.; El-Baz, A.; Suri, J.S. Accurate diabetes risk stratification using machine learning: Role of missing value and outliers. J. Med. Syst. 2018, 42, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Garberoglio, R.; Suri, J.S. Cost-effective and non-invasive automated benign & malignant thyroid lesion classification in 3D contrast-enhanced ultrasound using combination of wavelets and textures: A class of ThyroScan™ algorithms. Technol. Cancer Res. Treat. 2011, 10, 371–380. [Google Scholar]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Suri, J.S. ThyroScreen system: High resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. Comput. Methods Programs Biomed. 2012, 107, 233–241. [Google Scholar] [CrossRef]
- Pareek, G.; Acharya, U.R.; Sree, S.V.; Swapna, G.; Yantri, R.; Martis, R.J.; Saba, L.; Krishnamurthi, G.; Mallarini, G.; El-Baz, A. Prostate tissue characterization/classification in 144 patient population using wavelet and higher order spectra features from transrectal ultrasound images. Technol. Cancer Res. Treat. 2013, 12, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Saba, L.; Molinari, F.; Guerriero, S.; Suri, J.S. Ovarian tumor characterization and classification: A class of GyneScan™ systems. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 4446–4449. [Google Scholar] [PubMed]
- Liu, K.; Suri, J.S. Automatic Vessel Indentification for Angiographic Screening. U.S. Patent US20030166999A1, 18 January 2005. [Google Scholar]
- Acharya, O.S.U.R.; Vinitha, S.; Filippo, M.; Saba, L.; Nicolaide, A.; Suri, J.S. An accurate and generalized approach to plaque characterization in 346 carotid ultrasound scans. IEEE Trans. Instrum. Meas. 2011, 61, 1045–1053. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Alvin, A.; Krishnamurthi, G.; Seabra, J.C.; Sanches, J.; Suri, J.S. Understanding symptomatology of atherosclerotic plaque by image-based tissue characterization. Comput. Methods Programs Biomed. 2013, 110, 66–75. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Carriero, A.; Paschè, A.; Danna, P.S.; Columbu, M.; Saba, L.; Viskovic, K.; Mehmedović, A.; Agarwal, S. COVLIAS 1.0 vs. MedSeg: Artificial Intelligence-Based Comparative Study for Automated COVID-19 Computed Tomography Lung Segmentation in Italian and Croatian Cohorts. Diagnostics 2021, 11, 2367. [Google Scholar] [CrossRef]
- Battineni, G.; Chintalapudi, N.; Amenta, F.; Traini, E. A Comprehensive Machine-Learning Model Applied to Magnetic Resonance Imaging (MRI) to Predict Alzheimer’s Disease (AD) in Older Subjects. J. Clin. Med. 2020, 9, 2146. [Google Scholar] [CrossRef]
- Saba, L.; Suri, J.S. Multi-Detector CT Imaging: Abdomen, Pelvis, and CAD Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Sanches, J.M.; Laine, A.F.; Suri, J.S. Ultrasound Imaging; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Agarwal, M.; Saba, L.; Gupta, S.K.; Carriero, A.; Falaschi, Z.; Paschè, A.; Danna, P.; El-Baz, A.; Naidu, S.; Suri, J.S. A novel block imaging technique using nine artificial intelligence models for COVID-19 disease classification, characterization and severity measurement in lung computed tomography scans on an Italian cohort. J. Med. Syst. 2021, 45, 1–30. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Gupta, S.K.; Puvvula, A.; Biswas, M.; Saba, L.; Bit, A.; Tandel, G.S.; Agarwal, M.; Patrick, A. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput. Biol. Med. 2021, 130, 104210. [Google Scholar] [CrossRef]
- Paul, S.; Maindarkar, M.; Saxena, S.; Saba, L.; Turk, M.; Kalra, M.; Krishnan, P.R.; Suri, J.S. Bias Investigation in Artificial Intelligence Systems for Early Detection of Parkinson’s Disease: A Narrative Review. Diagnostics 2022, 12, 166. [Google Scholar] [CrossRef]
- Sibley, K.G.; Girges, C.; Hoque, E.; Foltynie, T. Video-based analyses of Parkinson’s disease severity: A brief review. J. Parkinson’s Dis. 2021, 11, S83–S93. [Google Scholar] [CrossRef]
- Dias, A.E.; Limongi, J.C.; Barbosa, E.R.; Hsing, W.T. Voice telerehabilitation in Parkinson’s disease. Codas 2016, 28, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suri, J.S.; Puvvula, A.; Biswas, M.; Majhail, M.; Saba, L.; Faa, G.; Singh, I.M.; Oberleitner, R.; Turk, M.; Chadha, P.S. COVID-19 pathways for brain and heart injury in comorbidity patients: A role of medical imaging and artificial intelligence-based COVID severity classification: A review. Comput. Biol. 2020, 124, 103960. [Google Scholar] [CrossRef]
- Alzubaidi, M.S.; Shah, U.; Zubaydi, H.D.; Dolaat, K.; Abd-Alrazaq, A.A.; Ahmed, A.; Househ, M. The Role of Neural Network for the Detection of Parkinson’s Disease: A Scoping Review. Healthcare 2021, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009, 72 (Suppl. S4), S1–S136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çubukçu, H.C.; Yurtdaş, M.; Durak, Z.E.; Aytaç, B.; Güneş, H.N.; Çokal, B.G.; Yoldaş, T.K.; Durak, İ. Oxidative and nitrosative stress in serum of patients with Parkinson’s disease. Neurol. Sci. 2016, 37, 1793–1798. [Google Scholar] [CrossRef]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Değirmenci, H.; Bakirci, E.M.; Hamur, H. Cardiac Effects of Parkinson’s Disease. Open J. Parkinson’s Dis. Treat. 2020, 3, 006–007. [Google Scholar]
- Scherder, E.; Herr, K.; Pickering, G.; Gibson, S.; Benedetti, F.; Lautenbacher, S. Pain in dementia. Pain 2009, 145, 276–278. [Google Scholar] [CrossRef]
- Günaydın, Z.Y.; Özer, F.F.; Karagöz, A.; Bektaş, O.; Karataş, M.B.; Vural, A.; Bayramoğlu, A.; Çelik, A.; Yaman, M. Evaluation of cardiovascular risk in patients with Parkinson disease under levodopa treatment. J. Geriatr. Cardiol. 2016, 13, 75. [Google Scholar]
- O’Suilleabhain, P.E.; Dewey, R.B., Jr. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson disease. Arch. Neurol. 2002, 59, 986–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisto, T.; Isola, J. Incidence of atherosclerosis in the internal mammary artery. Ann. Thorac. Surg. 1989, 47, 884–886. [Google Scholar] [CrossRef]
- Volterrani, M.; Scalvini, S.; Mazzuero, G.; Lanfranchi, P.; Colombo, R.; Clark, A.L.; Levi, G. Decreased heart rate variability in patients with chronic obstructive pulmonary disease. Chest 1994, 106, 1432–1437. [Google Scholar] [CrossRef]
- Malpas, S.C. What sets the long-term level of sympathetic nerve activity: Is there a role for arterial baroreceptors? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1–R12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studer, V.; Rocchi, C.; Motta, C.; Lauretti, B.; Perugini, J.; Brambilla, L.; Pareja-Gutierrez, L.; Camera, G.; Barbieri, F.R.; Marfia, G.A. Heart rate variability is differentially altered in multiple sclerosis: Implications for acute, worsening and progressive disability. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317701317. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, A.; Leys, F.; Falup-Pecurariu, C.; Thijs, R.; Wenning, G.K. Management of Orthostatic Hypotension in Parkinson’s Disease. J. Parkinson’s Dis. 2020, 10, S57–S64. [Google Scholar] [CrossRef]
- Potashkin, J.; Huang, X.; Becker, C.; Chen, H.; Foltynie, T.; Marras, C. Understanding the links between cardiovascular disease and Parkinson’s disease. Mov. Disord. 2020, 35, 55–74. [Google Scholar] [CrossRef]
- Firbank, M.J.; Yarnall, A.J.; Lawson, R.A.; Duncan, G.W.; Khoo, T.; Petrides, G.S.; O’Brien, J.; Barker, R.A.; Maxwell, R.; Brooks, D. Cerebral glucose metabolism and cognition in newly diagnosed Parkinson’s disease: ICICLE-PD study. J. Neurol. Neurosurg. Psychiatry 2017, 88, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Wiberg, B.; Lind, L.; Kilander, L.; Zethelius, B.; Sundelöf, J.E.; Sundström, J. Cognitive function and risk of stroke in elderly men. Neurology 2010, 74, 379–385. [Google Scholar] [CrossRef]
- Respondek, G.; Roeber, S.; Kretzschmar, H.; Troakes, C.; Al-Sarraj, S.; Gelpi, E.; Gaig, C.; Chiu, W.Z.; van Swieten, J.C.; Oertel, W.H. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov. Disord. 2013, 28, 504–509. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Joyner, M.J. Regulation of blood pressure by the arterial baroreflex and autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 89–102. [Google Scholar] [PubMed]
- Wong, K.K.; Raffel, D.M.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I.; Gilman, S. Pattern of cardiac sympathetic denervation in idiopathic Parkinson disease studied with 11C hydroxyephedrine PET. Radiology 2012, 265, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Most, A.; Ruocco, N., Jr.; Gewirtz, H. Effect of a reduction in blood viscosity on maximal myocardial oxygen delivery distal to a moderate coronary stenosis. Circulation 1986, 74, 1085–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, M.; Nassef, Y.E.; Shady, M.A.; Aziz, A.A.; El Malt, H.A. Metabolic syndrome and cardiovascular risk factors in obese adolescent. Open Access Maced. J. Med. Sci. 2016, 4, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlandi, G.; Fanucchi, S.; Strata, G.; Pataleo, L.; Pellegrini, L.L.; Prontera, C.; Martini, A.; Murri, L. Transient autonomic nervous system dysfunction during hyperacute stroke. Acta Neurol. Scand. 2000, 102, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Sofic, E.; Lange, K.W.; Jellinger, K.; Riederer, P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci. Lett. 1992, 142, 128–130. [Google Scholar] [CrossRef]
- Glick, G.; Braunwald, E.; Lewis, R.M. Relative roles of the sympathetic and parasympathetic nervous systems in the reflex control of heart rate. Circ. Res. 1965, 16, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Przedborski, S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017, 18, 251–259. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Anderson, A.S. The sympathetic nervous system and heart failure. Cardiol. Clin. 2014, 32, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Cuenca-Bermejo, L.; Almela, P.; Navarro-Zaragoza, J.; Villalba, E.F.; González-Cuello, A.-M.; Laorden, M.-L.; Herrero, M.-T. Cardiac Changes in Parkinson’s Disease: Lessons from Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 13488. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, D.-H.; Park, Y.-G.; Kwon, D.-Y.; Choi, M.; Jung, J.-H.; Han, K. Association of Parkinson disease with risk of cardiovascular disease and all-cause mortality: A nationwide, population-based cohort study. Circulation 2020, 141, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-Y.; He, Q.-F.; Lu, M.-Y.; Wang, S.-L.; Qi, Z.-Q.; Dong, H.-R. Association between carotid plaque and Parkinson’s disease. Ann. Transl. Med. 2019, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.A.; Fiorini, A.C.; Scorza, C.A.; Finsterer, J. Cardiac abnormalities in Parkinson’s disease and Parkinsonism. J. Clin. Neurosci. 2018, 53, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vikdahl, M.; Carlsson, M.; Linder, J.; Forsgren, L.; Håglin, L. Weight gain and increased central obesity in the early phase of Parkinson’s disease. Clin. Nutr. 2014, 33, 1132–1139. [Google Scholar] [CrossRef]
- Goldstein, D.S. Dysautonomia in Parkinson’s disease: Neurocardiological abnormalities. Lancet Neurol. 2003, 2, 669–676. [Google Scholar] [CrossRef]
- Pan, M.; Gao, H.; Long, L.; Xu, Y.; Liu, M.; Zou, J.; Wu, A.; Wei, X.; Chen, X.; Tang, B. Serum uric acid in patients with Parkinson’s disease and vascular parkinsonism: A cross-sectional study. Neuroimmunomodulation 2013, 20, 19–28. [Google Scholar] [CrossRef]
- Czarkowska, H.; Tutaj, M.; Rudzińska, M.; Motyl, M.; Bryś, M.; Bukowczan, S.; Kyrcz, A.; Zajdel, K.; Szczudlik, A. Cardiac responses to orthostatic stress deteriorate in Parkinson disease patients who begin to fall. Neurol. Neurochir. Pol. 2010, 44, 339–349. [Google Scholar] [CrossRef]
- Buob, A.; Winter, H.; Kindermann, M.; Becker, G.; Möller, J.; Oertel, W.; Böhm, M. Parasympathetic but not sympathetic cardiac dysfunction at early stages of Parkinson’s disease. Clin. Res. Cardiol. 2010, 99, 701–706. [Google Scholar] [CrossRef]
- Walter, B.L. Cardiovascular autonomic dysfunction in patients with movement disorders. Clevel. Clin. J. Med. 2008, 75, S54. [Google Scholar] [CrossRef] [Green Version]
- Ward, H.; Toledano, M.B.; Shaddick, G.; Davies, B.; Elliott, P. Oxford Handbook of Epidemiology for Clinicians; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A. Parkinson’s disease and its comorbid disorders: An analysis of Michigan mortality data 1970 to 1990. Neurology 1994, 44, 1865. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet Neurol. 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Hartmann, A.; Mast, H.; Mohr, J.; Koennecke, H.-C.; Osipov, A.; Pile-Spellman, J.; Duong, D.H.; Young, W.L. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke 1998, 29, 931–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobieszczyk, P.; Beckman, J. Carotid artery disease. Circulation 2006, 114, e244–e247. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.G.; Helft, G.; Worthley, S.G.; Badimon, J.J. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis 2000, 149, 251–266. [Google Scholar] [CrossRef]
- Hahnen, E.; Hauke, J.; Tränkle, C.; Eyüpoglu, I.Y.; Wirth, B.; Blümcke, I. Histone deacetylase inhibitors: Possible implications for neurodegenerative disorders. Expert Opin. Investig. Drugs 2008, 17, 169–184. [Google Scholar] [CrossRef]
- Mandat, T.; Hurwitz, T.; Honey, C. Hypomania as an adverse effect of subthalamic nucleus stimulation: Report of two cases. Acta Neurochir. 2006, 148, 895–898. [Google Scholar] [CrossRef]
- Kim, S.; Choi, B.Y.; Nam, J.H.; Kim, M.K.; Oh, D.H.; Yang, Y.J. Cognitive impairment is associated with elevated serum homocysteine levels among older adults. Eur. J. Nutr. 2019, 58, 399–408. [Google Scholar] [CrossRef]
- Mercuri, N.B.; Bernardi, G. The ‘magic’of L-dopa: Why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol. Sci. 2005, 26, 341–344. [Google Scholar] [CrossRef]
- Cao, C.; Li, D.; Zhan, S.; Zhang, C.; Sun, B.; Litvak, V. L-dopa treatment increases oscillatory power in the motor cortex of Parkinson’s disease patients. NeuroImage Clin. 2020, 26, 102–116. [Google Scholar] [CrossRef]
- Cenci, M.A.; Crossman, A.R. Animal models of l-dopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2018, 33, 889–899. [Google Scholar] [CrossRef]
- Chagraoui, A.; Boulain, M.; Juvin, L.; Anouar, Y.; Barrière, G.; Deurwaerdère, P.D. L-dopa in Parkinson’s disease: Looking at the “false” neurotransmitters and their meaning. Int. J. Mol. Sci. 2020, 21, 294. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, D.J.; Faggioni, M.; Wleklinski, M.J.; Gomez-Hurtado, N.; Venkataraman, R.; Gibbs, C.E.; Baudenbacher, F.J.; Gong, S.; Fishman, G.I.; Boyle, P.M. The Purkinje-myocardial junction is the anatomical origin of ventricular arrhythmia in CPVT. JCI Insight 2022, 7, e151893. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, Z.; Liang, C.; Fu, Y.; Wei, X.; Lu, J.; Pan, M.; Guo, Y.; Liao, X.; Xie, H. Trefoil factor 3, cholinesterase and homocysteine: Potential predictors for Parkinson’s disease dementia and vascular parkinsonism dementia in advanced stage. Aging Dis. 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poetini, M.R.; Musachio, E.A.S.; Araujo, S.M.; Bortolotto, V.C.; Meichtry, L.B.; Silva, N.C.; Janner, D.E.; Novo, D.L.R.; Mesko, M.F.; Roehrs, R. Improvement of non-motor and motor behavioral alterations associated with Parkinson-like disease in Drosophila melanogaster: Comparative effects of treatments with hesperidin and L-dopa. NeuroToxicology 2022, 89, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Dutta, A.; Phukan, B.C.; Mazumder, M.K.; Justin-Thenmozhi, A.; Manivasagam, T.; Bhattacharya, P.; Borah, A. Accumulation of cholesterol and homocysteine in the nigrostriatal pathway of brain contributes to the dopaminergic neurodegeneration in mice. Neuroscience 2018, 388, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.-P.; Bouhaddi, M.; Louisy, F.; Mikehiev, A.; Mourot, L.; Cappelle, S.; Vuillier, F.; Andre, P.; Rumbach, L.; Regnard, J. Side-effects of L-dopa on venous tone in Parkinson’s disease: A leg-weighing assessment. Clin. Sci. 2006, 110, 369–377. [Google Scholar] [CrossRef]
- Bello, F.D.; Giannella, M.; Giorgioni, G.; Piergentili, A.; Quaglia, W. Receptor ligands as helping hands to L-DOPA in the treatment of Parkinson’s disease. Biomolecules 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Lane, E.L. L-DOPA for Parkinson’s disease—A bittersweet pill. Eur. J. Neurosci. 2019, 49, 384–398. [Google Scholar] [CrossRef]
- Nutt, J.G. Motor fluctuations and dyskinesia in Parkinson’s disease. Parkinsonism Relat. Disord. 2001, 8, 101–108. [Google Scholar] [CrossRef]
- Mondal, B.; Choudhury, S.; Banerjee, R.; Chatterjee, K.; Ghosal, S.; Anand, S.S.; Kumar, H. Analysis of gait in Parkinson’s disease reflecting the effect of l-DOPA. Ann. Mov. Disord. 2019, 2, 21. [Google Scholar]
- Griffiths, R.I.; Kotschet, K.; Arfon, S.; Xu, Z.M.; Johnson, W.; Drago, J.; Evans, A.; Kempster, P.; Raghav, S.; Horne, M.K. Automated assessment of bradykinesia and dyskinesia in Parkinson’s disease. J. Parkinson’s Dis. 2012, 2, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keifman, E.; Ruiz-DeDiego, I.; Pafundo, D.E.; Paz, R.M.; Solís, O.; Murer, M.G.; Moratalla, R. Optostimulation of striatonigral terminals in substantia nigra induces dyskinesia that increases after L-DOPA in a mouse model of Parkinson’s disease. Br. J. Pharmacol. 2019, 176, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Bogetofte, H.; Alamyar, A.; Blaabjerg, M.; Meyer, M. Levodopa therapy for Parkinson’s disease: History, current status and perspectives. CNS Neurol. Disord. Drug Targets 2020, 19, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Tang, H.; Chen, S.; Ma, J. Stroke and coronary artery disease are associated with Parkinson’s disease. Can. J. Neurol. Sci. 2018, 45, 559–565. [Google Scholar] [CrossRef]
- Levine, J.; Greenwald, B.D. Fatigue in Parkinson disease, stroke, and traumatic brain injury. Phys. Med. Rehabil. Clin. 2009, 20, 347–361. [Google Scholar] [CrossRef]
- Rickards, H. Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S1), i48–i52. [Google Scholar] [CrossRef] [Green Version]
- Mastaglia, F.L.; Johnsen, R.D.; Kakulas, B.A. Prevalence of stroke in Parkinson’s disease: A postmortem study. Mov. Disord. 2002, 17, 772–774. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s. Front. Neuroanat. 2016, 9, 91. [Google Scholar]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. Pharmacological effects of natural Ganoderma and its extracts on neurological diseases: A comprehensive review. Int. J. Biol. Macromol. 2019, 121, 1160–1178. [Google Scholar] [CrossRef]
- Yu, J.; Park, S.; Kwon, S.-H.; Ho, C.M.B.; Pyo, C.-S.; Lee, H. AI-Based Stroke Disease Prediction System Using Real-Time Electromyography Signals. Appl. Sci. 2020, 10, 6791. [Google Scholar] [CrossRef]
- Emma, P.; Bennett, M.R. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic. Biol. Med. 2016, 100, 223–230. [Google Scholar]
- Wang, X.; Cao, G.; Ding, D.; Li, F.; Zhao, X.; Wang, J.; Yang, Y. Ferruginol prevents degeneration of dopaminergic neurons by enhancing clearance of α-synuclein in neuronal cells. Fitoterapia 2022, 156, 105066. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, F.; Martínez-Horta, S.; Horta-Barba, A.; Grothe, M.J.; Labrador-Espinosa, M.A.; Jesús, S.; Adarmes-Gómez, A.; Carrillo, F.; Puig-Davi, A.; Lora, F.R. Increased homocysteine levels correlate with cortical structural damage in Parkinson’s disease. J. Neurol. Sci. 2022, 434, 120148. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Frostegard, J.; Haegerstrand, A.; Gidlund, M.; Nilsson, J.J.A. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis 1991, 90, 119–126. [Google Scholar] [CrossRef]
- Chirkov, Y.Y.; Nguyen, T.H.; Horowitz, J.D. Impairment of Anti-Aggregatory Responses to Nitric Oxide and Prostacyclin: Mechanisms and Clinical Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 31042. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation 2002, 106, 666–671. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J. Am. Coll. Cardiol. 2003, 42, 2139–2143. [Google Scholar] [CrossRef] [Green Version]
- Kamal, R.M.; Razis, A.F.A.; Sukri, N.S.M.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Fang, S.; Hu, X.; Wang, T.; Yang, Y.; Xu, R.; Zhang, X.; Luo, J.; Ma, Y.; Patel, A.B.; Dmytriw, A.A. Parkinson’s Disease and Ischemic Stroke: A Bidirectional Mendelian Randomization Study. Transl. Stroke Res. 2022, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Jiang, Y.; Li, F.; Sun-Waterhouse, D.; Zhao, S.; Guan, X.; Li, D. Close association between the synergistic toxicity of zearalenone-deoxynivalenol combination and microRNA221-mediated PTEN/PI3K/AKT signaling in HepG2 cells. Toxicology 2022, 468, 153104. [Google Scholar] [CrossRef] [PubMed]
- Bellocchi, C.; Carandina, A.; Montinaro, B.; Targetti, E.; Furlan, L.; Rodrigues, G.D.; Tobaldini, E.; Montano, N. The Interplay between Autonomic Nervous System and Inflammation across Systemic Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 52449. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Carvalho, J.L.; Falquetto, B.; Takakura, A.C.; Vianna, L.C. Baroreflex dysfunction in Parkinson’s disease: Integration of central and peripheral mechanisms. J. Neurophysiol. 2021, 125, 1425–1439. [Google Scholar] [CrossRef]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F.; Dell’Oro, R.; Arenare, F.; Spaziani, D.; Mancia, G. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension 2009, 53, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.; Ehnvall, A.; Friberg, P.; Myredal, A. Arterial baroreflex dysfunction in major depressive disorder. Clin. Auton. Res. 2010, 20, 235–240. [Google Scholar] [CrossRef]
- Turkka, J.T.; Tolonen, U.; Myllylä, V.V. Cardiovascular reflexes in Parkinson’s disease. Eur. Neurol. 1987, 26, 104–112. [Google Scholar] [CrossRef]
- Rocchi, C.; Pierantozzi, M.; Galati, S.; Chiaravalloti, A.; Pisani, V.; Prosperetti, C.; Lauretti, B.; Bassi, M.S.; Olivola, E.; Schillaci, O. Autonomic function tests and MIBG in Parkinson’s disease: Correlation to disease duration and motor symptoms. CNS Neurosci. Ther. 2015, 21, 727–732. [Google Scholar] [CrossRef]
- Kemp, K.; Griffiths, J.; Campbell, S.; Lovell, K. An exploration of the follow-up up needs of patients with inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, e386–e395. [Google Scholar] [CrossRef] [Green Version]
- Maniruzzaman, M.; Suri, H.S.; Kumar, N.; Abedin, M.M.; Rahman, M.J.; El-Baz, A.; Bhoot, M.; Teji, J.S.; Suri, J.S. Risk factors of neonatal mortality and child mortality in Bangladesh. J. Glob. Health 2018, 8, 010417. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, N.; Azmanov, H.; Kesler, A.; Ilan, Y. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur. J. Hum. Genet. 2021, 29, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Banchhor, S.K.; Londhe, N.D.; Araki, T.; Saba, L.; Radeva, P.; Laird, J.R.; Suri, J.S. Wall-based measurement features provides an improved IVUS coronary artery risk assessment when fused with plaque texture-based features during machine learning paradigm. Comput. Biol. Med. 2017, 91, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Chou, Y.-J.; Tsai, T.-H.; Hsu, P.W.-C.; Li, C.-H.; Chan, Y.-H.; Tsai, S.-F.; Ng, S.-C.; Chou, K.-M.; Lin, Y.-C. Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease. Biomedicines 2022, 10, 116. [Google Scholar] [CrossRef]
- Saba, L.; Sanagala, S.S.; Gupta, S.K.; Koppula, V.K.; Johri, A.M.; Khanna, N.N.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M.; et al. Multimodality carotid plaque tissue characterization and classification in the artificial intelligence paradigm: A narrative review for stroke application. Ann. Transl. Med. 2021, 9, 1206. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Krishnan, M.M.R.; Molinari, F.; Garberoglio, R.; Suri, J.S. Non-invasive automated 3D thyroid lesion classification in ultrasound: A class of ThyroScan™ systems. Ultrasonics 2012, 52, 508–520. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Krishnan, M.M.R.; Molinari, F.; ZieleŸnik, W.; Bardales, R.H.; Witkowska, A.; Suri, J.S. Computer-aided diagnostic system for detection of Hashimoto thyroiditis on ultrasound images from a Polish population. J. Ultrasound Med. 2014, 33, 245–253. [Google Scholar] [CrossRef]
- Huang, S.-F.; Chang, R.-F.; Moon, W.K.; Lee, Y.-H.; Chen, D.-R.; Suri, J.S. Analysis of tumor vascularity using three-dimensional power Doppler ultrasound images. IEEE Trans. Med. Imaging 2008, 27, 320–330. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Kulshreshtha, S.; Molinari, F.; Koh, J.E.W.; Saba, L.; Suri, J.S. GyneScan: An improved online paradigm for screening of ovarian cancer via tissue characterization. Technol. Cancer Res. Treat. 2014, 13, 529–539. [Google Scholar] [CrossRef] [Green Version]
- McClure, P.; Elnakib, A.; El-Ghar, M.A.; Khalifa, F.; Soliman, A.; El-Diasty, T.; Suri, J.S.; Elmaghraby, A.; El-Baz, A. Ayman In-vitro and in-vivo diagnostic techniques for prostate cancer: A review. J. Biomed. Nanotechnol. 2014, 10, 2747–2777. [Google Scholar] [CrossRef]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Saba, L.; Araki, T.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M. A low-cost machine learning-based cardiovascular/stroke risk assessment system: Integration of conventional factors with image phenotypes. Cardiovasc. Diagn. Ther. 2019, 9, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Saba, L.; Laird, J.R.; Suri, J.S. Cardiovascular/stroke risk prevention: A new machine learning framework integrating carotid ultrasound image-based phenotypes and its harmonics with conventional risk factors. Indian Heart J. 2020, 72, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Jamthikar, A.D.; Gupta, D.; Johri, A.M.; Mantella, L.E.; Saba, L.; Kolluri, R.; Sharma, A.M.; Viswanathan, V.; Nicolaides, A.; Suri, J.S. Low-cost office-based cardiovascular risk stratification using machine learning and focused carotid ultrasound in an Asian-Indian cohort. J. Med. Syst. 2020, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jamthikar, A.D.; Gupta, D.; Mantella, L.E.; Saba, L.; Laird, J.R.; Johri, A.M.; Suri, J.S. Multiclass machine learning vs. conventional calculators for stroke/CVD risk assessment using carotid plaque predictors with coronary angiography scores as gold standard: A 500 participants study. Int. J. Cardiovasc. Imaging 2021, 37, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Jena, B.; Saxena, S.; Nayak, G.K.; Saba, L.; Sharma, N.; Suri, J.S. Artificial intelligence-based hybrid deep learning models for image classification: The first narrative review. Comput. Biol. Med. 2021, 137, 104803. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Sharma, N.; Giannopoulos, A.A.; Saba, L.; Nicolaides, A.; Suri, J.S. Hybrid deep learning segmentation models for atherosclerotic plaque in internal carotid artery B-mode ultrasound. Comput. Biol. Med. 2021, 136, 104721. [Google Scholar] [CrossRef] [PubMed]
- Suri, J.S.; Agarwal, S.; Pathak, R.; Ketireddy, V.; Columbu, M.; Saba, L.; Gupta, S.K.; Faa, G.; Singh, I.M.; Turk, M. COVLIAS 1.0: Lung Segmentation in COVID-19 Computed Tomography Scans Using Hybrid Deep Learning Artificial Intelligence Models. Diagnostics 2021, 11, 1405. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Saba, L.; Paraskevas, K.I.; Kalra, M.K.; Johri, A.; Nicolaides, A.N.; Suri, J.S. Automated deep learning-based paradigm for high-risk plaque detection in B-mode common carotid ultrasound scans: An asymptomatic Japanese cohort study. Int. Angiol. 2021, 41, 9–23. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Elavarthi, P.; Pathak, R.; Ketireddy, V.; Columbu, M.; Saba, L.; Gupta, S.K.; Faa, G.; Singh, I.M. Inter-Variability Study of COVLIAS 1.0: Hybrid Deep Learning Models for COVID-19 Lung Segmentation in Computed Tomography. Diagnostics 2021, 11, 2025. [Google Scholar] [CrossRef]
- Sudeep, P.; Palanisamy, P.; Rajan, J.; Baradaran, H.; Saba, L.; Gupta, A.; Suri, J.S. Speckle reduction in medical ultrasound images using an unbiased non-local means method. Biomed. Signal. Process. Control. 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Pewowaruk, R.J.; Tedla, Y.; Korcarz, C.E.; Tattersall, M.C.; Stein, J.H.; Chesler, N.C.; Gepner, A.D. Carotid Artery Stiffening with Aging: Structural Versus Load-Dependent Mechanisms in MESA (the Multi-Ethnic Study of Atherosclerosis). Hypertension 2022, 79, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Liboni, W.; Giustetto, P.; Badalamenti, S.; Suri, J.S. Automatic computer-based tracings (ACT) in longitudinal 2-D ultrasound images using different scanners. J. Mech. Med. Biol. 2009, 9, 481–505. [Google Scholar] [CrossRef]
- Suri, J.S.; Bhagawati, M.; Paul, S.; Protogeron, A.; Sfikakis, P.P.; Kitas, G.D.; Khanna, N.N.; Ruzsa, Z.; Sharma, A.M.; Saxena, S. Understanding the bias in machine learning systems for cardiovascular disease risk assessment: The first of its kind review. Comput. Biol. Med. 2022, 142, 105204. [Google Scholar] [CrossRef] [PubMed]
- Skandha, S.S.; Gupta, S.K.; Saba, L.; Koppula, V.K.; Johri, A.M.; Khanna, N.N.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M. 3-D optimized classification and characterization artificial intelligence paradigm for cardiovascular/stroke risk stratification using carotid ultrasound-based delineated plaque: Atheromatic™ 2.0. Comput. Biol. Med. 2020, 125, 103–114. [Google Scholar] [CrossRef]
- Skandha, S.S.; Nicolaides, A.; Gupta, S.K.; Koppula, V.K.; Saba, L.; Johri, A.M.; Kalra, M.S.; Suri, J.S. A hybrid deep learning paradigm for carotid plaque tissue characterization and its validation in multicenter cohorts using a supercomputer framework. Comput. Biol. Med. 2021, 141, 105131. [Google Scholar] [CrossRef]
- Saba, L.; Sanagala, S.S.; Gupta, S.K.; Koppula, V.K.; Laird, J.R.; Viswanathan, V.; Sanches, M.J.; Kitas, G.D.; Johri, A.M.; Sharma, N. A Multicenter Study on Carotid Ultrasound Plaque Tissue Characterization and Classification Using Six Deep Artificial Intelligence Models: A Stroke Application. IEEE Trans. Instrum. Meas. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Biswas, M.; Kuppili, V.; Saba, L.; Edla, D.R.; Suri, H.S.; Sharma, A.; Cuadrado-Godia, E.; Laird, J.R.; Nicolaides, A.; Suri, J.S. Deep learning fully convolution network for lumen characterization in diabetic patients using carotid ultrasound: A tool for stroke risk. Med. Biol. Eng. Comput. 2019, 57, 543–564. [Google Scholar] [CrossRef]
- Soun, J.; Chow, D.; Nagamine, M.; Takhtawala, R.; Filippi, C.; Yu, W.; Chang, P. Artificial intelligence and acute stroke imaging. Am. J. Neuroradiol. 2021, 42, 2–11. [Google Scholar] [CrossRef]
- Rava, R.A.; Seymour, S.E.; Snyder, K.V.; Waqas, M.; Davies, J.M.; Levy, E.I.; Siddiqui, A.H.; Ionita, C.N. Automated Collateral Flow Assessment in Patients with Acute Ischemic Stroke Using Computed Tomography with Artificial Intelligence Algorithms. World Neurosurg. 2021, 155, e748–e760. [Google Scholar] [CrossRef]
- Mouridsen, K.; Thurner, P.; Zaharchuk, G. Artificial intelligence applications in stroke. Stroke 2020, 51, 2573–2579. [Google Scholar] [CrossRef]
- Ain, K.; Hidayati, H.B.; Nastiti, O.A. Expert System for Stroke Classification Using Naive Bayes Classifier and Certainty Factor as Diagnosis Supporting Device. J. Phys. Conf. Ser. 2020, 1445, 012026. [Google Scholar] [CrossRef]
- Badriyah, T.; Sakinah, N.; Syarif, I.; Syarif, D.R. Machine Learning Algorithm for Stroke Disease Classification. In Proceedings of the 2020 International Conference on Electrical, Communication, and Computer Engineering (ICECCE), Istanbul, Turkey, 12–13 June 2020; pp. 1–5. [Google Scholar]
- Bikias, T.; Iakovakis, D.; Hadjidimitriou, S.; Charisis, V.; Hadjileontiadis, L. DeepFoG: An IMU-Based Detection of Freezing of Gait Episodes in Parkinson’s Disease Patients via Deep Learning. Front. Robot. 2021, 8, 537384. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Pradhan, R.; Nandy, P.; Bhoi, A.K.; Barsocchi, P. Machine Learning Methods with Decision Forests for Parkinson’s Detection. Appl. Sci. 2021, 11, 581–592. [Google Scholar] [CrossRef]
- Borzì, L.; Mazzetta, I.; Zampogna, A.; Suppa, A.; Olmo, G.; Irrera, F. Prediction of freezing of gait in Parkinson’s disease using wearables and machine learning. Sensors 2021, 21, 614. [Google Scholar] [CrossRef] [PubMed]
- Aich, S.; Youn, J.; Chakraborty, S.; Pradhan, P.M.; Park, J.-h.; Park, S.; Park, J. A supervised machine learning approach to detect the on/off state in Parkinson’s disease using wearable based gait signals. Diagnostics 2020, 10, 421. [Google Scholar] [CrossRef]
- Pramanik, M.; Pradhan, R.; Nandy, P.; Qaisar, S.M.; Bhoi, A.K. Assessment of Acoustic Features and Machine Learning for Parkinson’s Detection. J. Healthc. Eng. 2021, 3, 21–24. [Google Scholar] [CrossRef]
- Zahid, L.; Maqsood, M.; Durrani, M.Y.; Bakhtyar, M.; Baber, J.; Jamal, H.; Mehmood, I.; Song, O.-Y. A spectrogram-based deep feature assisted computer-aided diagnostic system for Parkinson’s disease. IEEE Access 2020, 8, 35482–35495. [Google Scholar] [CrossRef]
- Nissar, I.; Rizvi, D.; Masood, S.; Mir, A. Voice-based detection of Parkinson’s disease through ensemble machine learning approach: A Performance study. EAI Endorsed Trans. Pervasive Health Technol. 2019, 5, 162806. [Google Scholar] [CrossRef] [Green Version]
- Korczyn, A.D. Vascular Parkinsonism—Characteristics, pathogenesis and treatment. Nat. Rev. Neurol. 2015, 11, 319–326. [Google Scholar] [CrossRef]
- Rakhimbaeva, G.S.; Akramova, D.T. Role of Increasing Levels of The Hormone Cortisol in Cognitive Impairment in Parkinson’s Disease: Vascular Parkinsonism. Eur. J. Mol. Clin. Med. 2020, 7, 2987–2994. [Google Scholar]
- Winikates, J.; Jankovic, J. Clinical correlates of vascular parkinsonism. Arch. Neurol. 1999, 56, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Bay, A.A.; Ni, L.; Hackney, M.E. Apathy-Related Symptoms Appear Early in Parkinson’s Disease. Healthcare 2022, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kuruvilla, A. Vascular parkinsonism: What makes it different? Postgrad. Med. J. 2011, 87, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Tada, S.; Choudhury, M.E.; Kubo, M.; Ando, R.; Tanaka, J.; Nagai, M. Zonisamide Ameliorates Microglial Mitochondriopathy in Parkinson’s Disease Models. Brain Sci. 2022, 12, 268. [Google Scholar] [CrossRef]
- Pursiainen, V.; Korpelainen, T.; Haapaniemi, H.; Sotaniemi, A.; Myllylä, V.V. Selegiline and blood pressure in patients with Parkinson’s disease. Acta Neurol. Scand. 2007, 115, 104–108. [Google Scholar] [CrossRef]
- Sommer, S.; Aral-Becher, B.; Jost, W. Nondipping in Parkinson’s disease. Parkinson’s Dis. 2011, 2011, 897586. [Google Scholar] [CrossRef] [Green Version]
- Straus, S.M.; Kors, J.A.; de Bruin, M.L.; van der Hooft, C.S.; Hofman, A.; Heeringa, J.; Deckers, J.W.; Kingma, J.H.; Sturkenboom, M.C.; Stricker, B.H.C. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 2006, 47, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Seppi, K.; Chaudhuri, K.R.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Lloret, S.P.; Weintraub, D.; Sampaio, C.; Chahine, L. Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef] [Green Version]
- Pontico, M.; Brunotti, G.; Conte, M.; Corica, F.; Cosma, L.; de Angelis, C.; de Feo, M.S.; Lazri, J.; Matto, A.; Montebello, M. The prognostic value of 123 I-mIBG SPECT cardiac imaging in heart failure patients: A systematic review. J. Nucl. Cardiol. 2021, 1–11. [Google Scholar] [CrossRef]
- Seo, M.; Yamada, T.; Tamaki, S.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Nakamura, J. Prognostic Significance of Cardiac 123I-MIBG SPECT Imaging in Heart Failure Patients with Preserved Ejection Fraction. Cardiovasc. Imaging 2021, in press. [Google Scholar] [CrossRef]
- Braune, S.; Reinhardt, M.; Schnitzer, R.; Riedel, A.; Lücking, C. Cardiac uptake of [123I] MIBG separates Parkinson’s disease from multiple system atrophy. Neurology 1999, 53, 1020. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, H.; Nishida, H.; Matsuo, H.; Watanabe, S.; Nagashima, K.; Wada, H.; Noda, T.; Nishigaki, K.; Fujiwara, H. Cardiac sympathetic denervation from the early stage of Parkinson’s disease: Clinical and experimental studies with radiolabeled MIBG. J. Nucl. Med. 2000, 41, 71–77. [Google Scholar] [PubMed]
- Jellinger, K.A. Neuropathobiology of non-motor symptoms in Parkinson disease. J. Neural Transm. 2015, 122, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.S.; Dorfman, B.J.; Christos, P.J.; Khadem, N.R.; Henchcliffe, C.; Piboolnurak, P.; Nirenberg, M. Clinical characteristics of exacerbations in Parkinson disease. Neurologist 2012, 18, 120. [Google Scholar] [CrossRef] [Green Version]
- Brugger, F.; Erro, R.; Balint, B.; Kägi, G.; Barone, P.; Bhatia, K.P. Why is there motor deterioration in Parkinson’s disease during systemic infections—A hypothetical view. NPJ Parkinson’s Dis. 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Umemura, A.; Oeda, T.; Tomita, S.; Hayashi, R.; Kohsaka, M.; Park, K.; Sugiyama, H.; Sawada, H. Delirium and high fever are associated with subacute motor deterioration in Parkinson disease: A nested case-control study. PLoS ONE 2014, 9, e94944. [Google Scholar] [CrossRef] [Green Version]
- Kilkenny, M.F.; Olaiya, M.T.; Dalli, L.L.; Kim, J.; Andrew, N.E.; Sanfilippo, F.M.; Thrift, A.G.; Nelson, M.; Pearce, C.; Sanders, L. Treatment with Multiple Therapeutic Classes of Medication is Associated with Survival after Stroke. Neuroepidemiology 2022, 56, 66–74. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The impact of sex and gender on stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef]



| SN | Citations | Relation * | ME | PS | Outcome | TRE |
|---|---|---|---|---|---|---|
| 1 | Cuenca-Bermejo et al. [105] (2021) | Cardiac changes in PD | LBBM | NR | In PD patients with a lack of sympathetic innervation in the heart, cardiac abnormalities have also been identified. Post-prandial hypotension, supine hypertension, increasing blood pressure variability, reduced heart rate variability, and chronotropic incompetence are also symptoms. | NR |
| 2 | Park et al. [106] (2020) | PD with risk of CVD | Population-based cohort study | NR | PD was linked to an increased risk of cardiovascular disease. Physicians must also pay attention to CVD prevention in individuals with PD. | NR |
| 3 | Potashkin et al. [92] (2020) | Relation between CVD and PD | LBBM | 47 | Inflammation, insulin resistance, lipid metabolism, and oxidative stress are among the basic mechanisms that both CV disease and PD share. Physical exercise and moderate coffee intake are two modifiable risk variables that are inversely related to both CV disease and PD. | NR |
| 4 | Değirmenci et al. [83] (2020) | Cardiac effect of PD | LBBM | NR | Cardiac problems are frequent in PD patients. PD is associated with CVD, such as coronary artery disease, heart failure, cardiac autonomic dysfunction, heart failure, sudden death, and hypertension. | Levodopa, Monoamine oxidase B inhibitors, catechol-O-methyl transferase inhibitors, anticholinergic drugs, deep brain simulations |
| 5 | Fanciulli et al. [91] (2020) | Orthostatic hypertension in PD | LBBM | NR | Syncope, unexplained falls, lightheadedness, cognitive impairment, blurred vision, dyspnea, weariness, and shoulders, neck, or low-back discomfort are all symptoms of Orthostatic hypotension. They appear when you stand up and go away when you lie down. | Droxidopa, fludrocortisone, clonidine, transdermal nitroglycerin, nifedipine |
| 6 | Yan et al. [107] (2019) | Relation of Carotid plaque in PD | LBBM | 68 | As PD becoming worsening, the thickness of carotid plaques also increases. | NR |
| 7 | Scorza et al. [108] (2018) | Cardiac abnormalities in PD | LBBM | NR | Cardiovascular autonomic dysfunction, cardiomyopathy, coronary heart disease, arrhythmias, conduction abnormalities, and sudden cardiac death are all symptoms of PD/PS. | NR |
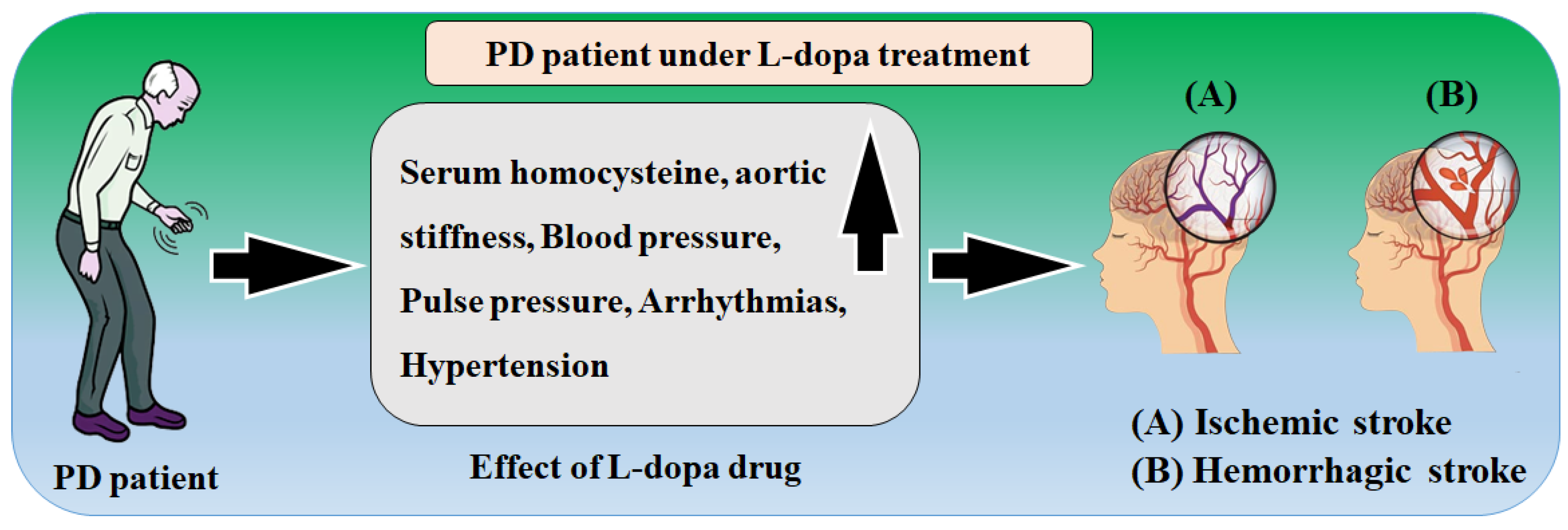
| 8 | Günaydın et al. [85] (2016) | CVD risk in PD under levodopa treatment | LBBM | 65 | Compared to healthy people, those with PD who use L-dopa have increased aortic stiffness and poor diastolic performance. Homocysteine levels in the blood may be a potential pathophysiological factor. | NR |
| 9 | Huang et al. [92] (2015) | plasma cholesterol risk in PD | LBBM | 156 | Statin usage has been linked to an increased risk of PD, although larger total cholesterol has been linked to a decreased risk. | Statins |
| 10 | Vikdahl et al. [109] (2015) | CVD risk in PD | LBBM | 147 | High blood cholesterol levels, smoking habits, and a high body mass index (BMI) have all been considered risk factors for PD. A moderate degree of physical exercise may help to lower the risk of heart disease. | NR |
| 11 | Goldstein [47] (2014) | Dystonia in PD | LBBM | 23 | Orthostatic hypotension in PD can be explained by the loss of sympathetic nerves and the associated failure of the baroreflex. During levodopa medication, hypotension might exacerbate after standing or after a substantial meal. | NR |
| 12 | Liang et al. [31] (2015) | Risk of CAD due to PD | LBBM | NR | PD is related to an increased risk of AMI; the mechanism needs to be explained. | NR |
| 13 | Goldstein [110] (2014) | Cardiac denervation in PD | LBBM | 40 | In individuals with PD and neurogenic orthostatic hypotension, cardiac sympathetic denervation is almost ubiquitous. Before the start of the movement disorder, baroreflex-cardiovagal failure and cardiac sympathetic denervation can occur, suggesting that neuroradiologic testing might be used as a biomarker for diagnosing presymptomatic or early PD and monitoring responses to possible neuroprotective therapies. | NR |
| 14 | Pan et al. [111] (2013) | Relation between Serum Uric acid with vascular PD | LBBM | 160 | Low uric acid levels are more likely to develop PD, and the inverse connection between uric acid and PD severity was strong for males but weak for women. There is no connection for uric acid found in vascular PD. | NR |
| 15 | Wong et al. [97] (2012) | PD with Cardiac Sympathetic Denervation | LBBM | 27 | In IPD, there is a sign of cardiac sympathetic denervation. | NR |
| 16 | Czarkowska et al. [112] (2010) | PD with Cardiac response | LBBM | 53 | With the progression of PD, cardiac responses to orthostatic stress worsen. The fall is caused by the detonation. | NR |
| 17 | Buob et al. [113] (2010) | Cardiac dysfunction in PD | LBBM | 07 | The chronotropic and contractile responses mediated by catecholamines rule out a functionally significant sympathetic malfunction. Sympathetic denervation maybe still not be complete, and the surviving fibers are enough to sustain autonomic control. | NR |
| 18 | Walter et al. [114] (2008) | PD with Cardiovascular autonomic dysfunction | LBBM | NR | Other parkinsonian illnesses are characterized by peripheral autonomic dysfunction. | Somatostatin, levodopa |
| SN | Citations | Relation | ME | PS | Outcome | TRE |
|---|---|---|---|---|---|---|
| 1 | Li et al. [140] (2018) | Stroke and CAD in PD | LBBM | 63 | Stroke risk was observed to be higher in people with PD. Cerebral small vessel disease has been linked to moderate parkinsonian symptoms. | NR |
| 2 | Studer et al. [90] (2017) | Heart rate variability and skin resonance in PD | LBBM | 73 | Both SSR and HRV measurements are sensitive in diagnosing ANS dysfunction, not only in the late stages of PD but also in the early stages and can be used to diagnose autonomic derangement in PD patients. | NR |
| 3 | Liu et al. [11] (2014) | Stroke in PD | Self-reporting a specialist for the diagnosis | 32 | Cerebral infarction is intimately linked to PD due to cerebrovascular and neurodegenerative disorders coincide. Although levodopa causes OH and raised homocysteine, which may increase the risk of stroke, it remains the most effective and essential symptomatic therapy for many people with PD. | NR |
| 4 | Becker et al. [18] (2009) | Risk of stroke in PD | LBBM | NR | Hyperhomocysteinemia might be a relationship between PD and an increased risk of ischemic stroke. Homocysteine levels beyond a certain threshold have been proven to increase the risk of stroke and coronary artery disease. vascular disease and dementia, as well as the fact that levodopa treatment is linked to both with a rise in homocysteine in the blood. | NR |
| 5 | Levine et al. [141] (2009) | Traumatic brain injury in PD | LBBM | NR | A potential technique for reducing both physical and cognitive weariness in people with neurologic diseases is exercise training. In people with PD, a cardiovascular exercise plan can help to reduce overall weariness. | NR |
| 6 | Rickards [142] (2005) | Stroke in PD | NR | NR | Depressive syndromes in chronic neurological illnesses are common and disabling. Their etiology is complex and may be multifactorial in individual patients. | NR |
| 7 | Mastaglia et al. [143] (2002) | Prevalence stroke in PD | Self-reporting a specialist for the diagnosis | 100 | Postmortem investigation, studies did not directly compare our findings to other studies of stroke-related mortality and morbidity in the PD population. | NR |
| SN | Citations | IC | DS | GT | FE | TOC | ML vs. DL | ACC % | AUC |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Suri et al. [189] (2022) | OBBM, CUSIP | 117 | CVD, Bias | NR | NR | ML | NR | NR |
| 2 | Kandha et al. [190] (2020) | OBBM, LBBM | 346 | Death | DCNN | NB, SVM, KNN, DT | DL | 83.33 | 0.833 |
| 3 | Jamthikar et al. [30] (2020) | OBBM, LBBM, CUSIP | 202 | CVD | SVM | NR | ML | 92.53 | 0.92 |
| 4 | Skandha et al. [191] (2020) | OBBM, LBBM | 246 | Stroke | 11 Models | NR | HDL | 98.30 | 0.983 |
| 5 | Saba et al. [192] (2020) | OBBM, LBBM, CUSIP | 246 | Death | 6 Models | NR | HDL | 89.00 | 0.898 |
| 6 | Jamthikar et al. [177] (2019) | OBBM, LBBM (US) | 395 | CVD | PCA | RF | ML | 95.00 | 0.80 |
| 7 | Biswas et al. [193] (2018) | OBBM, LBBM (US) | 407 | Stroke, Diabetes | NR | CNN | DL | 99.61 | 0.99 |
| SN | Citations | IC | DS | GT | FE | TOC | ML vs. DL | ACC % | AUC |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Soun et al. [194] (2021) | LBBM (CT) | 209 | Stroke | NN | AlexNet | DL | 96.09 | 0.96 |
| 2 | Reva et al. [195] (2021) | OBBM, LBBM | 200 | Stroke, CT | NB | DT, RF, SVM | ML | 85.32 | NR |
| 3 | Murray et al. [9] (2020) | OBBM, LBBM | 341 | LVO, Stroke | RF | CNN | HDL | 85.00 | NR |
| 4 | Mouridsen et al. [196] (2020) | OBBM, LBBM, CUSIP | 16 | Stroke, MRI | NR | CNN | DL | 74.00 | 0.74 |
| 5 | Yu et al. [147] (2020) | OBBM, LBBM (EMG) | 287 | Stroke, EMG | SVM | RF, LSTM | ML | 98.33 | 0.98 |
| 6 | Ain et al. [197] (2020) | OBBM, LBBM | 130 | Stroke, non-stroke | NB | NB | ML | 84.00 | NR |
| 7 | Badriyah et al. [198] (2020) | OBBM (CT) | 29 | Stroke | NB | DT, RF, SVM | HDL | 94.30 | NR |
| SN | Citations | IC | DS | GT | FE | TOC | ML vs. DL | ACC % | AUC |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bikias et al. [199] (2021) | LBBM (FoG) | 18 | PD vs. Non PD | SVM | CNN | DL | 90.00 | NR |
| 2 | Pramanik et al. [200] (2021) | LBBM (Voice) | 252 | PD vs. Non PD | NB | RF | ML | 95.00 | NR |
| 3 | Borzì et al. [201] (2021) | OBBM, LBBM (FoG) | 11 | PD vs. Non PD | RF | NB | ML | 84.10 | NR |
| 4 | Aich et al. [202] (2020) | OBBM, LBBM (FoG) | 20 | PD vs. Non PD | RF | SVM, RF, KNN | ML | 97.35 | 0.74 |
| 5 | Pramanik et al. [203] (2021) | LBBM (Voice) | 169 | PD vs. Non PD | NB | SVM, RF | ML | 78.97 | 0.78 |
| 6 | Zahid et al. [204] (2020) | LBBM (Voice) | 50 | PD vs. Non PD | SVM | RF | HDL | 99.1 | NR |
| 7 | Nissar et al. [205] (2019) | LBBM (Voice) | 188 | PD vs. Non PD | NB | XGBoost | ML | 92.76 | NR |
| SN | Citations | Year | PD | CVD | Stroke | AI | COVID-19 |
|---|---|---|---|---|---|---|---|
| 1 | Li et al. [70] | 2018 | Y | N | Y | N | N |
| 2 | Jamthikar et al. [18] | 2020 | N | Y | N | Y | N |
| 3 | Mouridsen et al. [122] | 2020 | N | N | Y | Y | N |
| 4 | Bikias et al. [119] | 2021 | Y | N | N | Y | N |
| 5 | Reva et al. [120] | 2021 | N | N | Y | Y | N |
| 6 | Bermejo et al. [72] | 2021 | Y | Y | N | N | N |
| 7 | Pramanik et al. [121] | 2021 | Y | N | N | Y | N |
| 8 | Suri et al. (Proposed) | 2022 | Y | Y | Y | Y | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, J.S.; Paul, S.; Maindarkar, M.A.; Puvvula, A.; Saxena, S.; Saba, L.; Turk, M.; Laird, J.R.; Khanna, N.N.; Viskovic, K.; et al. Cardiovascular/Stroke Risk Stratification in Parkinson’s Disease Patients Using Atherosclerosis Pathway and Artificial Intelligence Paradigm: A Systematic Review. Metabolites 2022, 12, 312. https://doi.org/10.3390/metabo12040312
Suri JS, Paul S, Maindarkar MA, Puvvula A, Saxena S, Saba L, Turk M, Laird JR, Khanna NN, Viskovic K, et al. Cardiovascular/Stroke Risk Stratification in Parkinson’s Disease Patients Using Atherosclerosis Pathway and Artificial Intelligence Paradigm: A Systematic Review. Metabolites. 2022; 12(4):312. https://doi.org/10.3390/metabo12040312
Chicago/Turabian StyleSuri, Jasjit S., Sudip Paul, Maheshrao A. Maindarkar, Anudeep Puvvula, Sanjay Saxena, Luca Saba, Monika Turk, John R. Laird, Narendra N. Khanna, Klaudija Viskovic, and et al. 2022. "Cardiovascular/Stroke Risk Stratification in Parkinson’s Disease Patients Using Atherosclerosis Pathway and Artificial Intelligence Paradigm: A Systematic Review" Metabolites 12, no. 4: 312. https://doi.org/10.3390/metabo12040312
APA StyleSuri, J. S., Paul, S., Maindarkar, M. A., Puvvula, A., Saxena, S., Saba, L., Turk, M., Laird, J. R., Khanna, N. N., Viskovic, K., Singh, I. M., Kalra, M., Krishnan, P. R., Johri, A., & Paraskevas, K. I. (2022). Cardiovascular/Stroke Risk Stratification in Parkinson’s Disease Patients Using Atherosclerosis Pathway and Artificial Intelligence Paradigm: A Systematic Review. Metabolites, 12(4), 312. https://doi.org/10.3390/metabo12040312










