Low-Concentrations of Fatty Acids Induce an Early Increase in IL-8 Levels in Normal Human Astrocytes
Abstract
:1. Introduction
2. Results
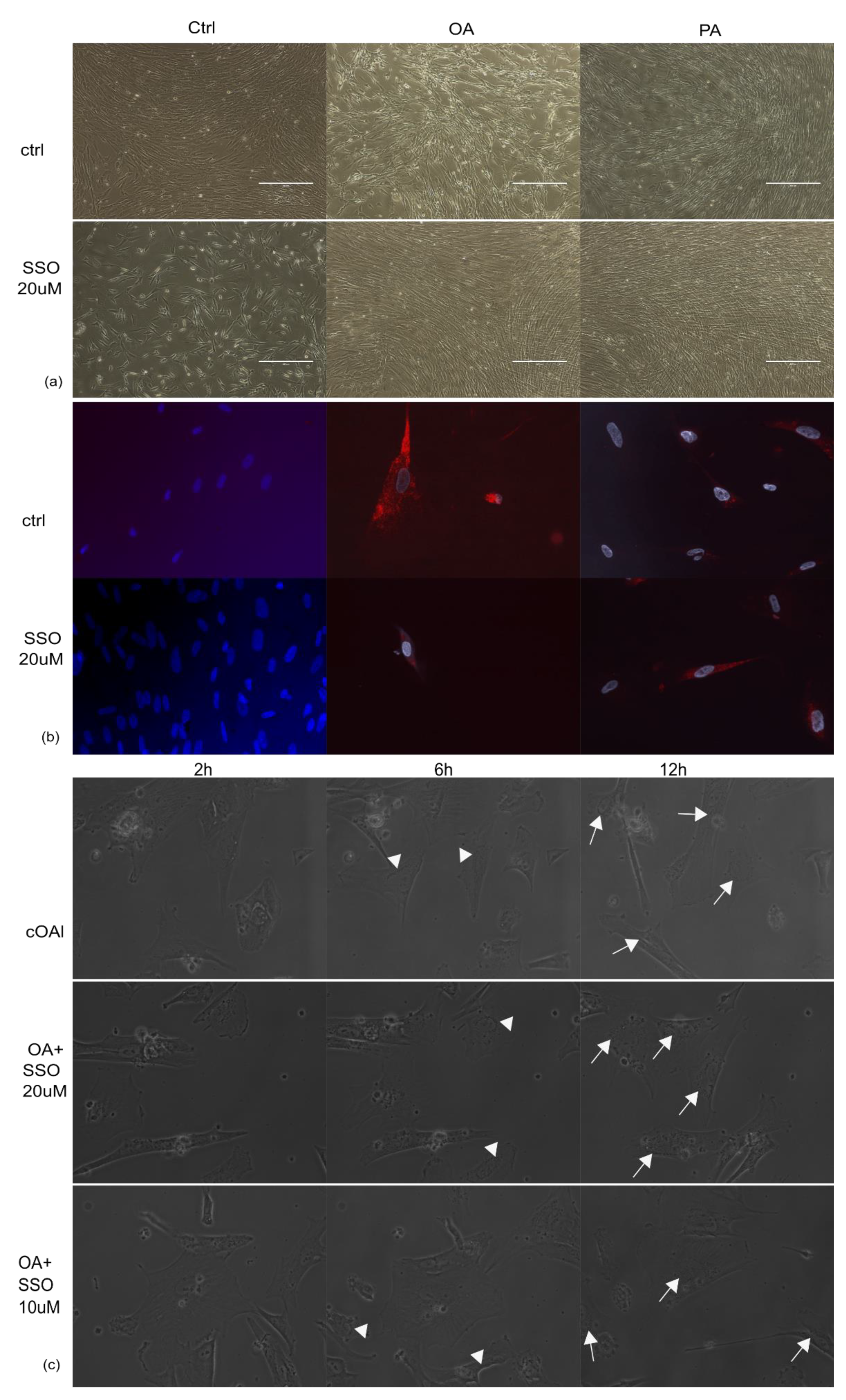
2.1. Astrocytes Are Able to Uptake Both Saturated and Unsaturated Fatty Acids, Which Are Not Impaired by Pre-Treatment with SSO
2.2. In the Presence of OA, SSO Short-Term Treatment Does Not Impair Long-Term Astrocytes Viability, as Assessed by Video Microscopy
2.3. Astrocytes Are Able to Produce Pro-Inflammatory Cytokines in Cell Culture, and Their Synthesis Is Lowered by SSO Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cell Treatments
4.2. Videomicroscopy
4.3. Electron Cryomicroscopy
4.4. ELISA
4.5. Multiplexing
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jump, D.B. Fatty Acid Regulation of Gene Transcription. Crit. Rev. Clin. Lab. Sci. 2004, 41, 41–78. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Zhao, W.; Bionaz, M.; Luo, J.; Loor, J.J. Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ? Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Ruiz, I.; Medina, M.Á.; Martínez-Poveda, B. From Food to Genes: Transcriptional Regulation of Metabolism by Lipids and Carbohydrates. Nutrients 2021, 13, 1513. [Google Scholar] [CrossRef]
- Daynes, R.A.; Jones, D.C. Emerging roles of PPARS in inflammation and immunity. Nat. Rev. Immunol. 2002, 2, 748–759. [Google Scholar] [CrossRef]
- Varga, T.; Nagy, L. Nuclear receptors, transcription factors linking lipid metabolism and immunity: The case of peroxisome proliferator-activated receptor gamma. Eur. J. Clin. Investig. 2008, 38, 695–707. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Dudau, M.; Vilceanu, A.C.; Codrici, E.; Mihai, S.; Popescu, I.D.; Albulescu, L.; Tarcomnicu, I.; Moise, G.; Ceafalan, L.C.; Hinescu, M.E.; et al. Sea-Buckthorn Seed Oil Induces Proliferation of both Normal and Dysplastic Keratinocytes in Basal Conditions and under UVA Irradiation. J. Pers. Med. 2021, 11, 278. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Saguie, B.O.; Pereira de Almeida Nogueira, N.; Paes, M.; dos Santos Valença, S.; Atella, G.C.; Monte-Alto-Costa, A. Oleic acid and hydroxytyrosol present in olive oil promote ROS and inflammatory response in normal cultures of murine dermal fibroblasts through the NF-κB and NRF2 pathways. Food Res. Int. 2020, 131, 108984. [Google Scholar] [CrossRef]
- Tanaka, S.; Saitoh, O.; Tabata, K.; Matsuse, R.; Kojima, K.; Sugi, K.; Nakagawa, K.; Kayazawa, M.; Teranishi, T.; Uchida, K.; et al. Medium-chain fatty acids stimulate interleukin-8 production in Caco-2 cells with different mechanisms from long-chain fatty acids1. J. Gastroenterol. Hepatol. 2001, 16, 748–754. [Google Scholar] [CrossRef]
- Chávez-Tapia, N.C.; Rosso, N.; Uribe, M.; Bojalil, R.; Tiribelli, C. Kinetics of the inflammatory response induced by free fatty acid accumulation in hepatocytes. Ann. Hepatol. 2014, 13, 113–120. [Google Scholar] [CrossRef]
- Pillon, N.J.; Arane, K.; Bilan, P.J.; Chiu, T.T.; Klip, A. Muscle cells challenged with saturated fatty acids mount an autonomous inflammatory response that activates macrophages. Cell Commun. Signal. 2012, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coraci, I.S.; Husemann, J.; Berman, J.W.; Hulette, C.; Dufour, J.H.; Campanella, G.K.; Luster, A.D.; Silverstein, S.C.; El Khoury, J.B. CD36, a Class B Scavenger Receptor, Is Expressed on Microglia in Alzheimer’s Disease Brains and Can Mediate Production of Reactive Oxygen Species in Response to β-Amyloid Fibrils. Am. J. Pathol. 2002, 160, 101–112. [Google Scholar] [CrossRef]
- Yamanaka, M.; Ishikawa, T.; Griep, A.; Axt, D.; Kummer, M.P.; Heneka, M.T. PPAR /RXR -Induced and CD36-Mediated Microglial Amyloid-Phagocytosis Results in Cognitive Improvement in Amyloid Precursor Protein/Presenilin 1 Mice. J. Neurosci. 2012, 32, 17321–17331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doens, D.; Valiente, P.A.; Mfuh, A.M.; X. T. Vo, A.; Tristan, A.; Carreño, L.; Quijada, M.; Nguyen, V.T.; Perry, G.; Larionov, O.V.; et al. Identification of Inhibitors of CD36-Amyloid Beta Binding as Potential Agents for Alzheimer’s Disease. ACS Chem. Neurosci. 2017, 8, 1232–1241. [Google Scholar] [CrossRef]
- Hou, J.; Jeon, B.; Baek, J.; Yun, Y.; Kim, D.; Chang, B.; Kim, S.; Kim, S. High fat diet-induced brain damaging effects through autophagy-mediated senescence, inflammation and apoptosis mitigated by ginsenoside F1-enhanced mixture. J. Ginseng Res. 2022, 46, 79–90. [Google Scholar] [CrossRef]
- Chen, Z.; Nie, S.-D.; Qu, M.-L.; Zhou, D.; Wu, L.-Y.; Shi, X.-J.; Ma, L.-R.; Li, X.; Zhou, S.-L.; Wang, S.; et al. The autophagic degradation of Cav-1 contributes to PA-induced apoptosis and inflammation of astrocytes. Cell Death Dis. 2018, 9, 771. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Rodriguez, A.; Acaz-Fonseca, E.; Boya, P.; Arevalo, M.A.; Garcia-Segura, L.M. Lipotoxic Effects of Palmitic Acid on Astrocytes Are Associated with Autophagy Impairment. Mol. Neurobiol. 2019, 56, 1665–1680. [Google Scholar] [CrossRef]
- Fatima, S.; Hu, X.; Gong, R.-H.; Huang, C.; Chen, M.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Palmitic acid is an intracellular signaling molecule involved in disease development. Cell. Mol. Life Sci. 2019, 76, 2547–2557. [Google Scholar] [CrossRef]
- Gupta, S.; Knight, A.G.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J. Neurochem. 2012, 120, 1060–1071. [Google Scholar] [CrossRef] [Green Version]
- Dhungana, H.; Huuskonen, M.T.; Jaronen, M.; Lemarchant, S.; Ali, H.; Keksa-Goldsteine, V.; Goldsteins, G.; Kanninen, K.M.; Koistinaho, J.; Malm, T. Sulfosuccinimidyl oleate sodium is neuroprotective and alleviates stroke-induced neuroinflammation. J. Neuroinflamm. 2017, 14, 237. [Google Scholar] [CrossRef] [Green Version]
- Kuda, O.; Pietka, T.A.; Demianova, Z.; Kudova, E.; Cvacka, J.; Kopecky, J.; Abumrad, N.A. Sulfo-N-succinimidyl Oleate (SSO) Inhibits Fatty Acid Uptake and Signaling for Intracellular Calcium via Binding CD36 Lysine 164. J. Biol. Chem. 2013, 288, 15547–15555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yan, Z.; Hu, J.; Shen, W.-J.; Azhar, S.; Kraemer, F.B. Scavenger receptor class B, type 1 facilitates cellular fatty acid uptake. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158554. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human Astrocytes: Secretome Profiles of Cytokines and Chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloisi, F.; Carè, A.; Borsellino, G.; Gallo, P.; Rosa, S.; Bassani, A.; Cabibbo, A.; Testa, U.; Levi, G.; Peschle, C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J. Immunol. 1992, 149, 2358–2366. [Google Scholar]
- Meeuwsen, S.; Persoon-Deen, C.; Bsibsi, M.; Ravid, R.; Noort, J.M. Van Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia 2003, 43, 243–253. [Google Scholar] [CrossRef]
- Kutsch, O.; Oh, J.-W.; Nath, A.; Benveniste, E.N. Induction of the Chemokines Interleukin-8 and IP-10 by Human Immunodeficiency Virus Type 1 Tat in Astrocytes. J. Virol. 2000, 74, 9214–9221. [Google Scholar] [CrossRef] [Green Version]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Aguirre-Rueda, D.; Guerra-Ojeda, S.; Aldasoro, M.; Iradi, A.; Obrador, E.; Ortega, A.; Mauricio, M.D.; Vila, J.M.; Valles, S.L. Astrocytes Protect Neurons from Aβ 1-42 Peptide-Induced Neurotoxicity Increasing TFAM and PGC-1 and Decreasing PPAR-γ and SIRT-1. Int. J. Med. Sci. 2015, 12, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.-L.; Wu, Y.-R.; Cheng, K.-S.; Chan, P.; Cheung, C.-W.; Lu, D.-Y.; Su, T.-H.; Liu, Z.-M.; Leung, Y.-M. Palmitic acid-induced lipotoxicity and protection by (+)-catechin in rat cortical astrocytes. Pharmacol. Rep. 2014, 66, 1106–1113. [Google Scholar] [CrossRef]
- Hrometz, S.L.; Ebert, J.A.; Grice, K.E.; Nowinski, S.M.; Mills, E.M.; Myers, B.J.; Sprague, J.E. Potentiation of Ecstasy-induced hyperthermia and FAT/CD36 expression in chronically exercised animals. Temperature 2016, 3, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Xu, W.; Yu, Q.; Yang, Q. 4,4′-Diaponeurosporene-Producing Bacillus subtilis Increased Mouse Resistance against Salmonella typhimurium Infection in a CD36-Dependent Manner. Front. Immunol. 2017, 8, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurvitz, A.; Rottensteiner, H. The biochemistry of oleate induction: Transcriptional upregulation and peroxisome proliferation. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 1392–1402. [Google Scholar] [CrossRef]
- Bento-Abreu, A.; Tabernero, A.; Medina, J.M. Peroxisome proliferator-activated receptor-alpha is required for the neurotrophic effect of oleic acid in neurons. J. Neurochem. 2007, 103, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Giménez, E.; Toscani, A.M. Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.-S.; Lee, D.H.; Lee, S.H.; Park, H.J.; Lee, D.; Kim, H. Neuroprotective effects of oleic acid in rodent models of cerebral ischaemia. Sci. Rep. 2019, 9, 10732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLarnon, J.G. Chemokine Interleukin-8 (IL-8) in Alzheimer’s and Other Neurodegenerative Diseases. J. Alzheimer’s Dis. Park. 2016, 6, 273. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Zhang, Y.; Hu, L.; Li, Q.; Zhou, H. Febuxostat Inhibits MPP+-Induced Inflammatory Response Through Inhibiting the JNK/NF-κB Pathway in Astrocytes. Neurotox. Res. 2021, 39, 566–574. [Google Scholar] [CrossRef]
- Phuagkhaopong, S.; Ospondpant, D.; Kasemsuk, T.; Sibmooh, N.; Soodvilai, S.; Power, C.; Vivithanaporn, P. Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-κB pathways. Neurotoxicology 2017, 60, 82–91. [Google Scholar] [CrossRef]
- Bahniwal, M.; Little, J.P.; Klegeris, A. High Glucose Enhances Neurotoxicity and Inflammatory Cytokine Secretion by Stimulated Human Astrocytes. Curr. Alzheimer Res. 2017, 14, 731–741. [Google Scholar] [CrossRef]
- Danik, M.; Puma, C.; Quirion, R.; Williams, S. Widely expressed transcripts for chemokine receptor CXCR1 in identified glutamatergic, γ-aminobutyric acidergic, and cholinergic neurons and astrocytes of the rat brain: A single-cell reverse transcription-multiplex polymerase chain reaction study. J. Neurosci. Res. 2003, 74, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Williams, W.; Wang, B.; Wei, J.; Lu, X.; Cheng, J.-W.; Gordon, J.R.; Li, J.-M.; Li, F. Cytotoxic effect of interleukin-8 in retinal ganglion cells and its possible mechanisms. Int. J. Ophthalmol. 2018, 11, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Puma, C.; Danik, M.; Quirion, R.; Ramon, F.; Williams, S. The chemokine interleukin-8 acutely reduces Ca2+ currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J. Neurochem. 2001, 78, 960–971. [Google Scholar] [CrossRef]
- Ryu, J.K.; Cho, T.; Choi, H.B.; Jantaratnotai, N.; McLarnon, J.G. Pharmacological antagonism of interleukin-8 receptor CXCR2 inhibits inflammatory reactivity and is neuroprotective in an animal model of Alzheimer’s disease. J. Neuroinflamm. 2015, 12, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bylicky, M.A.; Mueller, G.P.; Day, R.M. Mechanisms of Endogenous Neuroprotective Effects of Astrocytes in Brain Injury. Oxid. Med. Cell. Longev. 2018, 2018, 6501031. [Google Scholar] [CrossRef]
- Jay, A.G.; Simard, J.R.; Huang, N.; Hamilton, J.A. SSO and other putative inhibitors of FA transport across membranes by CD36 disrupt intracellular metabolism, but do not affect FA translocation. J. Lipid Res. 2020, 61, 790–807. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobri, A.-M.; Codrici, E.; Popescu, I.-D.; Albulescu, L.; Fertig, E.T.; Enciu, A.-M.; Tanase, C.; Hinescu, M.E. Low-Concentrations of Fatty Acids Induce an Early Increase in IL-8 Levels in Normal Human Astrocytes. Metabolites 2022, 12, 329. https://doi.org/10.3390/metabo12040329
Dobri A-M, Codrici E, Popescu I-D, Albulescu L, Fertig ET, Enciu A-M, Tanase C, Hinescu ME. Low-Concentrations of Fatty Acids Induce an Early Increase in IL-8 Levels in Normal Human Astrocytes. Metabolites. 2022; 12(4):329. https://doi.org/10.3390/metabo12040329
Chicago/Turabian StyleDobri, Ana-Maria, Elena Codrici, Ionela-Daniela Popescu, Lucian Albulescu, Emanuel Tudor Fertig, Ana-Maria Enciu, Cristiana Tanase, and Mihail E. Hinescu. 2022. "Low-Concentrations of Fatty Acids Induce an Early Increase in IL-8 Levels in Normal Human Astrocytes" Metabolites 12, no. 4: 329. https://doi.org/10.3390/metabo12040329
APA StyleDobri, A. -M., Codrici, E., Popescu, I. -D., Albulescu, L., Fertig, E. T., Enciu, A. -M., Tanase, C., & Hinescu, M. E. (2022). Low-Concentrations of Fatty Acids Induce an Early Increase in IL-8 Levels in Normal Human Astrocytes. Metabolites, 12(4), 329. https://doi.org/10.3390/metabo12040329






