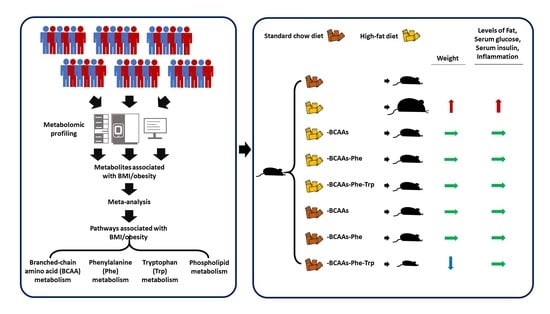
Restricting Branched-Chain Amino Acids within a High-Fat Diet Prevents Obesity
Abstract
1. Introduction
2. Results
2.1. BMI/Obesity Associated Metabolites and Pathways
2.2. Relationships between BMI and Obesity Associated Metabolites and Diabetes, and Levels of Serum Glucose, Insulin and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
2.3. Relationships between BMI and Obesity Associated Metabolites and Blood Lipids Profile in the CODING and NFOAS Cohorts
2.4. Relationships between BMI and Oobesity Associated Metabolites and Visceral Fat Mass in the CODING Cohort
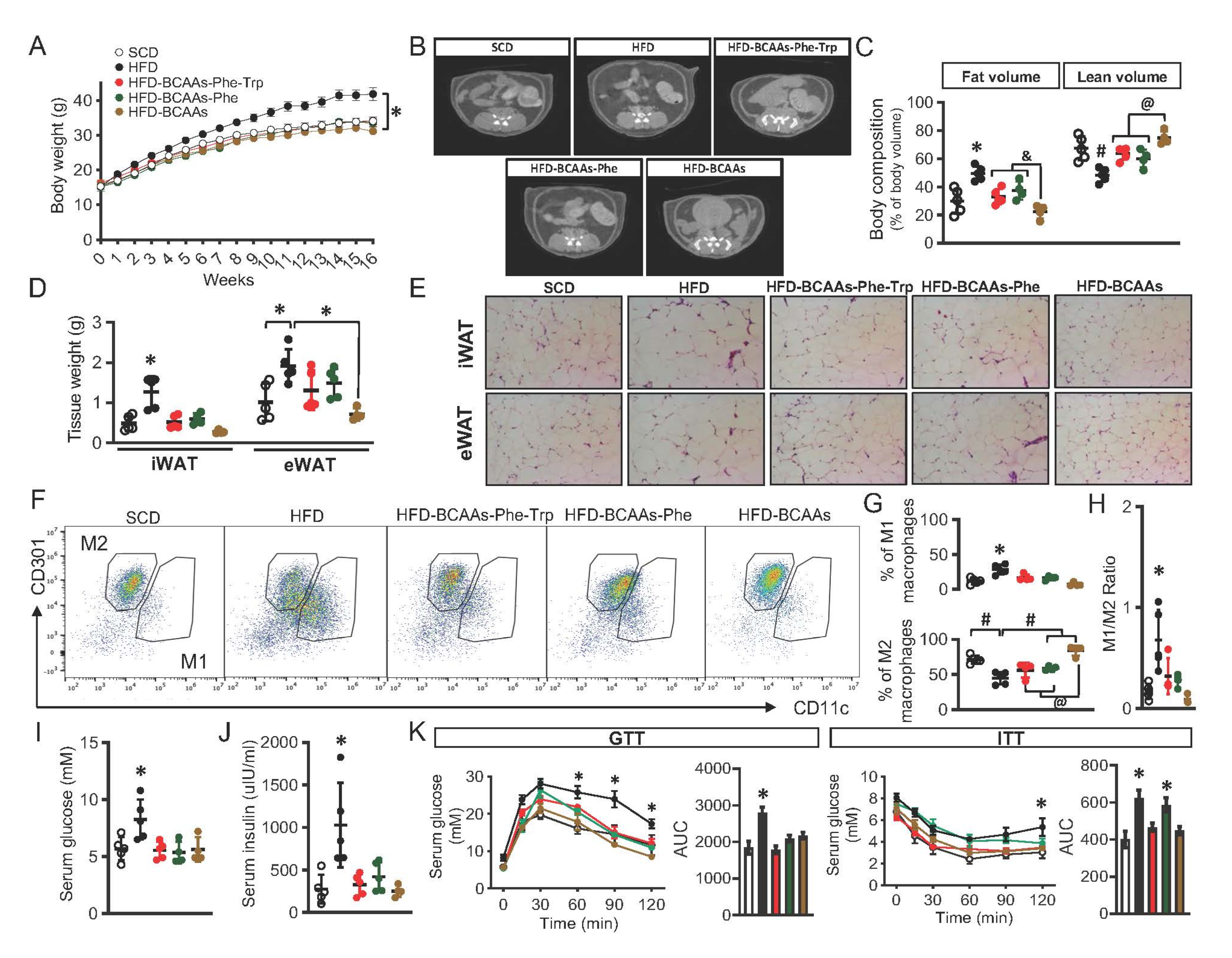
2.5. Amino Acids Restriction and Obesity in Mice
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Metabolomics Profiling
4.3. Demographic Data Collection and Biochemical Parameter Measurement
4.4. Experimental Animals and Dietary Regimens
4.5. Metabolic Assessment, Body Composition Evaluation, and Inflammation Marker Measurement in Mice
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, e65174. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Leeds, A.R.; Christensen, R. Osteoarthritis, obesity and weight loss: Evidence, hypotheses and horizons—A scoping review. Obes. Rev. 2014, 15, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Amiri, S.; Behnezhad, S. Obesity and anxiety symptoms: A systematic review and meta-analysis. Neuropsychiatr 2019, 33, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Kjellberg, J.; Tange Larsen, A.; Ibsen, R.; Hojgaard, B. The Socioeconomic Burden of Obesity. Obes. Facts 2017, 10, 493–502. [Google Scholar] [CrossRef]
- Divino, V.; Ramasamy, A.; Anupindi, V.R.; Eriksen, K.T.; Olsen, A.H.; DeKoven, M.; Meincke, H.H. Complication-specific direct medical costs by body mass index for 13 obesity-related complications: A retrospective database study. J. Manag. Care Spec. Pharm. 2021, 27, 210–222. [Google Scholar] [CrossRef]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef]
- McGranaghan, P.; Saxena, A.; Rubens, M.; Radenkovic, J.; Bach, D.; Schleussner, L.; Pieske, B.; Edelmann, F.; Trippel, T.D. Predictive value of metabolomic biomarkers for cardiovascular disease risk: A systematic review and meta-analysis. Biomarkers 2020, 25, 101–111. [Google Scholar] [CrossRef]
- Bereman, M.S.; Kirkwood, K.I.; Sabaretnam, T.; Furlong, S.; Rowe, D.B.; Guillemin, G.J.; Mellinger, A.L.; Muddiman, D.C. Metabolite Profiling Reveals Predictive Biomarkers and the Absence of beta-Methyl Amino-l-alanine in Plasma from Individuals Diagnosed with Amyotrophic Lateral Sclerosis. J. Proteome Res. 2020, 19, 3276–3285. [Google Scholar] [CrossRef]
- Kelly, R.S.; Vander Heiden, M.G.; Giovannucci, E.; Mucci, L.A. Metabolomic Biomarkers of Prostate Cancer: Prediction, Diagnosis, Progression, Prognosis, and Recurrence. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016, 55, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Lam, S.M.; Fan, X.; Cao, W.J.; Wang, S.Y.; Tian, H.; Chua, G.H.; Zhang, C.; Meng, F.P.; Xu, Z.; et al. Omics-Driven Systems Interrogation of Metabolic Dysregulation in COVID-19 Pathogenesis. Cell Metab. 2020, 32, 188–202. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Stroeve, J.H.; Saccenti, E.; Bouwman, J.; Dane, A.; Strassburg, K.; Vervoort, J.; Hankemeier, T.; Astrup, A.; Smilde, A.K.; van Ommen, B.; et al. Weight loss predictability by plasma metabolic signatures in adults with obesity and morbid obesity of the DiOGenes study. Obesity 2016, 24, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef]
- White, P.J.; Lapworth, A.L.; McGarrah, R.W.; Kwee, L.C.; Crown, S.B.; Ilkayeva, O.; An, J.; Carson, M.W.; Christopher, B.A.; Ball, J.R.; et al. Muscle-Liver Trafficking of BCAA-Derived Nitrogen Underlies Obesity-Related Glycine Depletion. Cell Rep. 2020, 33, 108375. [Google Scholar] [CrossRef]
- Chapman, K.A.; Bennett, M.J.; Sondheimer, N. Increased C3-carnitine in a healthy premature infant. Clin. Chem. 2008, 54, 1914–1917. [Google Scholar] [CrossRef][Green Version]
- Bagheri, M.; Djazayery, A.; Farzadfar, F.; Qi, L.; Yekaninejad, M.S.; Aslibekyan, S.; Chamari, M.; Hassani, H.; Koletzko, B.; Uhl, O. Plasma metabolomic profiling of amino acids and polar lipids in Iranian obese adults. Lipids Health Dis. 2019, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Hu, H.; Qu, S.; Wang, J.; Hua, C.; Zhang, J.; Wei, P.; He, X.; Hao, J.; Liu, P.; et al. SIRT5 deacylates metabolism-related proteins and attenuates hepatic steatosis in ob/ob mice. EBioMedicine 2018, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl coenzyme A: A central metabolite and second messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef]
- Mosaoa, R.; Kasprzyk-Pawelec, A.; Fernandez, H.R.; Avantaggiati, M.L. The Mitochondrial Citrate Carrier SLC25A1/CIC and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond. Biomolecules 2021, 11, 141. [Google Scholar] [CrossRef]
- Rauschert, S.; Uhl, O.; Koletzko, B.; Kirchberg, F.; Mori, T.A.; Huang, R.C.; Beilin, L.J.; Hellmuth, C.; Oddy, W.H. Lipidomics Reveals Associations of Phospholipids With Obesity and Insulin Resistance in Young Adults. J. Clin. Endocrinol. Metab. 2016, 101, 871–879. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Simonen, P.; Gylling, H.; Howard, A.N.; Miettinen, T.A. Introducing a new component of the metabolic syndrome: Low cholesterol absorption. Am. J. Clin. Nutr. 2000, 72, 82–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Bai, M.; Liu, Y.; Zhang, L.; Zhu, Q.; Bi, Y.; Ning, G.; Zhou, L.; Wang, X. The pivotal role of protein acetylation in linking glucose and fatty acid metabolism to beta-cell function. Cell Death Dis. 2019, 10, 66. [Google Scholar] [CrossRef]
- Felig, P.; Marliss, E.; Cahill, G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the Three Proteogenic Aromatic Amino Acids and Their Role in the Health of Plants and Animals. Front Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N.; et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. 2016, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Favennec, M.; Hennart, B.; Caiazzo, R.; Leloire, A.; Yengo, L.; Verbanck, M.; Arredouani, A.; Marre, M.; Pigeyre, M.; Bessede, A.; et al. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity 2015, 23, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Arriola Apelo, S.I.; et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021, 33, 905–922. [Google Scholar] [CrossRef]
- Thapa, B.; Lee, K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019, 52, 360–372. [Google Scholar] [CrossRef]
- Karusheva, Y.; Koessler, T.; Strassburger, K.; Markgraf, D.; Mastrototaro, L.; Jelenik, T.; Simon, M.C.; Pesta, D.; Zaharia, O.P.; Bodis, K.; et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2019, 110, 1098–1107. [Google Scholar] [CrossRef]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef]
- Sonoda, J.; Chen, M.Z.; Baruch, A. FGF21-receptor agonists: An emerging therapeutic class for obesity-related diseases. Horm. Mol. Biol. Clin. Investig. 2017, 19. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; Zhang, G.F.; Christopher, B.A.; Deleye, Y.; Walejko, J.M.; Page, S.; Ilkayeva, O.; White, P.J.; Newgard, C.B. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E216–E223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shao, J.; Wu, C.Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Wang, J.; et al. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, I.; Taylor, M.; Phillips, B.; Wilkinson, D.; Smith, K.; Hession, K.; Idris, I.; Atherton, P. A Novel Dietary Intervention Reduces Circulatory Branched-Chain Amino Acids by 50%: A Pilot Study of Relevance for Obesity and Diabetes. Nutrients 2020, 13, 95. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, W.; Wang, Y.; Pedram, P.; Cahill, F.; Zhai, G.; Randell, E.; Gulliver, W.; Sun, G. Serum metabolic biomarkers distinguish metabolically healthy peripherally obese from unhealthy centrally obese individuals. Nutr. Metab. 2016, 13, 33. [Google Scholar] [CrossRef]
- Power, J.D.; Perruccio, A.V.; Gandhi, R.; Veillette, C.; Davey, J.R.; Syed, K.; Mahomed, N.N.; Rampersaud, Y.R. Neuropathic pain in end-stage hip and knee osteoarthritis: Differential associations with patient-reported pain at rest and pain on activity. Osteoarthr. Cartil. 2018, 26, 363–369. [Google Scholar] [CrossRef]
- Raynauld, J.P.; Martel-Pelletier, J.; Bias, P.; Laufer, S.; Haraoui, B.; Choquette, D.; Beaulieu, A.D.; Abram, F.; Dorais, M.; Vignon, E.; et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: A first multicentre clinical trial using quantitative MRI. Ann. Rheum. Dis. 2009, 68, 938–947. [Google Scholar] [CrossRef]
- Zhai, G.; Pelletier, J.P.; Liu, M.; Aitken, D.; Randell, E.; Rahman, P.; Jones, G.; Martel-Pelletier, J. Activation of The Phosphatidylcholine to Lysophosphatidylcholine Pathway Is Associated with Osteoarthritis Knee Cartilage Volume Loss Over Time. Sci. Rep. 2019, 9, 9648. [Google Scholar] [CrossRef]
- Zhai, G.; Pelletier, J.P.; Liu, M.; Randell, E.W.; Rahman, P.; Martel-Pelletier, J. Serum lysophosphatidylcholines to phosphatidylcholines ratio is associated with symptomatic responders to symptomatic drugs in knee osteoarthritis patients. Arthritis Res. Ther. 2019, 21, 224. [Google Scholar] [CrossRef]
- Costello, C.A.; Hu, T.; Liu, M.; Zhang, W.; Furey, A.; Fan, Z.; Rahman, P.; Randell, E.W.; Zhai, G. Metabolomics Signature for Non-Responders to Total Joint Replacement Surgery in Primary Osteoarthritis Patients: The Newfoundland Osteoarthritis Study. J. Orthop. Res. 2020, 38, 793–802. [Google Scholar] [CrossRef]
- Liu, M.; Xie, Z.; Costello, C.A.; Zhang, W.; Chen, L.; Qi, D.; Furey, A.; Randell, E.W.; Rahman, P.; Zhai, G. Metabolomic analysis coupled with extreme phenotype sampling identified that lysophosphatidylcholines are associated with multisite musculoskeletal pain. Pain 2021, 162, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Liu, M.; Randell, E.W.; Rahman, P.; Jones, G.; Zhai, G. Sphingomyelin is involved in multisite musculoskeletal pain: Evidence from metabolomic analysis in two independent cohorts. Pain 2020, 162, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.A.; Nevitt, M.C.; Gross, K.D.; Hietpas, J.; Glass, N.A.; Lewis, C.E.; Torner, J.C. The Multicenter Osteoarthritis Study: Opportunities for rehabilitation research. PM R 2013, 5, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Sun, X.; Randell, E.W.; Liu, M.; Wang, N.; Tolstykh, I.; Rahman, P.; Torner, J.; Lewis, C.E.; Nevitt, M.C.; et al. Phenylalanine Is a Novel Marker for Radiographic Knee Osteoarthritis Progression: The MOST Study. J. Rheumatol. 2021, 48, 123–128. [Google Scholar] [CrossRef]
- Zhai, G.; Wang-Sattler, R.; Hart, D.J.; Arden, N.K.; Hakim, A.J.; Illig, T.; Spector, T.D. Serum branched-chain amino acid to histidine ratio: A novel metabolomic biomarker of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1227–1231. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-Moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The Bovine Metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef]
- Gao, X.; Randell, E.; Tian, Y.; Zhou, H.; Sun, G. Low serum choline and high serum betaine levels are associated with favorable components of metabolic syndrome in Newfoundland population. J. Diabetes Complicat. 2019, 33, 107398. [Google Scholar] [CrossRef]
- Gao, X.; Tian, Y.; Randell, E.; Zhou, H.; Sun, G. Unfavorable Associations Between Serum Trimethylamine N-Oxide and L-Carnitine Levels With Components of Metabolic Syndrome in the Newfoundland Population. Front Endocrinol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Liu, T.; Nie, X.; Wu, Z.; Zhang, Y.; Feng, G.; Cai, S.; Lv, Y.; Peng, X. Can statistic adjustment of OR minimize the potential confounding bias for meta-analysis of case-control study? A secondary data analysis. BMC Med. Res. Methodol. 2017, 17, 179. [Google Scholar] [CrossRef]
- Chang, W. R Graphics Cookbook; O’Reilly: Beijing, China; Sebastopol, CA, USA, 2013. [Google Scholar]



| Cohort | Population Location | N | Ethnicity (% of Caucasian) | Age (Years) | Sex (% of Females) | BMI (kg/m2) | Obese (%) | Normal Weight (%) | Prevalence of Diabetes (%) |
|---|---|---|---|---|---|---|---|---|---|
| CODING | Canada | 226 | 100% | 48.9 ± 12.7 | 60% | 28.9 ± 5.1 | 45% | 26% | 15% |
| LEAP-OA | Canada | 495 | 82% | 65.5 ± 8.4 | 57% | 30.8 ± 6.0 | 51% | 15% | 15% |
| Licofelone/naproxen clinical trial | Canada | 158 | 98% | 60.7 ± 8.0 | 69% | 31.8 ± 5.7 | 59% | 11% | 8% |
| MOST | U.S. | 1248 | 85% | 61.8 ± 7.8 | 62% | 30.6 ± 5.8 | 48% | 15% | - |
| NFOAS | Canada | 704 | 99% | 65.4 ± 9.6 | 55% | 33.3 ± 7.0 | 65% | 8% | 19% |
| TASOAC | Australia | 566 | 98% | 64.1 ± 6.6 | 52% | 27.8 ± 4.7 | 27% | 30% | 2% |
| Total | 3397 | 91% | 62.6 ± 9.4 | 58% | 30.7 ± 6.1 | 49% | 16% | 12% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Huang, Y.; Zhang, H.; Aitken, D.; Nevitt, M.C.; Rockel, J.S.; Pelletier, J.-P.; Lewis, C.E.; Torner, J.; Rampersaud, Y.R.; et al. Restricting Branched-Chain Amino Acids within a High-Fat Diet Prevents Obesity. Metabolites 2022, 12, 334. https://doi.org/10.3390/metabo12040334
Liu M, Huang Y, Zhang H, Aitken D, Nevitt MC, Rockel JS, Pelletier J-P, Lewis CE, Torner J, Rampersaud YR, et al. Restricting Branched-Chain Amino Acids within a High-Fat Diet Prevents Obesity. Metabolites. 2022; 12(4):334. https://doi.org/10.3390/metabo12040334
Chicago/Turabian StyleLiu, Ming, Yiheng Huang, Hongwei Zhang, Dawn Aitken, Michael C. Nevitt, Jason S. Rockel, Jean-Pierre Pelletier, Cora E. Lewis, James Torner, Yoga Raja Rampersaud, and et al. 2022. "Restricting Branched-Chain Amino Acids within a High-Fat Diet Prevents Obesity" Metabolites 12, no. 4: 334. https://doi.org/10.3390/metabo12040334
APA StyleLiu, M., Huang, Y., Zhang, H., Aitken, D., Nevitt, M. C., Rockel, J. S., Pelletier, J.-P., Lewis, C. E., Torner, J., Rampersaud, Y. R., Perruccio, A. V., Mahomed, N. N., Furey, A., Randell, E. W., Rahman, P., Sun, G., Martel-Pelletier, J., Kapoor, M., Jones, G., ... Zhai, G. (2022). Restricting Branched-Chain Amino Acids within a High-Fat Diet Prevents Obesity. Metabolites, 12(4), 334. https://doi.org/10.3390/metabo12040334