Development of Fermented Shrimp Shell Product with Hypoglycemic and Hypolipidemic Effects on Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Changes in the Contents of Components within Fermented SS
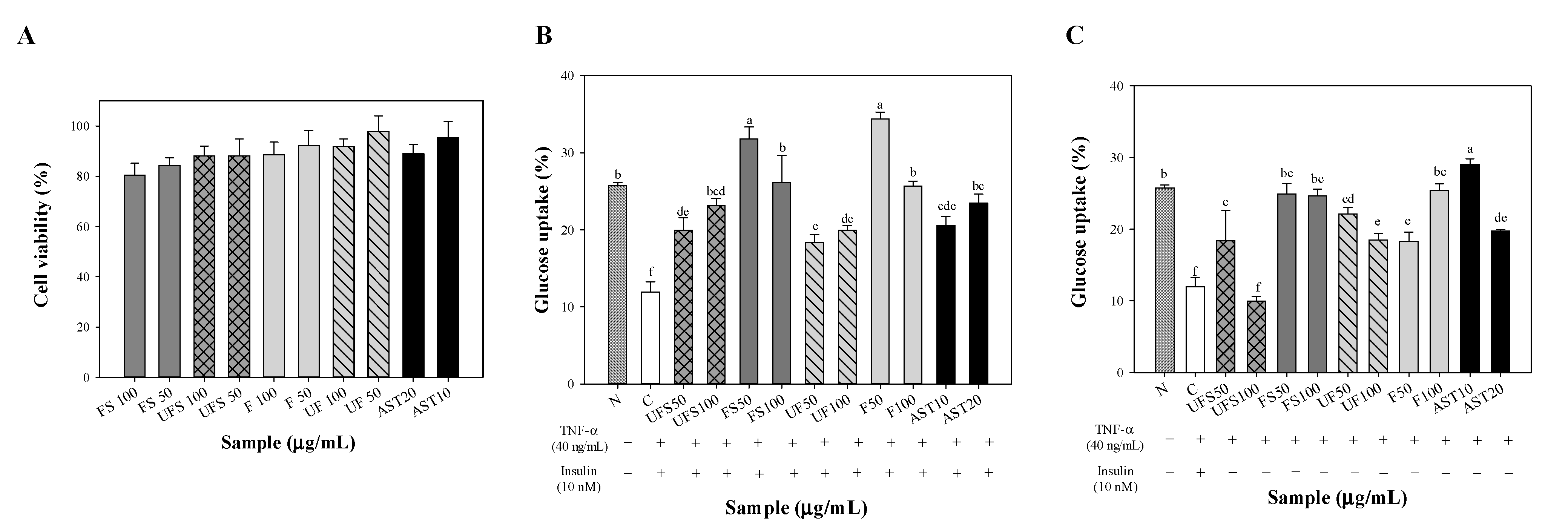
2.2. Effect of Fermented SS on Upregulating Glucose Uptake of FL83B Hepatocytes
2.3. Influence of Fermented SS on the General Physiological Parameters of Diabetic Rats
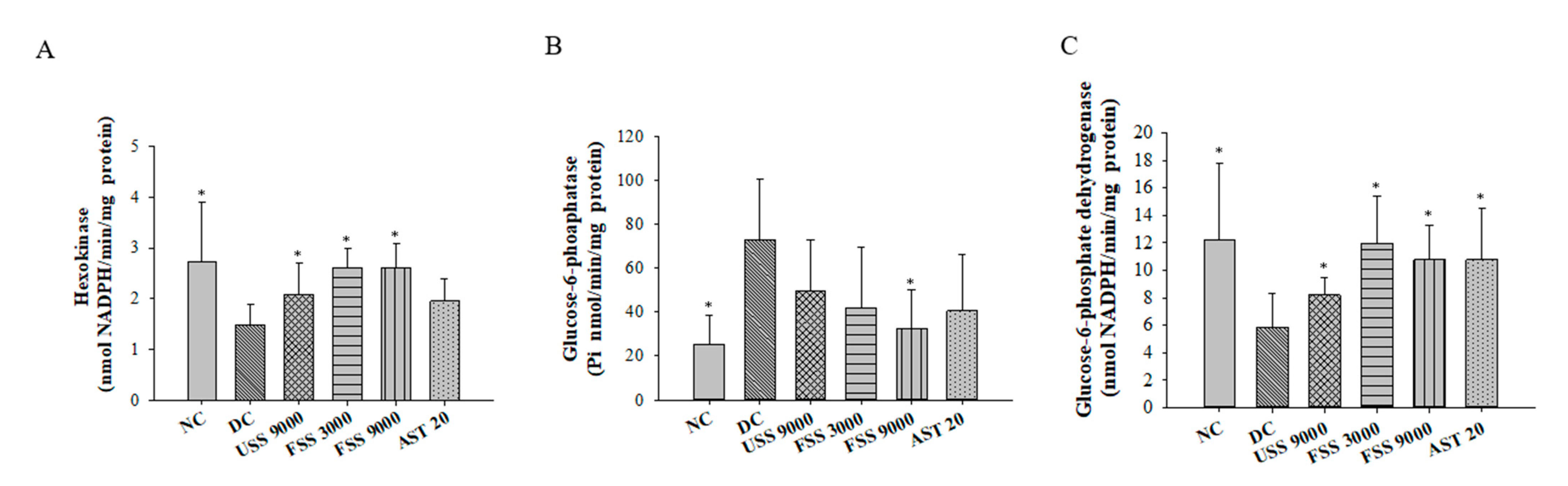
2.4. Oral Administation with Fermented SS Alleviated Hyperglycemia in Diabetic Rats
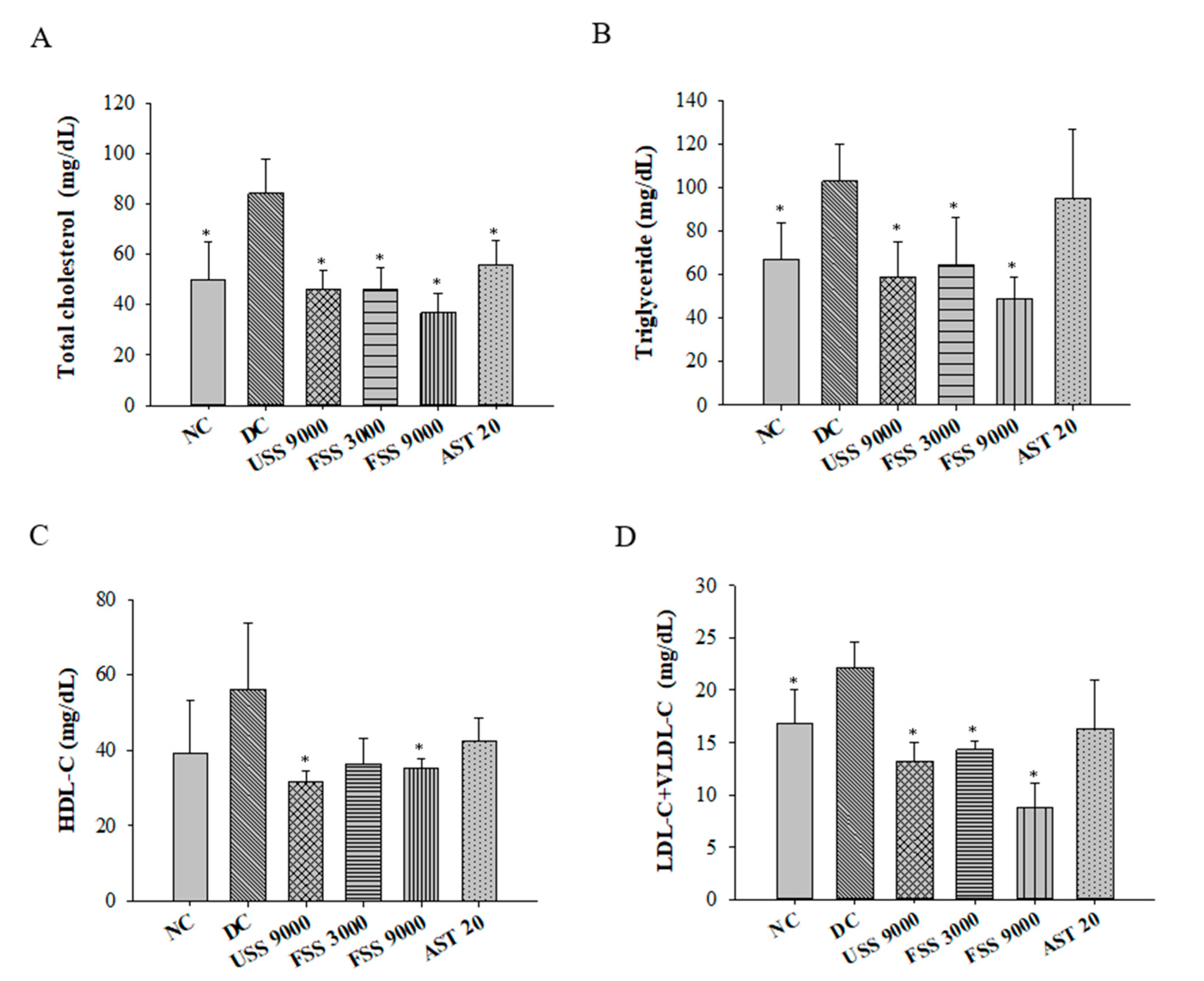
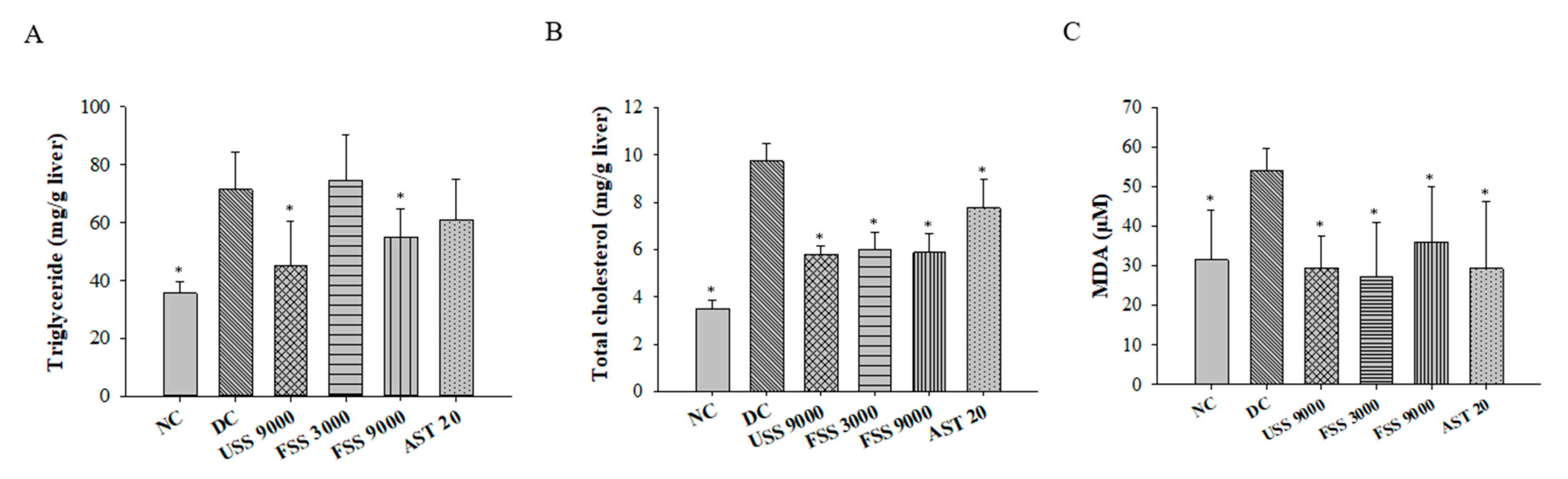
2.5. Oral Administation with Fermented SS Attenuated Dyslipidemia in Diabetic Rats
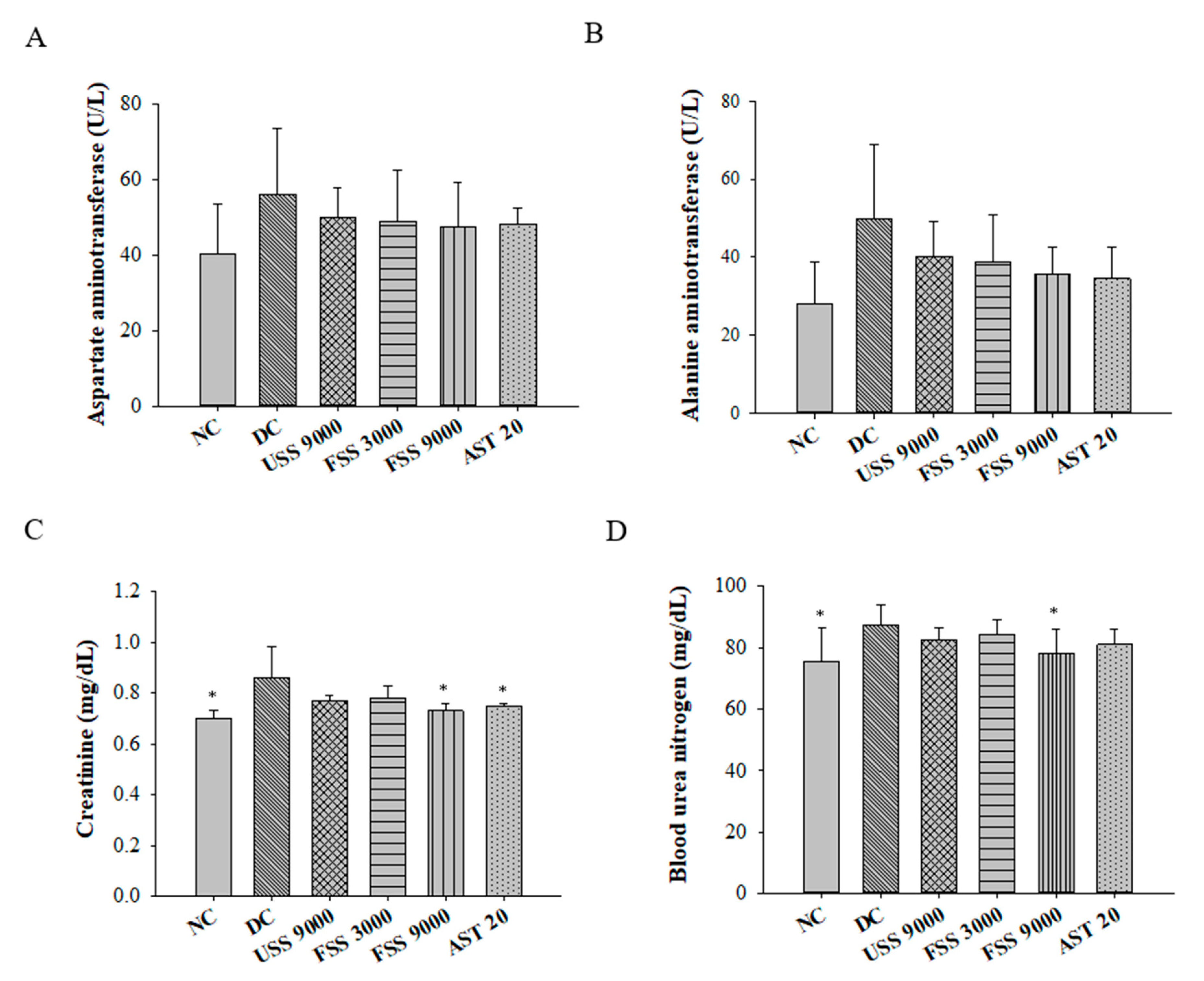
2.6. Oral Administation with Fermented SS Improves Kidney Function in Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Shrimp Shells (SSs), Microorganisms, Chemicals, Reagents, and Detection Kits
4.2. Fermentation of SS by Mixed Strains
4.3. Determination of Crude Protein, Ash, Total Phenolic Content, Total Peptides, and Astaxanthin Contents within Fermented SS and Amino Acid Analysis
4.4. Effect of Fermented SS on Glucose Uptake of Insulin Resistant FL83B Hepatocytes
4.5. Animals and Experimental Design
4.6. Measurement of Plasma Levels of Glucose, Insulin, Aspartate Amino Transferase, Alanine Amino Transferase, Creatinine, and Blood Urea Nitrogen
4.7. Plasma Levels of TGs, TC, and Lipoprotein Cholesterol Determination
4.8. Determination of TGs and TC in the Liver Tissues
4.9. Determination of Hexokinase, Glucose-6-Phosphatase, and Glucose-6-Phosphate Dehydrogenase in the Liver
4.10. Determination of Malondialdehyde (MDA) Levels in the Liver
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Tinker, L.; Howard, B.V.; Kuller, L.H.; Nathan, L.; Rifai, N.; Liu, S. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: The women’s health initiative observational study. Diabetes Care 2007, 30, 1747–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloop, K.W.; Showalter, A.D.; Cox, A.L.; Cao, J.X.; Siesky, A.M.; Zhang, H.Y.; Irizarry, A.R.; Murray, S.F.; Booten, S.L.; Finger, E.A. Specific reduction of hepatic glucose 6-phosphate transporter-1 ameliorates diabetes while avoiding complications of glycogen storage disease. J. Biol. Chem. 2007, 282, 19113–19121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymann, A.D.; Cohen, Y.; Chodick, G. Glucose-6-phosphate dehydrogenase deficiency and type 2 diabetes. Diabetes Care 2012, 35, e58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.; Abate, N. Body fat distribution and insulin resistance. Nutrients 2013, 5, 2019–2027. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care-Clin. Off. Pract. 2013, 40, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef] [Green Version]
- Davì, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal 2005, 7, 256–268. [Google Scholar] [CrossRef]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16, S267. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Salama, M.; El-Banna, H. Shrimp’s waste: Chemical composition, nutritional value and utilization. Food/Nahrung 1999, 43, 418–423. [Google Scholar] [CrossRef]
- Heu, M.S.; KIM, J.S.; Shahidi, F.; Jeong, Y.; Jeon, Y.J. Extraction, fractionation and activity characteristics of proteases from shrimp processing discards. J. Food Biochem. 2003, 27, 221–236. [Google Scholar] [CrossRef]
- Kannan, S.; Gariepy, Y.; Raghavan, G.V. Optimization and characterization of hydrochar derived from shrimp waste. Energy Fuels 2017, 31, 4068–4077. [Google Scholar] [CrossRef]
- Gimeno, M.; Ramírez-Hernández, J.Y.; Mártinez-Ibarra, C.; Pacheco, N.; García-Arrazola, R.; Bárzana, E.; Shirai, K. One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J. Agric. Food Chem. 2007, 55, 10345–10350. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Duan, S.; Li, L.; Zhuang, Z.; Wu, W.; Hong, S.; Zhou, J. Improved production of chitin from shrimp waste by fermentation with epiphytic lactic acid bacteria. Carbohydr. Polym. 2012, 89, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, J.; Kan, F.; Gao, Y.; Lan, J.; Zhang, X.; Hu, Z.; Li, Y.; Lin, H. Antioxidant production and chitin recovery from shrimp head fermentation with Streptococcus thermophilus. Food Sci. Biotechnol. 2013, 22, 1023–1032. [Google Scholar] [CrossRef]
- Aytekin, O.; Elibol, M. Cocultivation of Lactococcus lactis and Teredinobacter turnirae for biological chitin extraction from prawn waste. Bioprocess Biosyst. Eng. 2010, 33, 393–399. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Y.; Deng, Y.; Wang, D.; Zhao, Y. Production of chitin from shrimp shell powders using Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydr. Res. 2012, 362, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Seymour, T.A.; Li, S.J.; Morrissey, M.T. Characterization of a natural antioxidant from shrimp shell waste. J. Agric. Food Chem. 1996, 44, 682–685. [Google Scholar] [CrossRef]
- Arakaki, A.; Shimizu, K.; Oda, M.; Sakamoto, T.; Nishimura, T.; Kato, T. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: Organic molecular control of self-organization of hybrids. Org. Biomol. Chem. 2015, 13, 974–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K. Microbiological and chemical changes of shrimp Acetes vulgaris during Kapi production. J. Food Sci. Technol. 2017, 54, 3473–3482. [Google Scholar] [CrossRef]
- Jorge, L.; Marian, L.; Petr, H.; Petr, S. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Vital, P.; Larrieta, E.; Hiriart, M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. J. Endocrinol. 2006, 190, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Ana, D.; Raul, L.; Juan, G.; Daniel, M.; Cristina, M.; Kheira, M.A.; Jose, V.; Consuelo, B. Sex differences in age-associated type 2 diabetes in rats—Role of estrogens and oxidative stress. Oxid. Med. Cell. Longev. 2019, 2019, 6734836. [Google Scholar]
- Cruz, P.L.; Moraes-Silva, I.C.; Ribeiro, A.A.; Machi, J.F.; de Melo, M.D.T.; dos Santos, F.; da Silva, M.B.; Strunz, C.M.C.; Caldini, E.G.; Irigoyen, M. Nicotinamide attenuates streptozotocin-induced diabetes complications and increases survival rate in rats: Role of autonomic nervous system. BMC Endocr. Disord. 2021, 21, 133. [Google Scholar] [CrossRef]
- Xiong, H.; Wu, M.; Zou, H.; Jiang, S.; Yi, H.; Yi, T.; Wang, Q.; Liu, D.; Zhou, Y.; Wei, C. Chitosan inhibits inflammation and adipogenesis of orbital fibroblasts in Graves ophthalmopathy. Mol. Vis. 2018, 24, 509. [Google Scholar]
- Okita, K.; Iwahashi, H.; Kozawa, J.; Okauchi, Y.; Funahashi, T.; Imagawa, A.; Shimomura, I. Homeostasis model assessment of insulin resistance for evaluating insulin sensitivity in patients with type 2 diabetes on insulin therapy. Endocr. J. 2013, 60, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, C.M.; Garcia-Fernandez, N.; Escalada, J. Diabetic Kidney Disease, Cardiovascular Disease and Non-Alcoholic Fatty Liver Disease: A New Triumvirate? J. Clin. Med. 2021, 10, 2040. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects β-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Mathew, G.M.; Mathew, D.C.; Sukumaran, R.K.; Sindhu, R.; Huang, C.-C.; Binod, P.; Sirohi, R.; Kim, S.-H.; Pandey, A. Sustainable and eco-friendly strategies for shrimp shell valorization. Environ. Pollut. 2020, 267, 115656. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Yang, S.H. Microbial chitinases: Properties, current state and biotechnological applications. World J. Microbiol. Biotechnol. 2019, 35, 144. [Google Scholar] [CrossRef]
- Xie, J.; Xie, W.; Yu, J.; Xin, R.; Shi, Z.; Song, L.; Yang, X. Extraction of chitin from shrimp shell by successive two-step fermentation of Exiguobacterium profundum and Lactobacillus acidophilus. Front. Microbiol. 2021, 12, 677126. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Azwady, A.N.; Rusea, G.; Noormasshela, U.; Shaziera, A.N.; Azleen, A.; Muskhazli, M. The availability of astaxanthin from shrimp shell wastes through microbial fermentations, aeromonas hydrophila and cell disruptions. Int. J. Agric. Biol. 2014, 16, 277–284. [Google Scholar]
- Ketnawa, S.; Martínez-Alvarez, O.; Gómez-Estaca, J.; del Carmen Gómez-Guillén, M.; Benjakul, S.; Rawdkuen, S. Obtaining of functional components from cooked shrimp (Penaeus vannamei) by enzymatic hydrolysis. Food Biosci. 2016, 15, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Brewer, P.D.; Habtemichael, E.N.; Romenskaia, I.; Mastick, C.C.; Coster, A.C. Insulin-regulated Glut4 translocation: Membrane protein trafficking with six distinctive steps. J. Biol. Chem. 2014, 289, 17280–17298. [Google Scholar] [CrossRef] [Green Version]
- Bhuvaneswari, S.; Anuradha, C.V. Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice. Can. J. Physiol. Pharmacol. 2012, 90, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Xu, F.H.; Roh, S.-S.; Song, Y.O.; Uebaba, K.; Noh, J.S.; Yokozawa, T. Astaxanthin and Corni Fructus protect against diabetes-induced oxidative stress, inflammation, and advanced glycation end product in livers of streptozotocin-induced diabetic rats. J. Med. Food 2015, 18, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Ghlissi, Z.; Kamoun, Z.; Makni, M.; Nasri, M.; Bougatef, A.; Sahnoun, Z. Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. Eur. J. Nutr. 2015, 54, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-L.; Yao, H.-T.; Cheng, R.-S.; Chiang, M.-T. Chitosan reduces plasma adipocytokines and lipid accumulation in liver and adipose tissues and ameliorates insulin resistance in diabetic rats. J. Med. Food 2012, 15, 453–460. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflug. Arch.—Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Chen, R.-Y.; Chiang, M.-T. Effects and mechanisms of chitosan and chitosanoligosaccharide on hepatic lipogenesis and lipid peroxidation, adipose lipolysis, and intestinal lipid absorption in rats with high-fat diet-induced obesity. Int. J. Mol. Sci. 2021, 22, 1139. [Google Scholar] [CrossRef]
- Roberfroid, M. Dietary fiber, inulin, and oligofructose: A review comparing their physiological effects. Crit. Rev. Food Sci. Nutr. 1993, 33, 103–148. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, C.M.; Munion, J.; Hesslink, R., Jr.; Wise, J.; Gallaher, D.D. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutr. 2000, 130, 2753–2759. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, A.; Kiba, Y.; Akati, N.; Fukuzawa, K.; Terada, H. Molecular characteristics of astaxanthin and β-carotene in the phospholipid monolayer and their distributions in the phospholipid bilayer. Chem. Phys. Lipids 2001, 113, 11–22. [Google Scholar] [CrossRef]
- Dey, S.S.; Dora, K.C. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus). J. Food Sci. Technol. 2014, 51, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, D.-H.; Kim, S.-K. Antioxidant effects of chitin, chitosan, and their derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 2003. [Google Scholar]
- Bah, C.S.; Carne, A.; McConnell, M.A.; Mros, S.; Bekhit, A.E.-D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016, 202, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Huang, D.W.; Shen, S.C.; Wu, J.S. Effects of caffeic acid and cinnamic acid on glucose uptake in insulin-resistant mouse hepatocytes. J. Agric. Food Chem. 2009, 57, 7687–7692. [Google Scholar] [CrossRef]
- Masiello, P.; Broca, C.; Gross, R.; Roye, M.; Manteghetti, M.; Hillaire-Buys, D.; Novelli, M.; Ribes, G. Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 1998, 47, 224–229. [Google Scholar] [CrossRef]
- Szkudelski, T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp. Biol. Med. 2012, 237, 481–490. [Google Scholar] [CrossRef]
- Ghasemi, A.; Khalifi, S.; Jedi, S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta Physiol. Hung. 2014, 101, 408–420. [Google Scholar] [CrossRef]
- Assmann, G.; Schriewer, H.; Schmitz, G.; Hägele, E. Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clin. Chem. 1983, 29, 2026–2030. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Erickson, R.H.; Zakim, D.; Vessey, D.A. Preparation and properties of a phospholipid-free form of microsomal UDP-glucuronyltransferase. Biochemistry 1978, 17, 3706–3711. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]



| Free Amino Acids | Unfermented (mg/100 g) | Fermented (mg/100 g) |
|---|---|---|
| Phosphorserine | 2.20 | 2.99 |
| Taurine | 2.05 | 2.29 |
| Aspartic Acid | 3.59 | 3.52 |
| Threonine | 1.60 | 9.74 |
| Serine | 2.39 | 1.03 |
| Asparagine | 0.68 | N.D. |
| Glutamic Acid | 3.88 | 27.91 |
| L-2-Aminoadipic Acid | N.D. | 3.31 |
| Glycine | 5.26 | 16.13 |
| Alanine | 2.79 | 58.66 |
| Citrulline | N.D. | 12.73 |
| Valine | 2.58 | 38.79 |
| Methionine | N.D. | 4.58 |
| Isoleucine | 1.28 | 18.12 |
| Leucine | 2.49 | 21.49 |
| Tyrosine | 4.95 | 30.52 |
| Phenylalanine | N.D. | 26.49 |
| Γ-Aminobutyric Acid | 0.11 | 1.25 |
| Ethanolamine | 0.33 | 1.24 |
| Dl-Plus Allo-Δ-Hydroxylysine | 0.10 | 0.66 |
| Ornithine | 0.85 | 5.95 |
| Lysine | 3.19 | N.D. |
| Histidine | N.D. | 3.40 |
| Arginine | 6.11. | N.D. |
| Hydroxyproline | N.D. | 1.80 |
| Proline | N.D. | 17.19 |
| Total free amino acids | 46.52 | 309.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Lin, C.-H.; Huang, H.-H.; Tsai, G.-J. Development of Fermented Shrimp Shell Product with Hypoglycemic and Hypolipidemic Effects on Diabetic Rats. Metabolites 2022, 12, 695. https://doi.org/10.3390/metabo12080695
Huang C-H, Lin C-H, Huang H-H, Tsai G-J. Development of Fermented Shrimp Shell Product with Hypoglycemic and Hypolipidemic Effects on Diabetic Rats. Metabolites. 2022; 12(8):695. https://doi.org/10.3390/metabo12080695
Chicago/Turabian StyleHuang, Chung-Hsiung, Chih-Heng Lin, Hsiao-Han Huang, and Guo-Jane Tsai. 2022. "Development of Fermented Shrimp Shell Product with Hypoglycemic and Hypolipidemic Effects on Diabetic Rats" Metabolites 12, no. 8: 695. https://doi.org/10.3390/metabo12080695
APA StyleHuang, C.-H., Lin, C.-H., Huang, H.-H., & Tsai, G.-J. (2022). Development of Fermented Shrimp Shell Product with Hypoglycemic and Hypolipidemic Effects on Diabetic Rats. Metabolites, 12(8), 695. https://doi.org/10.3390/metabo12080695







