Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focal Question
2.2. Language
2.3. Databases
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
| Phytochemicals | In Vivo/In Vitro Model(s) | Effective Dose(s)/ Concentration(s) | Related Clinical Symptoms of IBD | NF-kB-Related Dysregulation Indicators | Related Molecular Mechanisms in Regulation of NF-kB in IBD | Ref. |
|---|---|---|---|---|---|---|
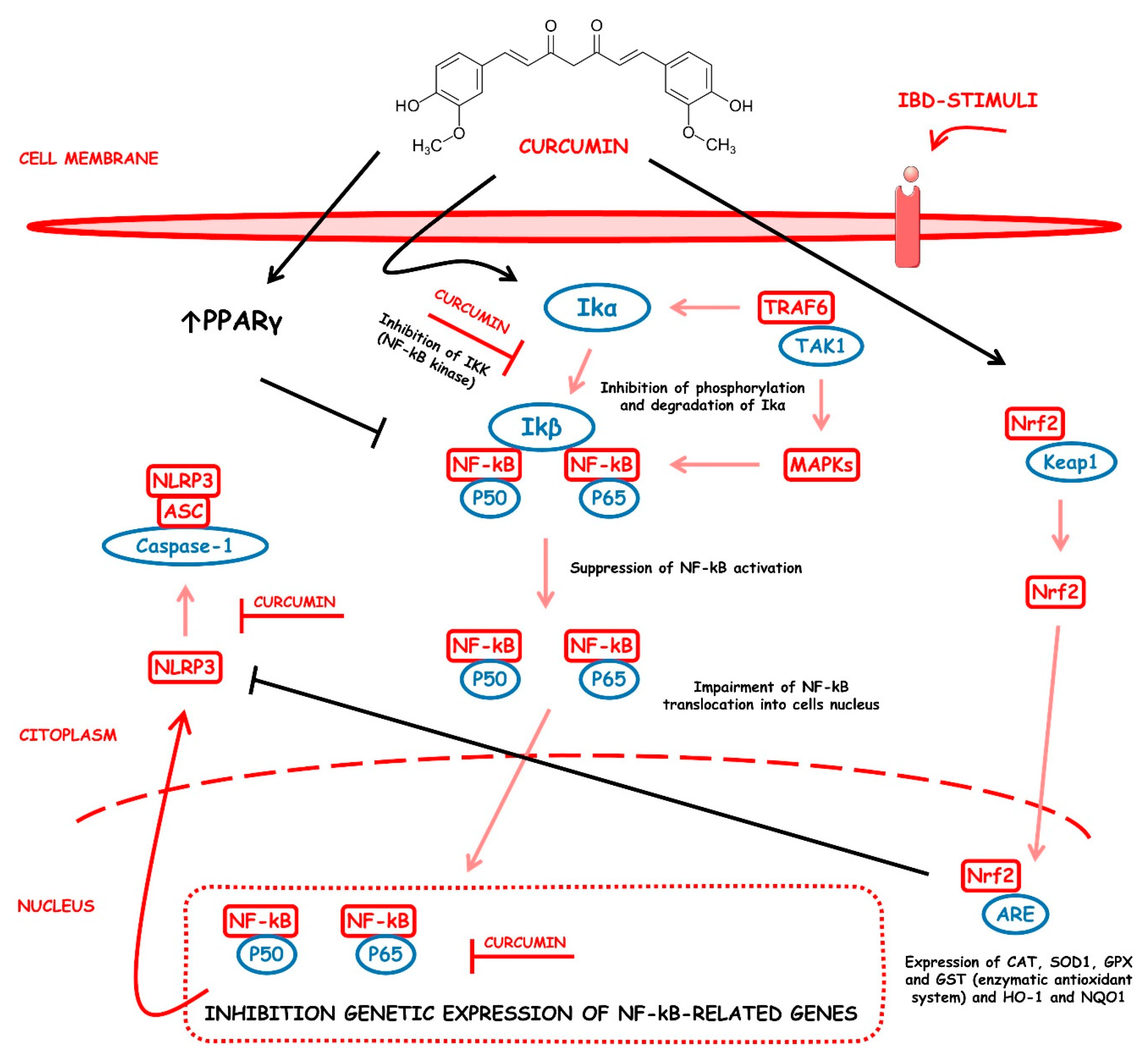
Curcumin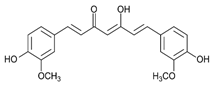 | DSS-induced FVB/NJ mice model of colitis and NFκB-RE-Luc transgenic mice model of colitis | 3 mg/day of TDNPs for 1 week orally | ↑Inflammatory cells infiltration in the lamina propria, ↑epithelial erosion, ↑interstitial edema, and ↓colonic goblet cells | ↑TNF-α, ↑IL-6, ↑IL-1β, ↑OS-related protein, ↓HO-1, and ↑MPO | ↓NF-kB-p65-dependent luciferase activity, ↓phospho-NF-kB-p65 and ↓nuclear translocation of p65 | [17] |
| TNBS-induced Wistar Hannover rats model of colitis | 20 mg/kg/day for 1 week orally | ↑Inflammatory cells infiltration and ↑ulcer and granuloma formation | ↑IL-6, ↑TNF-α, ↑MPO, ↑MDA, and ↑NO | ↓NF-kB-related proteins expression and ↓oxidative-related enzymes expression | [18] | |
| DSS-induced BALB/c mice model of colitis | 100 mg/kg mixed with olive oil in the chow | ↓Body weight, ↑DAI, and ↓colon length | ↑iNOS, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑nitrite, and ↑S-nitrosylation of IKKβ | ↓S-nitrosylation of IKKβ and ↓IκB phosphorylation | [19] | |
| TNBS-induced Sprague-Dawley rats model of colitis | 100 mg/kg/day for 1 week orally | ↑DAI score | ↑MPO, ↑NF-kB mRNA, ↑IL-27, ↑TLR4 expression, ↑NF-kB-p65, and ↑IL-27 p28 | ↓NF-kB mRNA and ↓NF-kB-p65 | [20] | |
| TNBS-induced Sprague-Dawley rats model of colitis | 100 mg/kg and 200/mg/kg orally 2 h prior to induction of colitis | ↓Body weight, ↑bloody diarrhea, thickened colon wall, ↓depletion of goblet cells, ↑hemorrhagic intestinal necrosis, ↑mucosal ulcerations, and ↑inflammatory cells infiltration | ↑MPO and ↑NF-kB expression | ↓NF-kB-related proteins expression and ↓oxidative-related enzymes expression | [21] | |
| Bacteria-induced Specific pathogen-free wild-type 129/SvEv mice and germ-free IL 10/mice models of colitis | 0.1, 0.5, and 1% curcumin-supplemented diets for 5 days | ↑Intestinal-associated ↑crypt hyperplasia, ↑lymphocytic and neutrophilic infiltrations, and ↑mucosal ulceration | ↑IL-12/23p40, ↑IFN-γ, ↑NF-kB activation, and ↑pSer276-p65 | ↓IFN-γ and IL-12/23p40 genetic expression, ↓phospho-p65-positive expression, ↑IL-10 mRNA, ↓NF-kB-related proteins expression | [22] | |
| DNCB-induced Wistar rats model of colitis | 25, 50, and 100 mg/kg/day of curcumin orally for 10 days | ↑Intestinal ulcers, ↑inflammation in the colon, and ↓colon length | ↑MPO, ↑LPO and ALP activities; ↑iNOS, and ↑NF-kB-related proteins expression | ↓NF-kB-related proteins expression and ↓iNOS expression | [23] | |
| TNBS-induced Sprague–Dawley rats model of colitis | 30 mg/kg/day intraperitoneally for 14 days | ↑Intestinal epithelial necrosis, ↑glandular destruction, ↑inflammatory cells infiltration, ↓body weight | ↑IL-1β mRNA, ↑TNF-α mRNA, ↑IFN-γ mRNA, ↑COX-2 mRNA, ↓PPAR-γ, ↓PGE2, ↑15d-PGJ2, ↓mRNA IL-4 | ↑mRNA do PPARγ, ↑15d-PGJ2, ↑PPAR-γ, ↓COX-2 mRNA, ↓IL-1β mRNA, ↓TNF-α mRNA, ↓IFN-γ mRNA, and ↑IL-4 mRNA | [24] | |
| TNBS-induced Wistar rats model of colitis | 2% of curcumin mixed with the chow for 14 days | ↓Body weight, intestinal ulcers, ↑inflammatory cells infiltration | ↑NF-kB DNA ligation activity, ↑IkB degradation, ↑IL-1β and ↓IL-10 | ↓NF-kB DNA ligation activity, ↓IkB degradation, and ↓IL-1 β mRNA | [25] | |
| TNBS-induced BALB/c mice model of colitis | 0.25% of curcumin mixed with the chow for 10 days | ↓Body weight, ↑inflammatory cellular infiltration, and ↑mucosal and muscle damage | ↑MPO, ↑IL-1 β, ↑NF-kB DNA ligation activity, and ↑p38 MAPK | ↓NF-kB DNA ligation activity and ↓p38 MAPK | [26] | |
| TNBS-induced C3H mice model of colitis | 25–300 mg/kg/day of curcumin orally for 10 days | ↑Hemorrhagic and ulcerative damage to the distal colon, ↑mucosal congestion, ↑leucocyte cellular infiltrate in the submucosa, and ↓body weight | ↑NO, ↑MPO, ↑MDA, ↑protease activities, ↑IFN-γ and IL-12 p40 mRNAs, and ↑iNOS | ↓Serine protease activities, ↓IFN-γ and IL-12 p40 mRNA levels, ↓NF-kB-related proteins expression | [27] | |
| TNBS-induced C57BL/6 and BALB/c mice models of colitis | 0.5%, 2.0%, or 5.0% of curcumin mixed with the chow for 7 days | ↓Body weight, ↑crypts distortion, ↓goblet cells, and mononuclear cells infiltration | ↑p65 nuclear expression, ↑IkB degradation, ↑macrophage infiltration, ↑IL-18, ↑NF-kB DNA ligation activity, ↑IL-6 mRNA, ↑IFN-γ mRNA, ↑TNF-α mRNA, and ↑IL-12 mRNA | ↓IkB degradation, ↓NF-kB DNA ligation activity, ↓IL-6 mRNA, ↓IFN-γ mRNA, ↓TNF-α mRNA, and ↓IL-12 mRNA | [28] | |
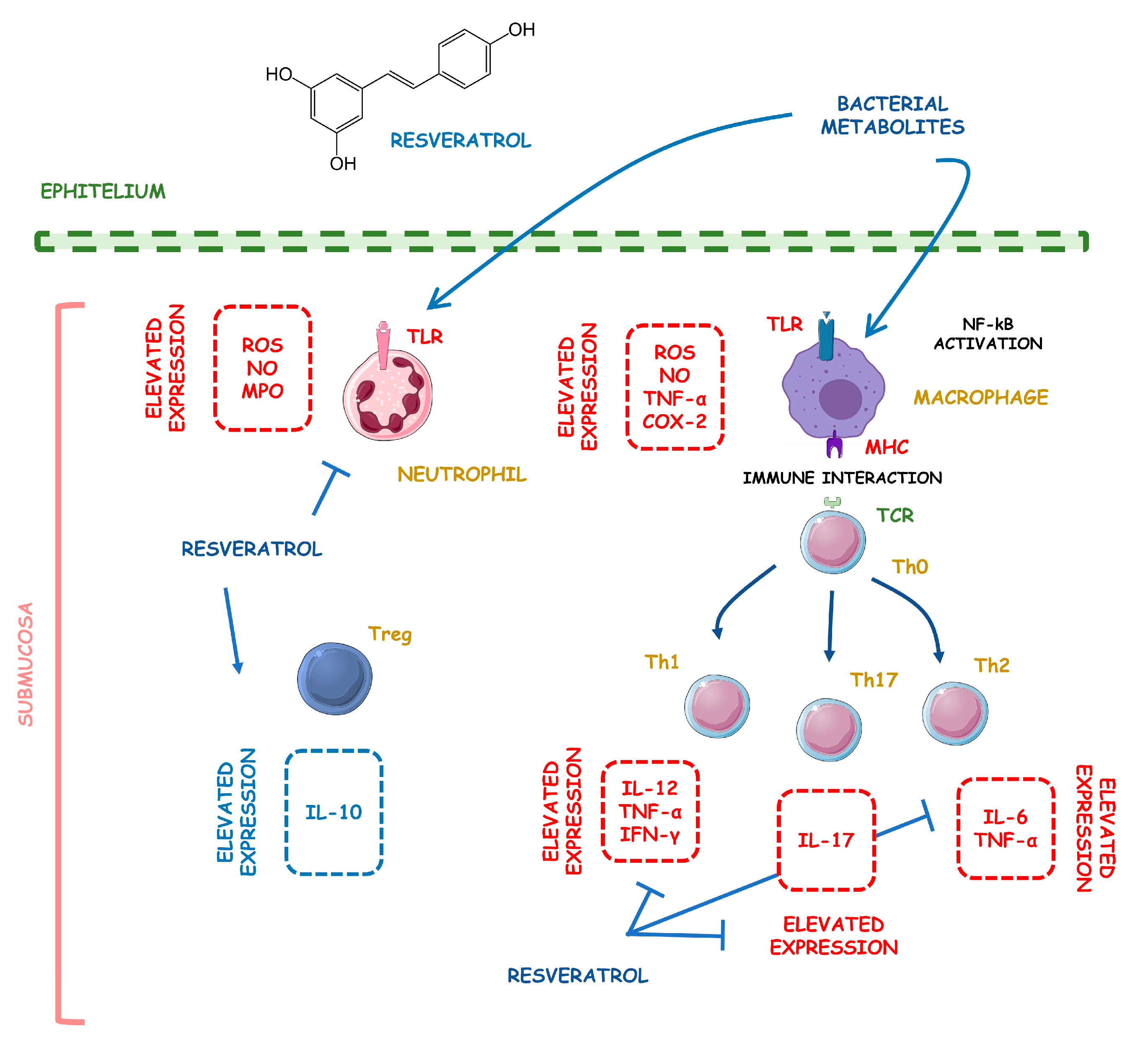
Resveratrol | TNBS-induced C57BL/6 mice model of colitis | 10 µL 4.5 mM and 10 µL 45 mM/day intraperitoneally for 4 days | Weight loss, diarrhea, bloody stool, ↑MPO activity, ↑DAI score, ↑colonic cytokine levels, and ↑visceral pain | ↑pNF-kB, ↑TNF-α mRNA, ↑TNF-α, ↑TGF-β mRNA, ↑TGF- | ↓pNF-Κb, ↓TNF-α mRNA, and ↓TGF-β mRNA | [29] |
| LPS-treated Caco-2 cells | 10–50 μM during 1 h of incubation | ↑Colon inflammation measured by COX-2 and PGE2 expression levels | ↑p65 nuclear translocation, ↑COX-2, ↑PGE2 | ↓p65 nuclear translocation, ↓IKK phosphorylation, ↓COX-2 mRNA, and ↓IkBα phosphorylation and degradation | [30] | |
| DSS-induced C57BL/6 mice model of colitis | 100 µL of 10, 50, and 100 mg/kg on alternate days orally for 7 days | Colon inflammation (lymphocyte infiltration and distortion of glands), weight loss, and ↑serum pro-inflammatory cytokines | ↑IL-6, ↑IL-1β, ↑IFN-γ, ↑TNF-α, ↑p-IkBα, ↓SIRT1 | ↓p-IkBα and ↑SIRT1 | [31] | |
| DSS-induced ICR mice model of colitis | 10 mg/kg/day orally for 7 days | ↑Histopathological score | ↑NF-kB-DNA binding complex, ↑IKKβ catalytic activity, ↑ERK phosphorylation, ↑iNOS expression, ↑STAT3 | ↓ERK phosphorylation, ↓NF-kB-DNA binding complex, and ↓IKKβ catalytic activity | [32] | |
| TNBS-induced Wistar mice model of colitis | 10 mg/kg/day orally for 14 days | ↑Macroscopic inflammation, presence of adhesions between the colon and small bowel and other organs, ulcers, crypt distortion, ↑leukocyte involvement, ↑pro-inflammatory cytokines production, and weight loss | ↑MPO, ↑TNF-α, ↑NF-kB p65, ↑COX-2, ↑PGD2 | ↑Inflammatory mucosa cells apoptosis and ↓NF-kB p65 | [33] | |
3-(4-Hydroxyphenyl)-propionic acid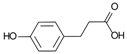 | DSS-induced colitis in antibiotics-treated pseudo-germ-free mice and LPS-stimulated RAW264.7 cells | - | ↑Intestinal inflammation and ↑OS both in vivo and in vitro | ↑NF-kB-related activation proteins and ↑MAPK | ↓NF-kB-related activation proteins and ↓MAPK | [34] |
Sesamol | DSS-induced C57BL/6 mice model of colitis | 100 mg/kg/day orally for 6 weeks | ↑DAI, histopathological changes, and ↓intestinal barrier integrity | ↑COX-2, ↑iNOS, ↑IL-6, ↑IL-1β, ↑TNF-α, ↑TLR4 | ↓COX-2 mRNA, ↓iNOS mRNA, ↓IL-6 mRNA, ↓IL-1β mRNA, ↓TNF-α mRNA, ↓TLR4 mRNA, and ↑p-NF-kB/NF-kB ratio | [35] |
Kaempferol | DSS-induced C57BL/6 mice model of colitis | 50 mg/kg/day orally for 14 days | ↑DAI, ↓colon length, ↑intestinal mucosal injury, and altered gut microbiota | ↑IL-6, ↑IL-1β, ↑TNF-α, ↑IL-1β mRNA, ↑IL-6 mRNA, ↑TNF-a mRNA, ↑COX-2 mRNA, ↑MCP-1 mRNA, ↑iNOS mRNA, ↓IL-10 mRNA, ↑TLR4, ↑NLRP3, ↑MAPK1, ↑NF-kB-related proteins expression, ↓ZO-1, ↓occludin, and ↓claudin-1 | IL-1β mRNA, ↓IL-6 mRNA, ↓TNF-a mRNA, ↓COX-2 mRNA, ↓MCP-1 mRNA, ↓iNOS mRNA, ↑IL-10 mRNA, ↓TLR4, ↓NLRP3, ↓MAPK1, ↓MyD88, ↓p-NF-kB-P65 | [36] |
Astragalin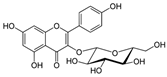 | DSS-induced C57BL/6 mice model of colitis | 200 µL of 50, 75, and 100 mg/kg/day orally for 7 days | ↑DAI, ↑intestinal mucosal injury, ↑inflammatory cells infiltration, and ↓colon length | ↑TLR4 mRNA,↑MCP-1 mRNA, ↑IL-1β mRNA, ↑TNF-α mRNA, ↑COX-2 mRNA, ↑IFN-γ mRNA, ↑p-IκBα, ↑p-IKKα/β, and ↑p-p65 | ↓TLR4 mRNA, ↑ZO-1 mRNA, ↑occludin mRNA, ↑Muc2 mRNA, ↓p-IκBα, ↓p-IKKα/β, and ↓p-p65, ↓MCP-1 mRNA, ↓IL-1β mRNA, ↓TNF-α mRNA, ↓COX-2 mRNA, ↓IFN-γ mRNA | [37] |
| TNF-α -stimulated HCT-116 and HT-29 human colonic epithelial cells in vitro and DSS-induced C57BL/6 mice model of colitis in vivo | 0, 20, 40, 60, 80, and 100 μM incubated for 24 h in vitro and 2 and 5 mg/kg/day orally for 7 days in vivo | ↑Pro-inflammatory cytokines production and ↑colon cells proliferation in vitro and ↓colon length, ↑pro-inflammatory cytokines production, and weight loss in vivo | ↑Cells proliferation, ↑TNF-α mRNA, ↑IL-8 mRNA, ↑IL-6 mRNA, ↑IκBα phosphorylation, and ↑NF-kB-DNA binding in vitro and ↑IκBα phosphorylation, ↑TNF-α mRNA, ↑IL-8 mRNA, and ↑IL-6 mRNA in vivo | ↓Cells proliferation, ↓TNF-α mRNA, ↓IL-8 mRNA, ↓IL-6 mRNA, ↓IκBα phosphorylation, and ↓NF-kB-DNA binding in vitro and ↓IκBα phosphorylation, ↓TNF-α mRNA, ↓IL-8 mRNA, and ↓IL-6 mRNA in vivo | [38] | |
Pinocembrin | LPS-stimulated RAW264.7 and Caco-2 cells in vitro and DSS-induced C57BL/6 mice model of colitis in vivo | 0–200 μM incubated for 24 h in vitro and 25, 50, and 100 mg/kg/day orally for 9 days in vivo | ↑Inflammation in vitro and weight loss, ↑intestinal tissue damage, ↑mucosa muscle thickness, ↑neutrophil infiltration, ↑diarrhea, ↑microbiota alterations, and ↑blood in stool in vivo | ↑TNF-α, ↑COX-2, ↑iNOS, ↑IFN-γ, ↑IL-6, ↑IL-15, ↑TLR4, ↑p65 phosphorylation, ↑IκBα phosphorylation, and ↓NO in vitro and ↑p65 phosphorylation, ↑TLR4 mRNA, ↑Myd88 mRNA, ↑iNOS mRNA, ↑COX-2 mRNA, ↑TNF-α mRNA | ↓Pro-inflammatory cytokines expression, ↓NF-kB-luciferase activity and ↓TLR4/MD2 · LPS interaction in vitro and ↓TLR4 mRNA, ↓Myd88 mRNA, ↓iNOS mRNA, ↓COX-2 mRNA, ↓TNF-α mRNA, and ↓p65 phosphorylation in vivo | [39] |
Oxyberberine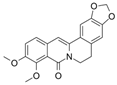 Bacterial metabolite | DSS-induced BALB/c mice model of colitis | 12.5, 25, and 50 mg/kg/day orally/7 days | Shaggy hair, low vitality, body weight loss, diarrhea, occult fecal blood, and ↑DAI | ↑MPO, ↓ZO-1, ↓ZO-2, ↓occludin, ↓JAM-A, ↓claudin-1, ↑IL-6, ↑IL-1β, ↑IL-17, ↑TNF-α, ↑IFN-γ, ↑TLR4, ↑MyD88, ↑p-IκBα, ↑p65 (nucleus), ↓IκBα, ↓p65 (cytoplasm) | ↓MPO, ↑ZO-1, ↑ZO-2, ↑occludin, ↑JAM-A, and ↑claudin-1 expressions, ↓IL-6, ↓IL-1β, ↓IL-17, ↓TNF-α, and IFN-γ expressions, ↑p65 (cytoplasm), ↓p65 (nucleus), ↓p-IκBα/IκBα ratio, ↓TLR4, ↓MyD88, ↓NF-kB-p65 translocation, ↓IκBα phosphorylation | [40] |
Berberine hydrochloride | DSS-induced Wistar mice model of colitis | 10, 30, and 50 mg/kg/day orally/6 weeks | Weight loss, ↓survival rate, ↓colon length, ↓colon weight, ↑DAI, ↓daily activity, anorexia, ↑inflammatory cells infiltration, ↑intestinal edema, and ↑microscopic damage scores | ↑IL-1 mRNA, ↑IL-1β mRNA, ↑IL-6 mRNA, ↑IL-12 mRNA, ↑TNF-α, ↑IFN-γ mRNA, ↓IL-4 mRNA, ↓IL-10 mRNA, ↑iNOS, ↑MPO, ↑MDA, ↑p-NF-kB | ↓IL-1 mRNA, ↓IL-1β mRNA, ↓IL-6 mRNA, ↓IL-12 mRNA, ↓TNF-α, ↓IFN-γ mRNA, ↑IL-4 mRNA, ↑IL-10 mRNA, ↓activity of iNOS, MPO, and MDA, ↓p-NF-kB, ↑p-STAT3 expression, ↑ZO-1 mRNA, ↑VCAM-1 mRNA, ↑occludin mRNA, and ↑claudin-1 mRNA | [41] |
Berberine | TNBS-induced C3H/HeN and C3H/HeJ mice models of colitis | 10 and 20 mg dissolved in 2% Tween 80 solution/day orally/5 days | Intestinal inflammation measured by shortened, thickened, and erythematous colon | ↑Lipid peroxidation, ↓SOD, ↓CAT, ↑TNF-α, ↑IL-1β, ↑IL-6, ↓IL-10, ↑iNOS, ↑COX-2, ↑TLR4, and ↑NF-kB activation (phosphorylation and nuclear translocation) | ↓Lipid peroxidation, ↑antioxidant SOD, and CAT expressions, ↓pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 expressions, ↑IL-10 expression, ↓iNOS, and ↓COX-2 activities, ↓TLR4 expression, and ↓NF-kB activation (phosphorylation and nuclear translocation) | [42] |
Eriodictyol | TNBS-induced Wistar mice models of colitis | 5, 20, and 50 mg/kg/day orally/7 days | Weight loss, colon crypt destruction, mucosal ulceration, and colon inflammatory cells infiltration | ↑MPO, ↑IL-6, ↑IL-1β, ↑IL 12, ↑IL-2, ↑TNF-α, ↓IL-10, ↓SOD, ↓CAT, ↓GSH-Px, ↑MDA, ↑TLR4, ↑p-IκBα, ↑p-p65, and ↓IκBα | ↓MPO activity, ↓pro-inflammatory cytokines IL-6, IL-1β, IL-12, IL-2, and TNF-α expressions, ↑IL-10 expression, ↑antioxidant enzymes SOD, CAT, and GSH-Px expressions, ↓MDA expression, ↓p65 phosphorylation, ↓IκBα phosphorylation, and ↑IκBα | [43] |
Betulin | Acetic acid-induced Sprague Dawley mice models of colitis | 8 mg/kg/day intraperitoneally for 14 days | Diffuse necrosis, congestion, and hemorrhage of the mucosal layer and submucosal edema, congestion, and immune/inflammatory cells infiltration | ↑CRP, ↑LDH activity, ↑TLR4, ↑CD68 cells infiltration, ↑IL-6, ↑NF-kB expression, ↑TNF-α, ↑IL-1β, ↑caspase-3, and ↑caspase-8 | ↓LDH activity, ↓TLR4 content, ↓CD68 cells infiltration, ↓IL-6 content, ↓NF-kB expression, ↓TNF-α expression, ↓IL-1β, ↓caspase-3 expression, and ↓caspase-8 expression | [44] |
Naringin | LPS-stimulated RAW264.7 cells in vitro and DSS-induced mice model of colitis in vivo | 20 μM incubated for 1 h in vitro and 25, 50, and 100 mg/kg/day orally for 7 days in vivo | ↑Intestinal inflammation in vitro and ↑intestinal mucosa injury and ↑DAI in vivo | ↑TNF-α, ↑NF-kB activation, ↓PPARγ expression in vitro and ↑TNF-α, ↑IL-1β, ↑IL-6, ↑NF-kB-p65 phosphorylation, ↑IκB phosphorylation, ↓PPARγ expression, ↑MAPK, ↑NLRP3, ↑ASC, and ↑caspase-1 in vivo | ↓TNF-α, ↓NF-kB activation, and ↑PPARγ expression in vitro and ↓pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 expressions, ↓NF-kB-p65 phosphorylation, ↓IκB phosphorylation, ↑PPARγ expression, ↓phosphorylation levels of p38, ERK, and JNK, ↓NLRP3, ↓ASC, and ↓caspase-1 in vivo | [45] |
5-Hydroxy-4-methoxycanthin-6-one | DSS-induced Sprague Dawley mice model of colitis | 25, 50, and 100 mg/kg/day orally for 11 days | Weight loss, ↑DAI, ↓colon length, epithelial crypts destruction, disruption of the mucosal barrier, and massive submucosal infiltration of inflammatory cells | ↑TNF-α, ↑IL-1β, ↑IL-6, ↓IL-10, ↓SOD, ↑MDA, ↑NF-kB/p65, ↑CD3, ↑MYD88, ↑p-IκBα, ↓IKKβ, ↓IκBα, ↑NF-kB/p65 nuclear translocation | ↑SOD, ↓MDA, ↓TNF-α, ↓IL-1β, ↓IL-6 and ↑IL-10 expression levels, ↓NF-kB/p65 and ↓CD3 pro-inflammatory phenotypes, ↓NF-kB/p65 mRNA, ↓MYD88, ↓p-IκBα, ↑IKKβ and ↑IκBα proteins expression, ↓NF-kB/p65 nuclear translocation | [46] |
Geniposide | LPS-stimulated RAW264.7 cells in vitro and DSS-induced ICR mice model of colitis in vivo | 200–1000 μM incubated for 24 h in vitro and 20 and 40 mg/kg/day orally/7 days in vivo | ↓Cells viability in vitro and weight loss, ↑erosion and ↑distortion of crypts, ↑loss of glandular epithelium, and ↑inflammatory cell infiltration in vivo | ↓SOD, ↑IL-1β, ↑IL-6, ↑TNF-α, ↑ROS, ↓HO-1, ↓Nrf2 activation, ↑p-NF-kBp65 and ↑p-IκBα in vitro and ↑MPO, ↓SOD, ↑IL-1β, ↑IL-6, ↑TNF-α, ↑inflammatory cells infiltration, ↓HO-1, ↓Nrf2 activation, ↑p-NF-kBp65 and ↑p-IκBα in vivo | ↑SOD, ↓IL-1β, ↓IL-6, ↓TNF-α, ↓ROS, ↑HO-1, ↑Nrf2 activation, ↓p-NF-kBp65, and ↓p-IκBα in vitro and ↓MPO, ↑SOD, ↓IL-1β, ↓IL-6, ↓TNF-α, ↓inflammatory cells infiltration, ↑HO-1, ↑Nrf2 activation, ↓p-NF-kBp65 and ↓p-IκBα in vivo | [47] |
Sesamin | DSS-induced C57BL/6 mice model of colitis | 50, 100, and 200 mg/kg/day orally/7 days | ↓Colon length and ↓body weight | ↑TNF-α, ↑IL-1β, ↑IL-6, ↑p-NF-kBp65, ↑p-IκBα, ↑NF-kB signaling and activity and ↑MAPK | ↓TNF-α, ↓IL-1β, ↓IL-6, ↓p-NF-kBp65, and ↓p-IκBα expression levels, ↓NF-kB signaling and activity, and ↓MAPK levels | [48] |
Taxifolin  | DSS-induced ICR mice model of colitis | 100 mg/kg/day orally for 14 days | ↑DAI, ↓colon length, ↓body weight, ↑crypt distortion, and ↑inflammatory cells infiltration | ↑TNF-α, ↑IL-1β, ↑IL-6, ↓SIgA, ↓IL-10, ↓SOD, ↑p-NF-kB-p65 and ↑p-IkBα | ↓TNF-α, ↓IL-1β and ↓IL-6 expression levels, ↑SIgA, ↑IL-10 and ↑SOD expression levels, ↓p-NF-kB-p65 and ↓p-IkBα | [49] |
Isobavachalcone | DSS-induced C57BL/6 mice model of colitis | 25 and 50 mg/kg/day orally/4 days | ↓Body weight, ↑DAI, ↑crypt distortion, ↑mucosal necrosis, ↑edema, ↑gland destruction, and ↑neutrophilic infiltration | ↑MPO, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑PGE2, ↑NO, ↑iNOS, ↑COX-2 and ↑p-NF-kB-p65 | ↓MPO, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓PGE2, ↓NO, ↓iNOS and ↓COX-2 expression levels and ↓p-NF-kB-p65 | [50] |
d-pinitol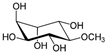 | DSS-induced BALB/c mice model of colitis | 10, 20, and 40 mg/kg/day orally/7 days | ↓Body weight, ↑DAI, ↑ulcer formation, ↑thickened bowel wall, ↑hyperemia, ↑edema, and ↑mucosal inflammatory cells infiltration | ↑MPO, ↑MDA, ↓GSH, ↓SOD, ↓CAT, ↑iNOS, ↑COX-2, ↑TNF-α, ↑IFN-γ, ↑IL-6, ↑IL-17, ↑IL-1β, ↓IL-10, ↓PPAR-γ and ↑NF-kB signaling | ↓MPO, ↓MDA, ↑GSH, ↑SOD, ↑CAT, ↓iNOS, ↓COX-2, ↓TNF-α, ↓IFN-γ, ↓IL-6, ↓IL-17, ↓IL-1β, ↑IL-10, ↑PPAR-γ and ↓NF-kB signaling | [51] |
Paeoniflorin-6’-O-benzene sulfonate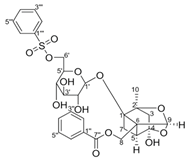 | DSS-induced mice model of colitis | 17.5, 35, and 70 mg/kg/day orally/6 days | ↑M1 macrophage polarization and ↑intestinal barrier dysfunction | ↑GRK2 activation and ↑TLR4-NF-kB-NLRP3 inflammasome signaling | ↓GRK2 translocation and ↓TLR4-NF-kB-NLRP3 inflammasome signaling in macrophages | [52] |
Thymol | AcOH-induced Wistar mice model of colitis | 10, 30, and 100 mg/kg/day orally/6 days | ↑Intestinal inflammation and ↑OS | ↑MPO, ↑TNF-α, and ↑p-NF-kB-p65 | ↓MPO, ↓TNF-α, and ↓p-NF-kB-p65 | [53] |
Tricin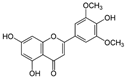 | DSS-induced BALB/c mice model of colitis and LPS-induced RAW 264.7 treated cells | 12.5, 25, and 50 µM incubated/30 min or 24 h in vitro and 100 and 150 mg/kg/day orally/7 days in vivo | ↑ DAI, ↓Body weight, ↓colon length, ↑Inflammatory cells infiltration, ↑epithelial cell disorganization, ↑mucosal thickening, ↓crypts, ↑spleen weight, and ↑myeloid-derived suppressor cells (MDSC, CD11b+Gr1+), ↑MPO, ↑IL-6, TNF-α, and IL-1β in colonic tissues in vivo | ↑NO, ↑IL-6, ↑TNF-α, ↑IL-1β, ↑MIP-2, ↑phosphorylated NF-kB-p65 in vitro | ↓IL-6 expression, ↓TNF-α expression, ↓MIP-2 expression, ↓IL-1β expression ↓Phosphorylated nuclear p65 in vitro and ↓NF-kB pathway in vivo | [54] |
Aesculin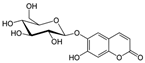 | DSS-induced BALB/c mice model of colitis and LPS-induced RAW 264.7 treated cells | 200, 300, 400, and 500 µM incubated for 1 h in vitro and 1 and 5 mg/kg/day intraperitoneally every two days after colitis induction for 12 days in vivo | ↓Body weight, ↑DAI, ↑colon length, ↑colon weight, ↑inflammatory cells infiltration (mononuclear macrophages and neutrophils), ↑mucosal and submucosal lesion, ↑degeneration, and ↑crypt cells necrosis | ↑p-p65, ↑IκBα phosphorylation and ↓PPAR-γ in vitro and ↑iNOS mRNA, ↑TNF-α mRNA, ↑IL-1β mRNA, ↑p-P65, ↑MAPKs protein and phosphorylation in vivo | ↓TNF-α mRNA, ↓IL-1β mRNA, ↓p-P65, ↓IκBα phosphorylation, ↑PPAR-γ, ↓NK-kB activation in vitro and ↓iNOS mRNA, ↓TNF-α mRNA, ↓IL-1β mRNA, ↓NK-kB activation in vivo | [55] |
Ginsenoside Rk3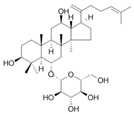 | HFD-induced obese C57BL/6 mice model of colitis | 30 and 60 mg/kg/day orally/8 weeks | ↑Body weight, ↑fat accumulation, ↑glucose tolerance, ↓colon length, ↑inflammatory cells infiltration and ↑crypt lesions | ↓ZO-1 mRNA, ↓claudin mRNA, ↓occludin mRNA,↑TLR4, ↑MYD88, and ↓IkBα | ↑ZO-1 mRNA, ↑claudin mRNA, ↑occludin mRNA, ↓TNF-α mRNA, ↓IL-1β mRNA, ↓IL-6 mRNA, ↓MCP-1 mRNA, ↓F4/80 mRNA, ↓NADPH mRNA, ↓STAMP2 mRNA, ↓TLR4, ↓JNK/phosphorylation JNK, ↓NF-kB, ↓TLRA4/MYD88 pathway, and ↑IkBα mRNA | [56] |
Lancemaside A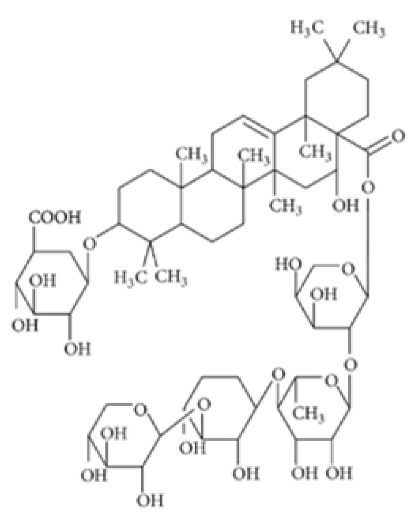 | TNBS-induced ICR mice model of colitis and LPS-induced 293-hTLR4A-hemagglutinin treated cells | 20 μM and 100μM incubated for 6 h in vitro and 10 or 20 mg/kg/day orally for 5 days in vivo | ↓Colon length, ↑thicken, ↑erythematous colon, ↑edema, ↑inflammatory cells infiltration, and ↑epithelial ulcers | ↑TLR4-linked NF-kB in vitro and ↑MPO, ↑TNF-α mRNA and ↑IL-1β mRNA, ↑IL-6 mRNA, ↑TLR4 mRNA, ↑NF-kB (pp65) mRNA, ↑COX-2 mRNA in vivo | ↓TLR4-linked NF-kB in vitro and ↓TNF-α mRNA, ↓IL-1β mRNA, ↓IL-6 mRNA, ↓TLR4 mRNA, ↓NF-kB-p65 mRNA and ↓COX-2 mRNA in vivo | [57] |
Tetramethylpyrazine 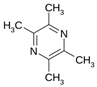 | Oxazolone-induced KM mice model of colitis and LPS-treated Caco-2 cells | 40 µg/mL incubated for 24 h in vitro and 80 mg/kg/day intraperitoneally/7 days in vivo | ↓Body weight, ↑diarrhea with or without hematochezia, ↑DAI, ↑inflammation of the mucosa, ↑fibrotic thickening, ↑ulcers, ↑edema, ↑microhemorrhages, and ↑ necrosis | ↑NF-kB translocation into the nucleus, ↓NF-kB P65 protein in the cytoplasm, ↑nuclear NF-kB P65 protein levels, ↑TNF- α, ↑IL-6, ↑IL-8, ↑INF-γ mRNA and ↑ROS in vitro, and ↑NF-kB P65 in the nucleus, ↓NF-kB in cytoplasmic, ↑C-MYC expression, ↑iNOS expression, ↑COX-2 expression in vivo | ↓NF-kB translocation into the nucleus, ↑NF-kB P65 protein in the cytoplasm, ↓nuclear NF-kB P65 protein levels, ↓INF-γ expression, ↑P65 in the cytoplasm, ↓P65 in the nucleus in vitro and ↓NF-kB P65 in the nucleus, ↓p-IKBα, ↑NF-kB in the cytoplasm, ↓nuclear NF-kB p65 protein levels, ↓C-MYC expression, ↓iNOS expression and ↓COX-2 expression in vivo | [58] |
Daurisoline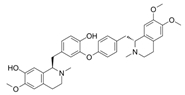 | DSS-induced BALB/c mice model of colitis and LPS-induced RAW 264.7 treated cells | 0, 0.5, 1, 2, 5, 10, 20, 50, and 100 μM incubated for 24 h in vitro and 10, 20, 40 mg/kg/day orally/7 days in vivo | ↑DAI, ↑diarrhea, ↑bleeding, ↓colon length, ↑edema, ↑congestion, ↑thickening, ↑erosion, ↑ulceration, ↑adhesions to adjacent tissues, ↑mucosal damage, ↑inflammatory cell infiltration, ↑crypt loss, and ↑TUNEL stained spots | ↑NO, ↑ROS, ↓GSH, ↑NF-kB-p65, ↑p65, ↓IkBα in vitro and ↑NO, ↑ COX-2, ↑PGE2, ↑IL-1β, ↑MMP-9, ↓IL-4, ↓IL-10, Gene expression of ↑Wnt-1, ↑β-Catenin, ↑cyclin-D1, ↑C-MYC, ↑Expression of Wnt-1, β-Catenin and LRP6, ↓Expression of p-GSK3β and ↑Expression of NF-kB p65 and p-IkBα in vivo | ↓NF-kB p65, ↓p65, ↑IkBα in vitro and Gene expression of ↓Wnt-1, ↓β-Catenin, ↓cyclin-D1, ↓C-MYC, ↓GSK3β, ↑Expression of TCF-4, LEF-1 and p-GSK3β, ↓Expression of Wnt-1, β-Catenin and LRP6 and ↓expression of NF-kB p65 and p-IkBα in vivo | [59] |
Tetrandrine | DSS-induced BALB/c mice model of colitis | 40 mg/kg/day orally/7 or 14 days | ↑DAI | ↑NF-kB DNA binding activity, ↑IL-1β mRNA and protein, ↑TNF-α mRNA and protein, and ↑MPO | ↓NF-kB DNA bindng activity, ↓IL-1β mRNA and protein, ↓TNF-α mRNA and protein | [60] |
Diosgenin  | TNBS-induced Sprague-Dawley rat model of colitis | 50, 100, or 200 mg/kg/day orally/14 days | ↑DAI, ↓body weight, ↑colonic damage, ↑ulceration, ↑stool consistency score, ↑destruction of colon tissue, ↑inflammatory cell infiltration, ↑necrosis and ↑edema | ↓GSH, ↓SOD, ↑MDA, ↑NO, ↑MPO, ↑hydroxyproline, ↑TNF-α, ↑IL-1β, ↑IL-6, ↓IL-10, ↑iNOs mRNA, ↑IFN-γ mRNA, ↑COX-2 mRNA, ↑LTB4 mRNA, ↑Bax, ↑Caspase-1, ↑NF-kB and ↑IκBα, | ↓iNOs mRNA, ↓COX-2 mRNA, ↓IFN-γ mRNA, ↓Bax, ↓Caspase-1, ↓NF-kB and ↓IκBα | [61] |
Mangiferin | TNBS-induced C57BL/6 mice model of colitis and LPS-induced peritoneal macrophages | 5, 10, and 20 μM incubated for 15 to 120 min in vitro and 10 or 20 mg/kg/day orally/3 days in vivo | ↓Colon length, ↑MPO | ↑IRAK1 phosphorylation and degradation, ↑degradation of IRAK1, 2, and 4, ↑NF-kB activation, ↑TAK1 phosphorylation and degradation, ↑IKKβ phosphorylation, ↑IκBα phosphorylation and degradation, ↑PGE2, ↑NO, ↑TNF-α expression, ↑IL-1β expression, ↑IL-6 expression, ↑IL-10 expression, ↑COX-2, ↑iNOS expression in vitro and ↑IRAK1 phosphorylation in vivo | ↓IRAK1 phosphorylation and degradation, ↓NF-kB activation, ↓IKKβ phosphorylation, ↓IκBα phosphorylation and degradation, ↓p65 translocation, ↓MAPK p38 phosphorylation, ↓ERK phosphorylation, ↓JNK phosphorylation, ↓TNF-α expression, ↓IL-1β expression, ↓IL-6 expression, ↓COX-2 expression, ↓iNOS expression and ↑IL-10 expression in vitro and ↓phosphorylation of IRAK1 and IKKβ, ↓NF-kB activation, ↓TNF-α expression, ↓IL-1β expression and ↓IL-6 expression in vivo | [62] |
Tryptanthrin | DSS-induced mice model of colitis | 39.2, 78.4, and 156.8 mg/kg twice a day orally/8 days | ↑CAS, ↓crypts and goblet cells, ↑erosive lesions, ↑inflammatory cell infiltration, and ↑atrophy | ↑TNF-α, ↑IL-1β, ↑IL-6, ↓IL-10, ↑NF-kBp65, ↑p-STAT3, ↓IκBα protein, ↑STAT3 and ↑p-STAT3 | ↓NF-kBp65, ↓p-STAT3, and ↓IκBα degradation | [63] |
l-Theanine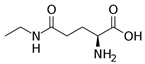 | DSS-induced C57BL/6J mice model of colitis | Water contained 0.1% of l-theanine for 14 days orally | ↓Body weight, ↓length of colon, ↓colon weight, ↑DAI, ↑inflammatory infiltrates, and ↑epithelial injury | ↑TNF-α, ↑IL-1β, ↑IL-6, ↑COX2 mRNA, ↑iNOS mRNA, ↓Ki67-positive cells, ↑TUNEL-positive cells, ↓Occludin mRNA, ↓Claudin1 mRNA, ↓Ecadherin mRNA, ↑ p65, ↑p-p65, ↑p53, ↑p-p53 and ↑p-AKT expression and ↑lipid metabolic perturbation | ↓COX2 mRNA, ↓iNOS mRNA, ↑Occludin mRNA, ↑Claudin1 mRNA, ↑Ecadherin mRNA, ↓p65, ↓p-p65, ↓p53, ↓p-p53, and ↓p-AKT expression | [64] |
| Koreanaside A | LPS-induced RAW 264.7 and peritoneal macrophages treated cells and DSS-induced ICR mice model of colitis | 20, 40, or 80 µM in vitro incubated/4 days and 5 or 20 mg/kg/day orally/7 days in vivo | ↑DAI, ↑body weight loss, ↑stool consistency, ↑occult fecal blood, ↓colon length, ↑spleen index, ↑mucosal layer, ↑ulceration, ↑crypt loss, and ↑inflammatory cell infiltration | ↑NO, ↑PGE2, ↑expression of iNOS and ↑ expression of COX-2, ↑IL-6 mRNA, ↑TNF-α mRNA, ↑AP-1, ↑DNA-binding activity of NF-kB in vitro and ↑F4/80 mRNA, ↑Ly6G mRNA, ↓ZO-1 mRNA, ↓occludin mRNA, ↑claudin-1 mRNA, ↓E-cadherin mRNA, ↑N-cadherin mRNA, ↑vimentin mRNA, ↑iNOS mRNA, ↑COX-2 mRNA, ↑IL-6 mRNA, ↑TNF-α mRNA, ↑c-Fos, ↑p65, STAT1 and ↑STAT3 phosphorylation in vivo | ↓iNOS expression and ↓COX-2 expression, ↓IL-6 mRNA, ↓TNF-α mRNA, ↓MyD88-dependent TLR4 pathway, ↓DNA binding of AP-1, ↓DNA-binding activity of NF-kB, ↓c-Fos phosphorylation, ↓phosphorylation and nuclear translocation of p65. ↓phosphorylation and degradation of IκBα, ↓phosphorylation of IKKα/β, ↓phosphorylation of TAK1, ↓STAT1 (Y701 and S727), ↓STAT3 (Y705), ↓JAK1 (Y1022), JAK2 (Y1007/1008) phosphorylation in vitro and ↓F4/80 mRNA, ↓Ly6G mRNA, ↑ZO-1 mRNA, ↑occludin mRNA, ↓claudin-1 mRNA, ↑E-cadherin mRNA, ↓N-cadherin, ↓iNOS mRNA, ↓COX-2 mRNA, ↓IL-6 mRNA, ↓TNF-α mRNA, ↓vimentin mRNA, ↓↑c-Fos, p65, STAT1 and ↑STAT3 phosphorylation in vivo | [65] |
6-gingerol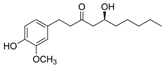 | DSS-induced BALB/c mice model of colitis | 100 and 250 mg/kg/day orally/14 days | ↓Body weight, ↓crypt cells, ↓goblet, ↑granulation, ↑hyperplasia, and ↑inflammatory cells infiltration | ↑IL-17, ↓IL-10, ↑IkBα, ↑p65, ↑p-IκBα and ↑p-p65 | ↓IkBα, ↓p65, ↓p-IκBα and ↓p-p65 | [66] |
Lycopene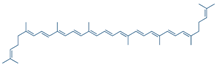 | DSS-induced C57BL/6 mice model of colitis | 5, 10, and 20 mg/kg/day orally/14 days | ↓Body weight, ↑DAI, ↑colon length, ↑colon weight, ↑glandular disorder, and ↑inflammatory cell infiltration | ↑MPO, ↓SOD, ↓ CAT, ↓GSH-Px, ↑MDA, ↑IFN-γ, ↑TNF-α, ↑IL-6, ↑IL-1β, ↑TLR4, ↑TRIF, and ↑p-NF-kB p65 expression, ↓ZO-1, ↓occludin, and ↓claudin-1 expressions | ↓TLR4, ↓TRIF, ↓p-NF-kB p65 expression, ↑ZO-1, ↑occludin and ↑claudin-1 expressions | [67] |
α-mangostin | DSS-induced mice model of colitis | 30 and 100 mg/kg/day orally/14 days | ↓Body weight, ↑DAI, ↑diarrhea, ↑bleeding, ↓colon length, ↑ulceration, ↑erosion, ↑crypt distortion, ↑inflammatory cell infiltration, and ↑edema | ↑MPO, ↑phosphorylation of IKKα and IκB, ↑activated NF-kB, ↑MAPK, ↑phosphorylation of ERK1/2, SAPK/JNK and p38 | ↓IKKα phosphorylation, ↓IκBα phosphorylation, ↓activated NF-kB, ↓phosphorylation of ERK1/2, SAPK/JNK and ↓p38 | [68] |
Ophiopogonin D | DSS-induced C57BL/6J mice model of colitis and LPS-induced IEC-6 treated cells | 10 mg/kg and 40 mg/kg/day orally/7 days in vivo 20 μmol/L incubated for 24 h in vitro | ↑Ulceration, ↑congestion, ↑edema, ↑inflammatory cell infiltration, ↓colon length, and ↓body weight | ↑cl-caspase3 and ↑COX-2, ↑MLCK and ↑iNOS in vitro and ↑TNF-α, ↑IL-6, ↑IL-1β, ↓Bcl-2, ↓occludin, ↑NF-Κb-p65, ↑cl-caspase3, ↑Bax, ↑MLCK, ↑MDA, ↓GSH, ↓SOD, ↑iNOS, ↑COX-2 in vivo | ↓NF-Κb-p65 in vivo and in vitro | [69] |
Alantolactone | DSS-induced C57BL/6 mice model of colitis and LPS-induced RAW 264.7 and HT-29 colorectal treated cells | 0–25 μM incubated for 2 h in vitro 50 mg/kg/day orally/9 days in vivo | ↓Body weight, ↓bloody diarrhea, ↓colon length, ↑histological injury, ↑inflammatory cell infiltration | ↑p-p65 nuclear translocation in vitro and ↑NF-kB p65 phosphorylation, ↑IκBα phosphorylation/degradation, ↑iNOS expression, ↑ICAM-1 expression, ↑MCP-1 expression, ↑COX-2 expression, ↑TNF-α expression, ↑IFN-γ expression, ↑IL-6 expression, ↑MPO, ↑ NO, ↑PGE2, ↑TNF-α, ↑IL-6 in vivo | ↓p-p65 nuclear translocation, ↑hPXR via binding to hPXR-LBD in vitro and ↓NF-kB p65 phosphorylation, ↓IκBα phosphorylation/degradation, ↓iNOS expression, ↓ICAM-1 expression, ↓COX-2 expression, ↓TNF-α expression, ↓IFN-γ expression and ↓IL-6 expression in vivo | [70] |
Sinomenine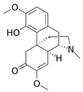 | DSS-induced BALB/c mice model of colitis | 30, 90, 270, 180, 540 mg/kg/day and 1.6 g/kg/day orally/9 days | ↓Body weight, ↓food intake, ↑pasty stools, ↑DAI, ↓colon length, and ↑inflammatory cell infiltration | ↑MyD88, ↑NF-kBp65, ↑TLR4, ↓SIGIRR expression, ↑TLR/NF-kB | ↓MyD88 expression, ↓NF-kBp65 expression, ↓TLR expression, ↑SIGIRR expression, ↓TLR/NF-kB, ↓expression of IFN-γ, IL-1β, TNF-α, IL-6, and IL-12 | [71] |
Convallatoxin | LPS-induced RAW264.7 and BMDMs macrophages and DSS-induced C57BL/6 mice model of colitis | 10–50 nM incubated for 12 or 24 h in vitro and 50 or 150 μg/kg/day orally/10 days in vivo | ↓Colon length, ↑colon and spleen weights, ↓body weight, ↑inflammatory cell infiltration, ↑ulceration, ↑necrosis, ↑congestion and ↑edema, ↑IL-1β, IL-6, and TNF-α in the colon | ↑NF-kB, ↑COX-2, ↑iNOS, ↑ IL-1β, ↑ IL-6, ↑TNF-α, ↑NF-kB-p65 and ↓PPARγ in vitro and ↑COX-2, ↑iNOS, ↑IL-1β, ↑IL-6, ↑TNF-α, ↑nuclear NF-kB-p65, ↓PPARγ protein in vivo | ↓NF-kB p65, ↑PPARγ, ↑PPARγ mRNA, ↓NF-kB mRNA, ↓IL-1β mRNA, ↓IL-6 mRNA, ↓TNF-α mRNA, ↓p-IκBα, ↑PPARγ siRNA in vitro and ↓nuclear NF-kB-p65, ↑PPARγ expression, ↓nuclear translocation of NF-kB-p65, ↓cytoplasmic p-IκBα expression, ↑PPARγ mRNA and ↓NF-kB mRNA in vivo | [72] |
Fisetin | DSS-induced Balb/C mice model of colitis and LPS-induced macrophages treated cells | 5 and 10 mg/kg/day orally/8 days in vivo and 0–50 μM incubated for 24 h in vitro | ↑DAI, ↓body weight, ↓colon length, ↓crypts, ↓goblet cells, ↑inflammatory cell infiltration, | ↑Nitrites, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑COX-2, ↑iNOS, ↑NF-kB-p65 nuclear translocation, ↑IkBα phosphorylation and degradation in vitro and ↑MPO, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑Nitrites, ↑COX-2, ↑iNOS, ↑nuclear NF-kB (p65), ↑phosphorylation of IκBα (p-IκBα/IκBα), ↑NF-kB (p65)-DNA binding activity, ↑p-p38/p38, ↑p-ERK/ERK, ↑Akt phosphorylation, ↓GSH, and ↑TBARS in vivo | ↓NF-kB-p65e expression, ↓IkBα phosphorylation and degradation in vitro and ↓nuclear NF-kB (p65), ↓phosphorylation of IκBα (p-IκBα/IκBα), ↑NF-kB (p65)-DNA binding activity, ↓p-p38/p38, ↑p-ERK/ERK, and ↓Akt phosphorylation in vivo | [73] |
Genipin | DSS-induced C57BL/6 mice model of colitis | 2.5, 5, 10 mg/kg/day orally/14 days | ↓Body weight, ↑intestinal epithelial destruction, ↑crypt abscesses, and ↑goblet cells loss | ↑MPO, ↑MDA, ↑TNF-α, ↑IL-1β, ↑NF-kB signaling, ↓Nrf2 signaling and ↓HO-1 | ↓MPO, ↓MDA, ↓TNF-α and ↓IL-1β expression, ↓NF-kB signaling, ↑Nrf2 signaling and ↑HO-1 expression | [74] |
Piperine | TNBS-induced Sprague–Dawley mice model of colitis | 10, 20, and 40 mg/kg/day orally/14 days | ↓Body weight, ↑colon weight-to-length ratio, and ↑ulceration | ↑Oxide-nitrosative stress, ↑iNOS, ↑TNF-α, ↑IL-1β, ↑IFN-γ, ↑COX-2 mRNA, ↑LTB4, ↑IkBα, ↑NF-kB signaling, ↓occludin, ↓claudin-1, ↓zonula occludens-1, ↑caspase-1 and ↓IL-10 | ↓Oxide-nitrosative stress, ↓iNOS, ↓TNF-α, ↓IL-1β, ↓IFN-γ, ↓COX-2 mRNA, ↓LTB4 and ↓IkBα expression levels, ↓NF-kB signaling, ↑occludin, ↑claudin-1, ↑zonula occludens-1 and ↑IL-10 expression levels and ↓caspase-1 | [75] |
Ligustilide  | DSS-induced C57BL/6 mice model of colitis | 15, 30, and 60 mg/kg/day orally/14 days | ↓Body weight and ↓colon length, ↑diarrhea, ↑rectal bleeding, ↑ulceration, and ↑inflammatory cells infiltration | ↑MPO, ↑iNOS, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑IL-12, ↑MIP-1α, ↑IL-17, ↓PPARγ and ↑NF-kB-p65 | ↓MPO, ↓iNOS, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓IL-12, ↓MIP-1α and ↓IL-17 expression levels, ↑PPARγ expression and signaling and ↓NF-kB-p65 expression | [76] |
Evodiamine | DSS-induced C57BL/6 mice model of colitis | 20, 40, and 80 mg/kg/day orally/10 days | ↑Diarrhea, ↑fecal bleeding, ↑colon shortening, and ↓body weight | ↑MPO, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑p-NF-kB p65, ↑p-IkB, ↑NLRP3, ↑ASC, ↑caspase-1, ↓ZO-1 and ↓occludin | ↓MPO, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓p-NF-kB p65, ↓p-IkB, ↓NLRP3, ↓ASC, ↓caspase-1, ↑ZO-1 and ↑occludin | [77] |
Chrysin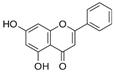 | TNBS-induced C57BL/6 mice model of colitis | 25 mg/kg/day orally/10 days | ↓Body weight, ↑diarrhea, ↑fecal bleeding, ↑crypt distortion, and ↑inflammatory exudate | ↑p-65, ↑IkBα phosphorylation and degradation, ↑NF-kB nuclear translocation, ↑iNOS mRNA, ↑ICAM-1 mRNA, ↑MCP-1 mRNA, ↑COX-2 mRNA, ↑TNF-α mRNA, ↑IL-6 mRNA, and ↑MPO | ↓p-65, ↓IkBα phosphorylation and degradation, ↓NF-kB nuclear translocation, ↓iNOS mRNA, ↓ICAM-1 mRNA, ↓MCP-1 mRNA, ↓COX-2 mRNA, ↓TNF-α mRNA, ↓IL-6 mRNA, and ↓MPO | [78] |
Wogonoside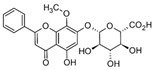 | DSS-induced BALB/c mice model of colitis and LPS-induced Human acute monocytic leukemia THP-1 treated cells | 12.5, 25, or 50 mg/kg/day orally/10 days in vivo and 0.1 mM incubated for 4 h in vitro | ↓Body weight, ↓colon length, ↓spleen weight, ↑inflammatory cell infiltration, ↑ulcers, ↑edema and ↑congestion, ↑CD11b+ F4/80+ macrophages and ↑CD11b+ Gr-1+ neutrophils, | ↑NLRP3 mRNA and ↑pro-caspase-1 mRNA in vitro and ↑IL-1β, ↑TNF-α, ↑IL-6, ↑NF-kB p65, ↑cleaved caspase-1 (p10), ↑cleaved-IL-1β, ↑NLRP3 and ↑ASC in vivo | ↓IL-1β mRNA, ↓TNF-α mRNA, ↓IL-6 mRNA, ↓NF-kB nuclear translocation, ↓IkBa phosphorylation, ↓phosphorylation of p65, ↓NF-kB DNA binding activity, ↓NLRP3 mRNA and ↓pro-caspase-1 mRNA in vitro and ↓NF-kB, ↓NF-kB-p65, ↓IkBa phosphorylation, ↓p65, ↓p65 phosphorylation and ↓NF-kB DNA binding activity in vivo | [79] |
Oxymatrine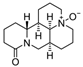 | TNBS-induced rats model of colitis | 10, 30, or 60 mg/kg/day intraperitoneally/7 days | ↓Body weight, ↓colon length, ↑DAI, ↑ulcers, ↓goblet cells, and ↑inflammatory cell infiltration | ↓ZO-1 mRNA, ↓occludin mRNA, ↓claudin-2 mRNA, ↑IL-6, ↑TLR9, ↑Myd88 and ↑p-NF-kB P65 | ↓IL-1β mRNA, ↓TNF-α mRNA, ↓IL-6 mRNA, ↓NF-kB, ↓TLR9 expression, ↓Myd88, ↓TLR9/Myd88/NF-kB pathway | [80] |
Epicatechin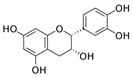 | DSS-induced C57BL/6J mice model of colitis and LPS-induced RAW264.7 and IEC-6 treated cells | 100, 200, or 300 mg/kg/day orally/7 days in vivo and 0.1 µM, 1 µM or 10 µM incubated for 4 h in vitro | ↓Body weight, ↓colon length, ↑intestinal bleeding, ↑DAI, and ↑CMDI scores | ↑TNF-α, ↑IL-6, ↑NO, ↑MPO and ↑NF-kB | ↓NF-kB expression | [81] |
Thymoquinone | DSS-induced C57BL/6J mice model of colitis and TNF-α-induced HT-29 treated cells | 20 and 40 mg/kg/day orally for 8 days in vivo and 0, 12.5, 50, 100, 150, and 200 µM incubated for 24 h in vitro | ↑DAI, ↑inflammatory cells infiltration, ↑MPO, ↓crypts, ↓villi, ↑submucosal edema, and ↑epithelium destruction | ↑CXCL-1 mRNA, ↑IL-8 mRNA and COX-2 mRNA in vitro and ↑IL-1β expression, ↑TNF-α expression, ↑IL-6, expression ↑IL-6 mRNA, ↑IL-1β mRNA, ↑TNF-α mRNA, ↑COX-2, ↑iNOS, ↑COX-2 mRNA, ↑iNOS mRNA, ↑p-ERK, ↑p-JNK, ↑p-p38, ↑phosphorylation of the NF-kB protein and ↓PPAR-γ expression in vivo | ↓CXCL-1 mRNA, IL-8 mRNA, and COX-2 mRNA, ↑PPAR-γ expression both at protein and mRNA in vitro and ↓IL-6 mRNA, ↓IL-1β mRNA, ↓TNF-α mRNA, ↓p-ERK, ↓p-JNK, ↓p-p38, ↓phospho-NF-kB protein and ↑PPAR-γ in vivo | [82] |
Fraxinellone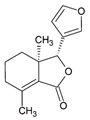 | DSS-induced C57BL/6J mice model of colitis and LPS-induced Human THP-1 treated cells | 7.5, 15, 30 mg/kg/ day intraperitoneally/9 days in vivo and 10, 30 µM incubated for 24 h in vitro | ↓Body weight, ↑diarrhea, ↑loose feces, ↑visible fecal blood, ↑mortality, ↑gross bleeding, ↑ulcerations, colon length, ↑DAI, ↑inflammatory cell infiltration at mucosa and submucosa, ↑crypts distortion, and ↓goblet cells | ↑IL-1β, ↑IL-18, ↑TNF-α, ↑IL-6 in vivo and ↑IL-1β, ↑IL-18 and ↑NO in vitro | ↓VCAM1 mRNA, ↓iNOS mRNA, and ↓COX-2 mRNA in vivo and ↓IL-1β expression, ↓IL-18 expression, ↓phosphorylation of IKKα/β, ↓IκBα, ↓phosphorylation of the p65, ↓p65, ↓Caspase-1 activation and ↓NLRP3 inflammasome in vitro | [83] |
Artesunate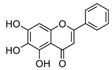 | DSS-induced Sprague-Dawley rats model of colitis and LPS-induced RAW264.7 treated cells | 10, 30, and 50 mg/kg/day orally/5 days in vivo and 5, 10, and 20 µg/mL incubated for 24 h in vitro | ↑DAI, ↓hemoglobin, ↓colon length, and ↑cell destruction | ↑TNF- α, ↑IL-8, ↑IFN-γ, ↑TLR4, ↑p-NF-kB, ↑p-p38, ↑Bax, ↑caspase-9 and ↓Bcl-2 | ↓TLR4, ↓p-NF-kB, ↓p-p38, ↓Bax, ↓caspase-9 and ↑Bcl-2 | [84] |
Aesculetin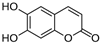 | DSS-induced C57BL/6J mice model of colitis and LPS-induced RAW264.7 treated cells | 20 mg/kg/day orally/7 days in vivo and 10, 25, 50 µM incubated for 4 h in vitro | ↓Colon length, ↓body weight, ↑DAI, and ↑inflammatory cell infiltration | ↑NO, ↑iNOS expression, ↑p–NF–κB-P65 expression, ↑NF-kB P65 nuclear translocation, ↑p38 phosphorylation, ↑JNK phosphorylation, ↑ERK phosphorylation, ↑NLRP3 expression in vitro and ↑NF-kB P65, ↑TNF-α and ↑IL-6 in vivo | ↓iNOS expression, ↓p–NF–κB P65 expression, ↓NF-kB-P65 nuclear translocation, ↓p38 phosphorylation, ↓JNK phosphorylation, ↓ERK phosphorylation and ↓NLRP3 expression in vitro and ↓NF-kB-P65, ↓p38 phosphorylation, ↓JNK phosphorylation and ↓ERK phosphorylation in vivo | [85] |
Euphol | DSS and TNBS-induced CD1 mice model of colitis and LPS-induced BMDMs treated cells | 3, 10, and 30 mg/kg twice a day orally for 3, 4 or 7 days in vivo and 1 and 10 µM incubated for 24 h in vitro | ↑Hemorrhage in the colonic lumen, ↓body weight, ↑diarrhea with bloody stools, ↑DAI, ↑mucosal neutrophils infiltration, ↓crypts, ↓goblet cells, ↑mucosal hyperemia, ↑mucosal necrosis | ↑IL-1β, ↑CXCL1, ↑MIP-2, ↑MCP-1, ↑IL-1β mRNA, ↑CXCL1 mRNA, ↑TNF-α mRNA, ↑IL-6 mRNA, ↑NOS2 expression, ↑VEGF expression, ↑Ki67 expression, ↑NF-kB-p65 phosphorylation, ↑ICAM-1 mRNA, ↑VCAM-1 mRNA and ↑LFA-1 mRNA | ↓NOS2 expression, ↓VEGF expression and ↓p65 NF-kB activation | [86] |
Nobiletin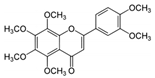 | TNBS-induced Sprague-Dawley rats model of colitis and LPS-induced Caco-2 treated cells | 20 and 40 mg/kg/day orally/7 days in vivo and 0, 10, 20, 40, or 80 incubated for 0–36 h µM in vitro | ↑DAI, ↓body weight, ↑colon weight-to-length Ratio, ↑intestinal permeability, ↑MPO, ↑TNF-α, ↑IL-1β, ↑IL-6, ↑NO, ↑PGE2, ↑iNOS expression, ↑COX-2 expression in vivo | ↑Akt, ↑MLCK mRNA, ↑MLCK protein and ↑NF-kB p65 protein expression in vitro and ↑MLCK, ↑NF-kB, ↑PI3K, ↑Akt and ↑NF-kB p65 protein Expression in vivo | ↓Akt, ↓MLCK mRNA, ↓MLCK protein and ↓NF-kB p65 protein expression in vitro and ↓MLCK, ↓NF-kB, ↓phosphatidylinositol 3-kinase (PI3K), ↓Akt, ↓NF-kB p65 protein expression, ↓iNOS expression and ↓COX-2 expression in vivo | [87] |
Galangin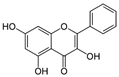 | DSS-induced Swiss albino mice model of colitis | 40 mg/kg/day orally for 20 days | ↑Mucosal ulceration, ↑mucosal necrosis, ↑inflammatory cell infiltration in the lamina propria and submucosa | ↑TLR4 mRNA, ↑NF-kB p65 nuclear translocation, ↑TNF-α and ↑IL-6 | ↓TLR4 mRNA, ↓NF-kB-p65 nuclear translocation, ↓TNF-α expression, and ↓IL-6 expression | [88] |
3. Results
4. Discussion
4.1. Physiopathology of Ulcerative Colitis
4.2. Physiopathology of Crohn’s Disease
4.3. NF-kB and Its Related Molecular Insights into Inflammation
4.4. NF-kB and Its Implications on IBD
4.5. Phytochemicals That Influence the NF-kB Signaling during IBD: An Overview
4.5.1. Curcumin
4.5.2. Resveratrol
4.5.3. 3-(4-Hydroxyphenyl)-propionic Acid
4.5.4. Sesamol
4.5.5. Kaempferol
4.5.6. Astragalin
4.5.7. Pinocembrin
4.5.8. Oxyberberine
4.5.9. Berberine Hydrochloride
4.5.10. Berberine
4.5.11. Eriodictyol
4.5.12. Betulin
4.5.13. Naringin
4.5.14. 5-Hydroxy-4-methoxycanthin-6-one
4.5.15. Geniposide
4.5.16. Sesamin
4.5.17. Taxifolin
4.5.18. Isobavachalcone
4.5.19. d-Pinitol
4.5.20. Paeoniflorin-6′-O-benzene Sulfonate
4.5.21. Thymol
4.5.22. Tricin
4.5.23. Aesculin
4.5.24. Ginsenoside Rk3
4.5.25. Lancemaside A
4.5.26. Tetramethylpyrazine
4.5.27. Daurisoline
4.5.28. Tetrandrine
4.5.29. Diosgenin
4.5.30. Mangiferin
4.5.31. Tryptanthrin
4.5.32. l-Theanine
4.5.33. Koreanaside A
4.5.34. 6-Gingerol
4.5.35. Lycopene
4.5.36. α-Mangostin
4.5.37. Ophiopogonin D
4.5.38. Alantolactone
4.5.39. Sinomenine
4.5.40. Convallatoxin
4.5.41. Fisetin
4.5.42. Genipin
4.5.43. Piperine
4.5.44. Ligustilide
4.5.45. Evodiamine
4.5.46. Chrysin
4.5.47. Wogonoside
4.5.48. Oxymatrine
4.5.49. Epicatechin
4.5.50. Thymoquinone
4.5.51. Fraxinellone
4.5.52. Artesunate
4.5.53. Aesculetin
4.5.54. Euphol
4.5.55. Nobiletin
4.5.56. Galangin
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Na, J.E.; Kim, T.J.; Lee, Y.C.; Kim, J.E.; Kim, E.R.; Hong, S.N.; Chang, D.K.; Kim, Y.H. Risk of prostate cancer in patients with inflammatory bowel disease: A nationwide cohort study in South Korea. Ther. Adv. Gastroenterol. 2022, 15, 17562848221137430. [Google Scholar] [CrossRef] [PubMed]
- Matias, J.N.; Lima, V.M.; Nutels, G.S.; Laurindo, L.F.; Barbalho, S.M.; de Alvares Goulart, R.; Araújo, A.C.; Suzuki, R.B.; Guiguer, E.L. The use of vitamin D for patients with inflammatory bowel diseases. Int. J. Vitam. Nutr. Res. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.A.; Barbalho, S.M. Can vitamin D induce remission in patients with inflammatory bowel disease? Ann. Gastroenterol. 2022, 35, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Pan, C.; Cai, Q.; Zhao, Y.; He, D.; Wei, W.; Zhang, N.; Shi, S.; Chu, X.; Zhang, F. Assessing the effect of interaction between gut microbiome and inflammatory bowel disease on the risks of depression. Brain Behav. Immun.—Health 2022, 26, 100557. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, R.S.; Alaklabi, A.; Alsitary, Y.M.; Khunayn, M.A.B.; Hijazi, S.O.; Alshagary, R.I.; Rajendram, R. Clinical profile, course and outcomes of adults with inflammatory bowel disease over a decade: A single center experience. Ann. Saudi Med. 2022, 42, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.A.; Araújo, A.C.; Bechara, M.D. Curcumin, autoimmune and inflammatory diseases: Going beyond conventional therapy—A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef]
- Goulart, R.A.; Barbalho, S.M.; Lima, V.M.; Souza, G.A.; Matias, J.N.; Araújo, A.C.; Rubira, C.J.; Buchaim, R.L.; Buchaim, D.V.; Carvalho, A.C.A.; et al. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J. Med. Food 2021, 24, 675–685. [Google Scholar] [CrossRef]
- Abdul Khaliq, H.; Alhouayek, M.; Quetin-Leclercq, J.; Muccioli, G.G. 5′AMP-activated protein kinase: An emerging target of phytochemicals to treat chronic inflammatory diseases. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–26. [Google Scholar] [CrossRef]
- Goulart, R.A.; Barbalho, S.M.; Rubira, C.J.; Araújo, A.C.; Lima, V.M.; Rogerio Leoni, B.; Guiguer, E.L. Curcumin therapy for ulcerative colitis remission: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1171–1179. [Google Scholar] [CrossRef]
- Ritmejerytė, E.; Ryan, R.Y.M.; Byatt, B.J.; Peck, Y.; Yeshi, K.; Daly, N.L.; Zhao, G.; Crayn, D.; Loukas, A.; Pyne, S.G.; et al. Anti-inflammatory properties of novel galloyl glucosides isolated from the Australian tropical plant Uromyrtus metrosideros. Chem.-Biol. Interact. 2022, 368, 110124. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Zubair, H.M.; Almutairi, M.H.; Abbas, M.; Akhtar, M.F.; Aleya, L.; Kamel, M.; Saleem, A.; Jabeen, Q.; Noreen, S.; et al. Hepatoprotective effect of Cordia rothii extract against CCl(4)-induced oxidative stress via Nrf2-NFκB pathways. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 156, 113840. [Google Scholar] [CrossRef]
- Batalha, A.; Souza, D.C.M.; Ubiera, R.D.; Chaves, F.C.M.; Monteiro, W.M.; da Silva, F.M.A.; Koolen, H.H.F.; Boechat, A.L.; Sartim, M.A. Therapeutic Potential of Leaves from Fridericia chica (Bonpl.) L. G. Lohmann: Botanical Aspects, Phytochemical and Biological, Anti-Inflammatory, Antioxidant and Healing Action. Biomolecules 2022, 12, 1208. [Google Scholar] [CrossRef]
- Qazi, N.G.; Khan, A.U.; Abbasi, S.W.; Shah, F.A.; Rasheed, F.; Ali, F.; Hassan, S.S.U.; Bungau, S. Pharmacological Basis of Rumex hastatus D. Don in Gastrointestinal Diseases with Focusing Effects on H(+)/K(+)-ATPase, Calcium Channels Inhibition and PDE Mediated Signaling: Toxicological Evaluation on Vital Organs. Molecules 2022, 27, 5919. [Google Scholar] [CrossRef] [PubMed]
- Chera, E.I.; Pop, R.M.; Pârvu, M.; Sorițău, O.; Uifălean, A.; Cătoi, F.A.; Cecan, A.; Negoescu, A.G.; Achimaș-Cadariu, P.; Pârvu, A.E. Flaxseed Ethanol Extracts’ Antitumor, Antioxidant, and Anti-Inflammatory Potential. Antioxidants 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef]
- Altinel, Y.; Yalçın, Ş.; Ercan, G.; Yavuz, E.; Erçetin, C.; Gülçiçek, O.B.; Çelik, A.; Özkaya, G.; Uzun, H. The efficacy of curcumin on PDGF expression and NF-kappa B pathway: TNBS-induced colitis. Ulus. Travma Ve Acil Cerrahi Derg. Turk. J. Trauma Emerg. Surg. TJTES 2020, 26, 663–670. [Google Scholar] [CrossRef]
- Kao, N.J.; Hu, J.Y.; Wu, C.S.; Kong, Z.L. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. Int. Immunopharmacol. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhan, L.; Liao, H.; Chen, L.; Lv, X. Curcumin improves TNBS-induced colitis in rats by inhibiting IL-27 expression via the TLR4/NF-kB signaling pathway. Planta Med. 2013, 79, 102–109. [Google Scholar] [CrossRef]
- Lubbad, A.S.; Oriowo, M.A.; Khan, I. Curcumin reverses attenuated carbachol-induced contraction of the colon in a rat model of colitis. Scand. J. Gastroenterol. 2009, 44, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Larmonier, C.B.; Uno, J.K.; Lee, K.M.; Karrasch, T.; Laubitz, D.; Thurston, R.; Midura-Kiela, M.T.; Ghishan, F.K.; Sartor, R.B.; Jobin, C.; et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 295, G1079–G1091. [Google Scholar] [CrossRef]
- Venkataranganna, M.V.; Rafiq, M.; Gopumadhavan, S.; Peer, G.; Babu, U.V.; Mitra, S.K. NCB-02 (standardized Curcumin preparation) protects dinitrochlorobenzene-induced colitis through down-regulation of NFkappa-B and iNOS. World J. Gastroenterol. 2007, 13, 1103–1107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Deng, C.; Zheng, J.; Xia, J.; Sheng, D. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int. Immunopharmacol. 2006, 6, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.T.; Mai, G.F.; Wang, J.D.; Zhang, Y.L.; Luo, R.C.; Fang, Y.X. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J. Gastroenterol. 2005, 11, 1747–1752. [Google Scholar] [CrossRef] [PubMed]
- Salh, B.; Assi, K.; Templeman, V.; Parhar, K.; Owen, D.; Gómez-Muñoz, A.; Jacobson, K. Curcumin attenuates DNB-induced murine colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G235–G243. [Google Scholar] [CrossRef]
- Ukil, A.; Maity, S.; Karmakar, S.; Datta, N.; Vedasiromoni, J.R.; Das, P.K. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br. J. Pharmacol. 2003, 139, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Hanai, H.; Tozawa, K.; Aoshi, T.; Uchijima, M.; Nagata, T.; Koide, Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 2002, 123, 1912–1922. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xu, H.M.; Han, Y.; Zhang, Y.L. Analgesic effect of resveratrol on colitis-induced visceral pain via inhibition of TRAF6/NF-kB signaling pathway in the spinal cord. Brain Res. 2019, 1724, 146464. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-kB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. Vitro 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J. Pharmacol. Exp. Ther. 2010, 332, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, J.; Lee, J.S.; Na, H.K.; Kundu, J.K.; Surh, Y.J. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer 2009, 61, 847–854. [Google Scholar] [CrossRef]
- Martín, A.R.; Villegas, I.; Sánchez-Hidalgo, M.; de la Lastra, C.A. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br. J. Pharmacol. 2006, 147, 873–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Zhang, Y.; Liu, X.; Zhao, C.; Yin, J.; Li, X.; Zhang, X.; Wang, J.; Wang, S. Distinctive anti-inflammatory effects of resveratrol, dihydroresveratrol, and 3-(4-hydroxyphenyl)-propionic acid on DSS-induced colitis in pseudo-germ-free mice. Food Chem. 2023, 400, 133904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xia, B.; Li, X.; Zhang, L.; Liu, X.; Shi, R.; Kou, R.; Liu, Z.; Liu, X. Sesamol Supplementation Attenuates DSS-Induced Colitis via Mediating Gut Barrier Integrity, Inflammatory Responses, and Reshaping Gut Microbiome. J. Agric. Food Chem. 2020, 68, 10697–10708. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Xu, F.; Zhao, S.; Wu, X.; Wang, Y.; Xie, J. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-kB Axis. Front. Immunol. 2021, 12, 679897. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-kB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef]
- Han, Y.M.; Koh, J.; Kim, J.H.; Lee, J.; Im, J.P.; Kim, J.S. Astragalin Inhibits Nuclear Factor-κB Signaling in Human Colonic Epithelial Cells and Attenuates Experimental Colitis in Mice. Gut Liver 2021, 15, 100–108. [Google Scholar] [CrossRef]
- Yue, B.; Ren, J.; Yu, Z.; Luo, X.; Ren, Y.; Zhang, J.; Mani, S.; Wang, Z.; Dou, W. Pinocembrin alleviates ulcerative colitis in mice via regulating gut microbiota, suppressing TLR4/MD2/NF-kB pathway and promoting intestinal barrier. Biosci. Rep. 2020, 40, BSR20200986. [Google Scholar] [CrossRef]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-kB pathway. Pharmacol. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 2019, 68, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.A.; Hyun, Y.J.; Kim, D.H. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-kB activation. Eur. J. Pharmacol. 2010, 648, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.H.; Liu, J.Y.; Yin, J.B. Eriodictyol attenuates TNBS-induced ulcerative colitis through repressing TLR4/NF-kB signaling pathway in rats. Kaohsiung J. Med. Sci. 2021, 37, 812–818. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.; Eisa, N.H.; Abo El-Magd, N.F.; Elsherbiny, N.M.; Said, E.; Khodir, A.E. Anti-inflammatory/anti-apoptotic impact of betulin attenuates experimentally induced ulcerative colitis: An insight into TLR4/NF-kB/caspase signalling modulation. Environ. Toxicol. Pharmacol. 2021, 88, 103750. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yao, Y.; Lu, Z.; Zhang, Q.; Liu, C.; Zhu, C.; Lin, C. 5-Hydroxy-4-methoxycanthin-6-one alleviates dextran sodium sulfate-induced colitis in rats via regulation of metabolic profiling and suppression of NF-kB/p65 signaling pathway. Phytomedicine 2021, 82, 153438. [Google Scholar] [CrossRef]
- Yang, H.; Yue, Y.; Li, Y.; Su, L.; Yan, S. Geniposide attenuates dextran sulfate sodium-induced colitis in mice via Nrf-2/HO-1/NF-kB pathway. Ann. Palliat. Med. 2020, 9, 2826–2836. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, C.L.; Shen, H.Q.; Zhou, X.F.; Li, J.H.; Yu, J.L.; An, Q.; Fu, B.D.; Yi, P.F. Sesamin protects against DSS-induced colitis in mice by inhibiting NF-kB and MAPK signaling pathways. Food Funct. 2021, 12, 1688–1694. [Google Scholar] [CrossRef]
- Hou, J.; Hu, M.; Zhang, L.; Gao, Y.; Ma, L.; Xu, Q. Dietary Taxifolin Protects Against Dextran Sulfate Sodium-Induced Colitis via NF-kB Signaling, Enhancing Intestinal Barrier and Modulating Gut Microbiota. Front. Immunol. 2020, 11, 631809. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, B.; Min, X.; Hou, Y.; Lin, L.; Wu, Q.; Shi, J.; Chen, X. Therapeutic potential of isobavachalcone, a natural flavonoid, in murine experimental colitis by inhibiting NF-kB p65. Phytother. Res. 2021, 35, 5861–5870. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Y.; Su, J.; Wang, M.; Wu, X.; Su, Z.; Yi, X.; Wei, L.; Cai, J.; Sun, Z. Therapeutic role of d-pinitol on experimental colitis via activating Nrf2/ARE and PPAR-γ/NF-kB signaling pathways. Food Funct. 2021, 12, 2554–2568. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, M.Y.; Chen, J.Y.; Xu, Z.W.; Zhang, J.W.; Li, T.; Zhang, L.L.; Wei, W. CP-25 exerts therapeutic effects in mice with dextran sodium sulfate-induced colitis by inhibiting GRK2 translocation to downregulate the TLR4-NF-kB-NLRP3 inflammasome signaling pathway in macrophages. IUBMB Life 2021, 73, 1406–1422. [Google Scholar] [CrossRef] [PubMed]
- Chamanara, M.; Abdollahi, A.; Rezayat, S.M.; Ghazi-Khansari, M.; Dehpour, A.; Nassireslami, E.; Rashidian, A. Thymol reduces acetic acid-induced inflammatory response through inhibition of NF-kB signaling pathway in rat colon tissue. Inflammopharmacology 2019, 27, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Chen, S.G.; Yue, G.G.; Kwok, H.F.; Lee, J.K.; Zheng, T.; Shaw, P.C.; Simmonds, M.S.J.; Lau, C.B. Natural flavone tricin exerted anti-inflammatory activity in macrophage via NF-kB pathway and ameliorated acute colitis in mice. Phytomedicine 2021, 90, 153625. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Peng, Z.; Luo, S.; Zhang, S.; Li, B.; Zhou, C.; Fan, H. Aesculin protects against DSS-Induced colitis though activating PPARγ and inhibiting NF-kB pathway. Eur. J. Pharmacol. 2019, 857, 172453. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, H.; Deng, J.; Fan, D. Ginsenoside Rk3 Ameliorates Obesity-Induced Colitis by Regulating of Intestinal Flora and the TLR4/NF-kB Signaling Pathway in C57BL/6 Mice. J. Agric. Food Chem. 2021, 69, 3082–3093. [Google Scholar] [CrossRef]
- Joh, E.H.; Lee, I.A.; Han, S.J.; Chae, S.; Kim, D.H. Lancemaside A ameliorates colitis by inhibiting NF-kappaB activation in TNBS-induced colitis mice. Int. J. Colorectal Dis. 2010, 25, 545–551. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, M.; Chen, W.; Yin, L.; Zhu, J.; Chen, N.; Chen, W. Tetramethylpyrazine improves oxazolone-induced colitis by inhibiting the NF-kB pathway. Clin. Investig. Med. Med. Clin. Et Exp. 2014, 37, E1–E9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wu, H.; Hou, J.; Wang, J.; Wang, J.; Li, M.; Yao, X.; Gao, J.; Zhang, Q. Daurisoline alleviated experimental colitis in vivo and in vitro: Involvement of NF-kB and Wnt/β-Catenin pathway. Int. Immunopharmacol. 2022, 108, 108714. [Google Scholar] [CrossRef]
- Zhang, D.K.; Cheng, L.N.; Huang, X.L.; Shi, W.; Xiang, J.Y.; Gan, H.T. Tetrandrine ameliorates dextran-sulfate-sodium-induced colitis in mice through inhibition of nuclear factor -kappaB activation. Int. J. Colorectal Dis. 2009, 24, 5–12. [Google Scholar] [CrossRef]
- Tang, X.; Huang, G.; Zhang, T.; Li, S. Elucidation of colon-protective efficacy of diosgenin in experimental TNBS-induced colitis: Inhibition of NF-kB/IkB-α and Bax/Caspase-1 signaling pathways. Biosci. Biotechnol. Biochem. 2020, 84, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.J.; Jang, S.E.; Hyam, S.R.; Han, M.J.; Kim, D.H. Mangiferin ameliorates colitis by inhibiting IRAK1 phosphorylation in NF-kB and MAPK pathways. Eur. J. Pharmacol. 2014, 740, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X.; Wang, C.L.; Wang, L.; Sun, C.; Zhang, D.B.; Liu, J.L.; Liang, Y.N.; Tang, D.X.; Tang, Z.S. Tryptanthrin Protects Mice against Dextran Sulfate Sodium-Induced Colitis through Inhibition of TNF-α/NF-kB and IL-6/STAT3 Pathways. Molecules 2018, 23, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yao, X.; Ma, M.; Ding, Y.; Zhang, H.; He, X.; Song, Z. Protective Effect of l-Theanine against DSS-Induced Colitis by Regulating the Lipid Metabolism and Reducing Inflammation via the NF-kB Signaling Pathway. J. Agric. Food Chem. 2021, 69, 14192–14203. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Shin, J.S.; Chung, K.S.; Lee, Y.G.; Baek, N.I.; Lee, K.T. Anti-Inflammatory Mechanisms of Koreanaside A, a Lignan Isolated from the Flower of Forsythia koreana, against LPS-Induced Macrophage Activation and DSS-Induced Colitis Mice: The Crucial Role of AP-1, NF-kB, and JAK/STAT Signaling. Cells 2019, 8, 1163. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Wu, T.; Dai, Y.; Xu, L.; Zhong, Y.; Xue, Y.; Tian, Y. 6-gingerol alleviates inflammatory injury in DSS-induced ulcerative colitis mice by regulating NF-kB signaling. Ann. Palliat. Med. 2020, 9, 1944–1952. [Google Scholar] [CrossRef]
- Li, Y.; Pan, X.; Yin, M.; Li, C.; Han, L. Preventive Effect of Lycopene in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice through the Regulation of TLR4/TRIF/NF-kB Signaling Pathway and Tight Junctions. J. Agric. Food Chem. 2021, 69, 13500–13509. [Google Scholar] [CrossRef]
- You, B.H.; Chae, H.S.; Song, J.; Ko, H.W.; Chin, Y.W.; Choi, Y.H. α-Mangostin ameliorates dextran sulfate sodium-induced colitis through inhibition of NF-kB and MAPK pathways. Int. Immunopharmacol. 2017, 49, 212–221. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Qiao, L.; Liu, J.; Liao, X.; Huang, H.; Dong, J.; Chen, J.; Chen, D.; Wang, J. Ophiopogonin D Inhibiting Epithelial NF-kB Signaling Pathway Protects Against Experimental Colitis in Mice. Inflammation 2022, 45, 1720–1731. [Google Scholar] [CrossRef]
- Ren, Y.; Yue, B.; Ren, G.; Yu, Z.; Luo, X.; Sun, A.; Zhang, J.; Han, M.; Wang, Z.; Dou, W. Activation of PXR by alantolactone ameliorates DSS-induced experimental colitis via suppressing NF-kB signaling pathway. Sci. Rep. 2019, 9, 16636. [Google Scholar] [CrossRef]
- Xiong, H.; Tian, L.; Zhao, Z.; Chen, S.; Zhao, Q.; Hong, J.; Xie, Y.; Zhou, N.; Fu, Y. The sinomenine enteric-coated microspheres suppressed the TLR/NF-kB signaling in DSS-induced experimental colitis. Int. Immunopharmacol. 2017, 50, 251–262. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhang, Z.H.; Wang, Z.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Jin, C.H.; Xu, G.H.; Piao, L.X.; Ma, J.; et al. Convallatoxin protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-kB signaling through activation of PPARγ. Pharmacol. Res. 2019, 147, 104355. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kumar, J.M.; Sistla, R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of NF-kB signaling. J. Nutr. Biochem. 2016, 28, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, T.; Zhang, W.; Shang, Y.; Zhang, Y.; Ma, Y. Genipin attenuates dextran sulfate sodium-induced colitis via suppressing inflammatory and oxidative responses. Inflammopharmacology 2020, 28, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Shi, F.; Zhu, J.; Shao, Y.; Gong, W.; Zhou, G.; Wu, H.; She, J.; Shi, W. Piperine, a functional food alkaloid, exhibits inhibitory potential against TNBS-induced colitis via the inhibition of IκB-α/NF-kB and induces tight junction protein (claudin-1, occludin, and ZO-1) signaling pathway in experimental mice. Hum. Exp. Toxicol. 2020, 39, 477–491. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Wan, T.; Mei, Y.; Wang, Z.; Xue, J.; Luo, Y.; Li, M.; Fang, S.; Pan, H.; et al. Systems pharmacology approach uncovers Ligustilide attenuates experimental colitis in mice by inhibiting PPARγ-mediated inflammation pathways. Cell Biol. Toxicol. 2021, 37, 113–128. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Z.; Zhu, K.; Cao, H.; Liu, J.; Lu, X.; Li, Y.; Jing, Y.; Yuan, X.; Fu, Y.; et al. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-kB and NLRP3 inflammasome. Biomed. Pharmacother. 2019, 110, 786–795. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Zhang, E.; Sun, A.; Ding, L.; Chou, G.; Wang, Z.; Mani, S. Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-kB signaling pathway. J. Pharmacol. Exp. Ther. 2013, 345, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhao, Y.; Yao, J.; Zhao, L.; Wu, Z.; Wang, Y.; Pan, D.; Miao, H.; Guo, Q.; Lu, N. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-kB and NLRP3 inflammasome activation. Biochem. Pharmacol. 2015, 94, 142–154. [Google Scholar] [CrossRef]
- Li, S.; Feng, G.; Zhang, M.; Zhang, X.; Lu, J.; Feng, C.; Zhu, F. Oxymatrine attenuates TNBS-induced colinutis in rats through TLR9/Myd88/NF-kB signal pathway. Hum. Exp. Toxicol. 2022, 41, 9603271221078866. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, A.; Zhang, Z.; Yu, Z.; Liu, Y.; Peng, S.; Wu, L.; Qin, H.; Wang, W. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-kB pathway. Pharmacol. Rep. 2016, 68, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Alhassani, A.T.; Alhassani, A.S.; Ahmed, K.J.; Subramanian, V.S.; Ojha, S.K.; Attoub, S.; Adrian, T.E.; et al. Thymoquinone, a Dietary Bioactive Compound, Exerts Anti-Inflammatory Effects in Colitis by Stimulating Expression of the Colonic Epithelial PPAR-γ Transcription Factor. Nutrients 2021, 13, 1343. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Ouyang, Z.J.; Feng, L.L.; Chen, G.; Guo, W.J.; Shen, Y.; Wu, X.D.; Sun, Y.; Xu, Q. Suppression of NF-kB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol. Appl. Pharmacol. 2014, 281, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Zhang, X.Q.; Yu, C.G.; Huang, S.L.; Xie, Y.; Dou, X.T.; Liu, W.J.; Zou, X.P. Artesunate exerts protective effects against ulcerative colitis via suppressing Toll-like receptor 4 and its downstream nuclear factor-κB signaling pathways. Mol. Med. Rep. 2019, 20, 1321–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.K.; Chen, T.X.; Wang, W.; Xu, L.L.; Zhang, Y.Q.; Jin, Z.; Liu, Y.B.; Tang, Y.Z. Aesculetin exhibited anti-inflammatory activities through inhibiting NF-kB and MAPKs pathway in vitro and in vivo. J. Ethnopharmacol. 2022, 296, 115489. [Google Scholar] [CrossRef]
- Dutra, R.C.; Claudino, R.F.; Bento, A.F.; Marcon, R.; Schmidt, E.C.; Bouzon, Z.L.; Pianowski, L.F.; Calixto, J.B. Preventive and therapeutic euphol treatment attenuates experimental colitis in mice. PLoS ONE 2011, 6, e27122. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef]
- Gerges, S.H.; Tolba, M.F.; Elsherbiny, D.A.; El-Demerdash, E. The natural flavonoid galangin ameliorates dextran sulphate sodium-induced ulcerative colitis in mice: Effect on Toll-like receptor 4, inflammation and oxidative stress. Basic Clin. Pharmacol. Toxicol. 2020, 127, 10–20. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Segal, J.P.; LeBlanc, J.F.; Hart, A.L. Ulcerative colitis: An update. Clin. Med. 2021, 21, 135–139. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A. Use of Synbiotics for Ulcerative Colitis Treatment. Curr. Clin. Pharmacol. 2020, 15, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Zheng, C.Q.; Sang, L.X. Mucosal lesions of the upper gastrointestinal tract in patients with ulcerative colitis: A review. World J. Gastroenterol. 2021, 27, 2963–2978. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Veauthier, B.; Hornecker, J.R. Crohn’s Disease: Diagnosis and Management. Am. Fam. Physician 2018, 98, 661–669. [Google Scholar] [PubMed]
- Yang, F.; Ni, B.; Liu, Q.; He, F.; Li, L.; Zhong, X.; Zheng, X.; Lu, J.; Chen, X.; Lin, H.; et al. Human umbilical cord-derived mesenchymal stem cells ameliorate experimental colitis by normalizing the gut microbiota. Stem Cell Res. Ther. 2022, 13, 475. [Google Scholar] [CrossRef]
- Torres, J.; Burisch, J.; Riddle, M.; Dubinsky, M.; Colombel, J.F. Preclinical disease and preventive strategies in IBD: Perspectives, challenges and opportunities. Gut 2016, 65, 1061–1069. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal. 2022. ahead of print. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-kB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-kB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, J.P.; Carmody, R.J. NF-kB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-kB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Palazzo, I.; Todd, L.J.; Hoang, T.V.; Reh, T.A.; Blackshaw, S.; Fischer, A.J. NFkB-signaling promotes glial reactivity and suppresses Müller glia-mediated neuron regeneration in the mammalian retina. Glia 2022, 70, 1380–1401. [Google Scholar] [CrossRef]
- Maity, A.; Wollman, R. Information transmission from NFkB signaling dynamics to gene expression. PLoS Comput. Biol. 2020, 16, e1008011. [Google Scholar] [CrossRef]
- Nisr, R.B.; Shah, D.S.; Ganley, I.G.; Hundal, H.S. Proinflammatory NFkB signalling promotes mitochondrial dysfunction in skeletal muscle in response to cellular fuel overloading. Cell. Mol. Life Sci. 2019, 76, 4887–4904. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, K.; Karin, M. NF-kB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Li, B.; Wang, Y.; Xu, L.; Li, D.; Li, F.; Sun-Waterhouse, D. Attenuation of Palmitic Acid-Induced Intestinal Epithelial Barrier Dysfunction by 6-Shogaol in Caco-2 Cells: The Role of MiR-216a-5p/TLR4/NF-κB Axis. Metabolites 2022, 12, 1028. [Google Scholar] [PubMed]
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.M.; Koh, J.; Kim, J.W.; Lee, C.; Koh, S.J.; Kim, B.; Lee, K.L.; Im, J.P.; Kim, J.S. NF-kappa B activation correlates with disease phenotype in Crohn’s disease. PLoS ONE 2017, 12, e0182071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Liu, R.; Huang, L.; Xu, Y.; Su, M.; Chen, J.; Geng, L.; Xu, W.; Gong, S. CD147 Aggravated Inflammatory Bowel Disease by Triggering NF-kB-Mediated Pyroptosis. Biomed. Res. Int. 2020, 2020, 5341247. [Google Scholar] [CrossRef]
- Qiu, S.; Li, P.; Zhao, H.; Li, X. Maresin 1 alleviates dextran sulfate sodium-induced ulcerative colitis by regulating NRF2 and TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2020, 78, 106018. [Google Scholar] [CrossRef]
- Wang, G.; Xu, B.; Shi, F.; Du, M.; Li, Y.; Yu, T.; Chen, L. Protective Effect of Methane-Rich Saline on Acetic Acid-Induced Ulcerative Colitis via Blocking the TLR4/NF-kB/MAPK Pathway and Promoting IL-10/JAK1/STAT3-Mediated Anti-inflammatory Response. Oxid. Med. Cell. Longev. 2019, 2019, 7850324. [Google Scholar] [CrossRef] [Green Version]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Burge, K.; Gunasekaran, A.; Eckert, J.; Chaaban, H. Curcumin and Intestinal Inflammatory Diseases: Molecular Mechanisms of Protection. Int. J. Mol. Sci. 2019, 20, 1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; Santos, A.R.d.O.d.; Carvalho, A.C.A.d.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.d.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. https://doi.org/10.3390/metabo13010096
Laurindo LF, Santos ARdOd, Carvalho ACAd, Bechara MD, Guiguer EL, Goulart RdA, Vargas Sinatora R, Araújo AC, Barbalho SM. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites. 2023; 13(1):96. https://doi.org/10.3390/metabo13010096
Chicago/Turabian StyleLaurindo, Lucas Fornari, Ana Rita de Oliveira dos Santos, Antonelly Cassio Alves de Carvalho, Marcelo Dib Bechara, Elen Landgraf Guiguer, Ricardo de Alvares Goulart, Renata Vargas Sinatora, Adriano Cressoni Araújo, and Sandra Maria Barbalho. 2023. "Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects" Metabolites 13, no. 1: 96. https://doi.org/10.3390/metabo13010096
APA StyleLaurindo, L. F., Santos, A. R. d. O. d., Carvalho, A. C. A. d., Bechara, M. D., Guiguer, E. L., Goulart, R. d. A., Vargas Sinatora, R., Araújo, A. C., & Barbalho, S. M. (2023). Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites, 13(1), 96. https://doi.org/10.3390/metabo13010096










