Hyperbaric Oxygen Therapy Counters Oxidative Stress/Inflammation-Driven Symptoms in Long COVID-19 Patients: Preliminary Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. HBOT Protocol
2.3. Fatigue Severity Scale
2.4. Saliva and Urine Samples Collection
2.5. ROS by Electron Paramagnetic Resonance (EPR)
2.6. Total Antioxidant Capacity (TAC)
2.7. 8-Isoprostane (8-iso-PGF2α)
2.8. 8-Hydroxy-2′-deoxyguanosine (8-OH-dG)
2.9. NO Metabolites (Nitrite and Nitrate)
2.10. Quantification of Inflammatory Markers Levels in Saliva
2.11. Creatinine, Neopterin, and Uric Acid Concentration in Urine
2.12. Secondary Outcomes
2.13. Spirometry
2.14. Statistical Analysis
3. Results
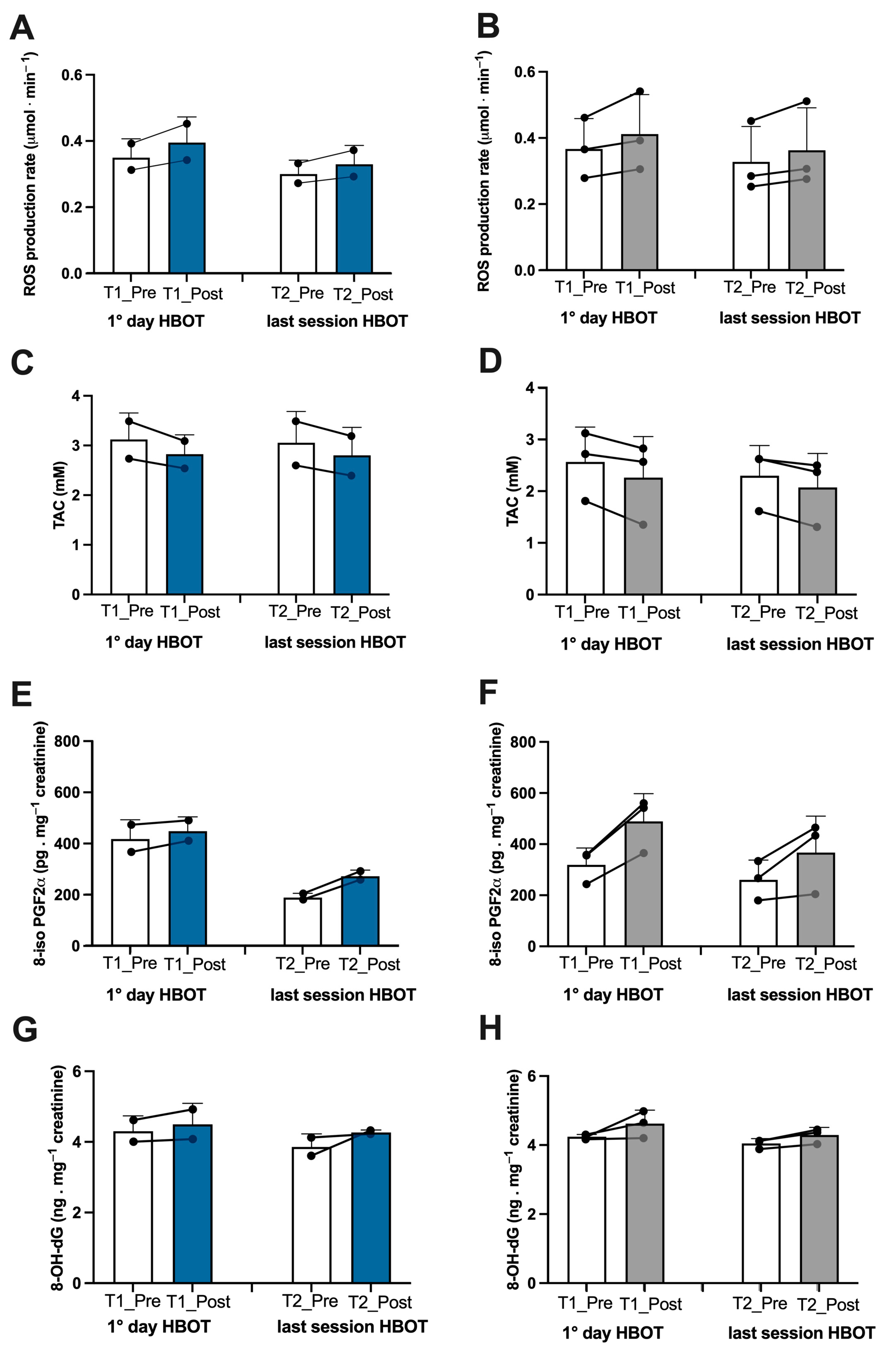
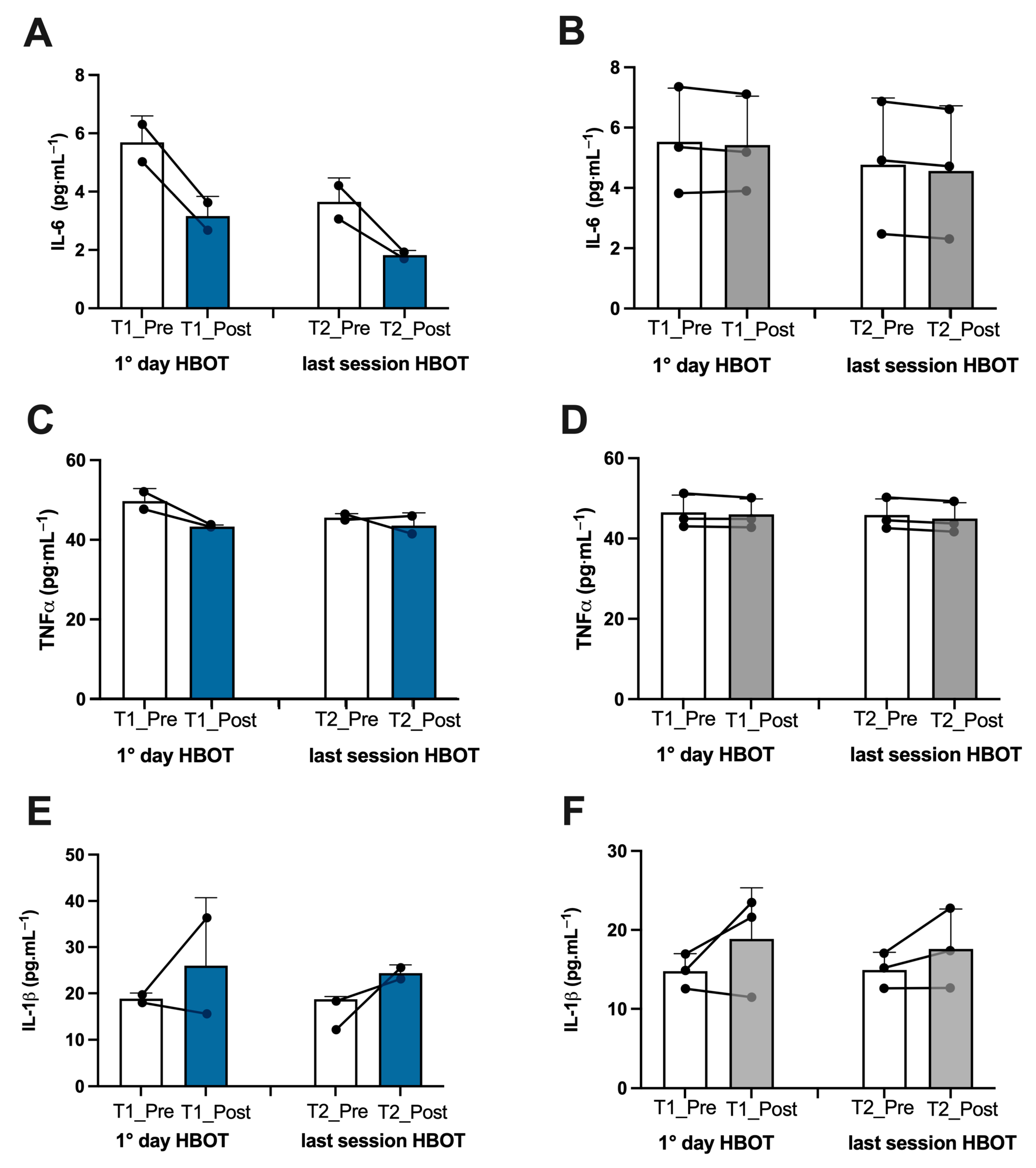
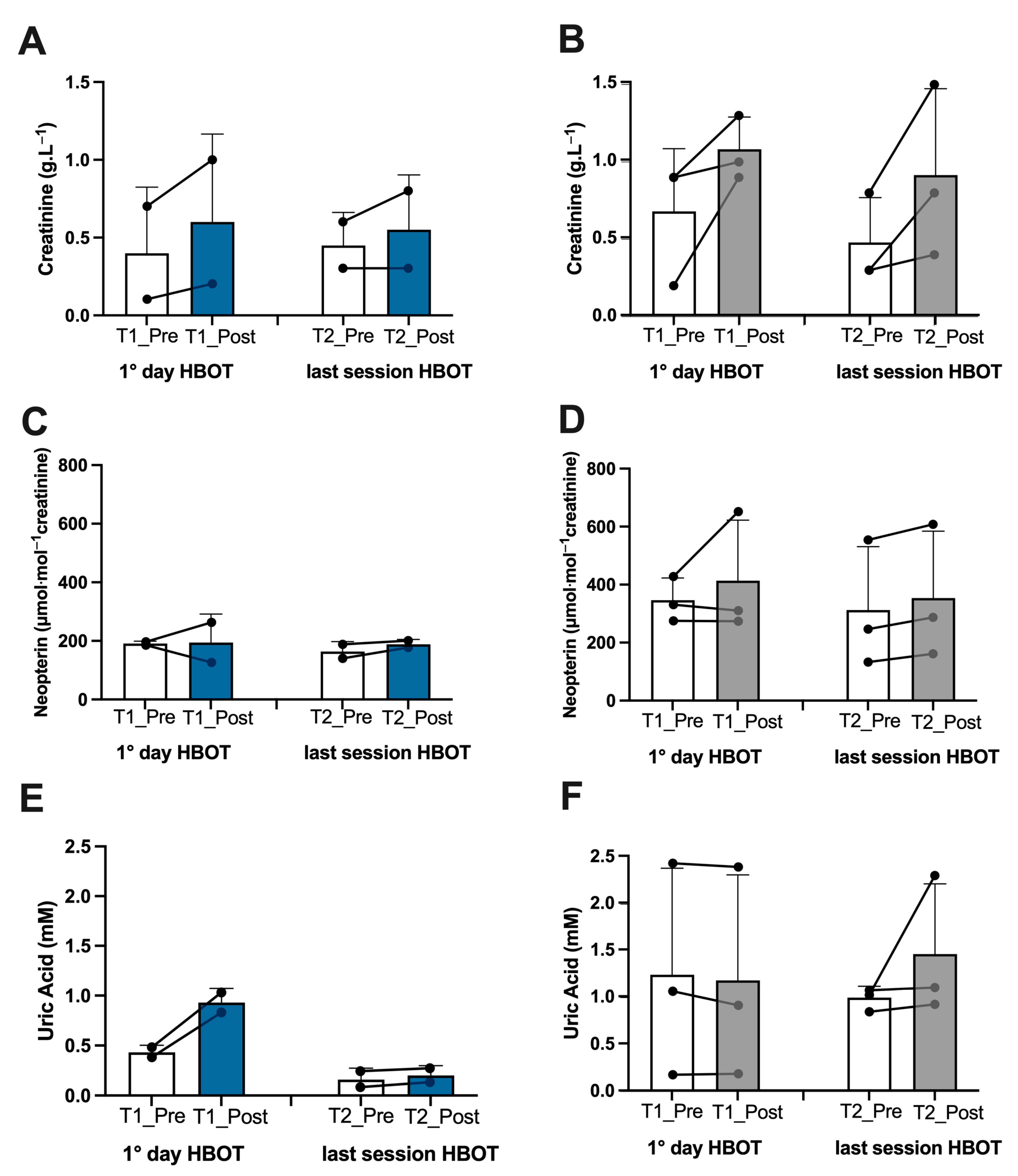
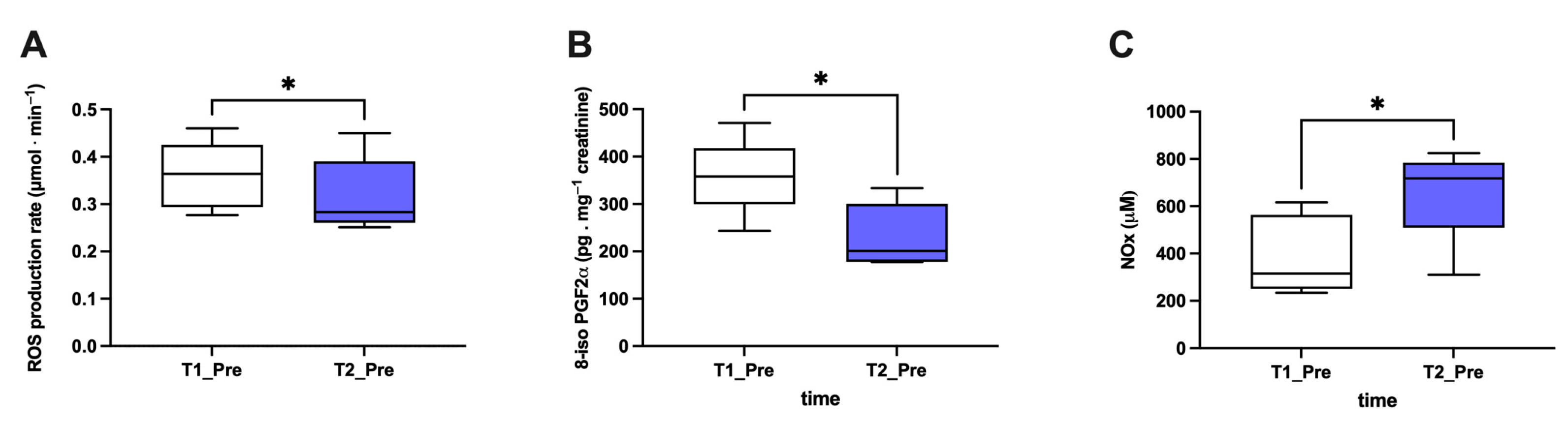
3.1. Biomarker Oxy-Inflammation
3.2. Hematological and Biochemical Analysis
3.3. Spirometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATA | Atmosphere absolute |
| BMI | Body Mass Index |
| DBP | Diastolic Blood Pressure |
| EPR | Electron Paramagnetic Resonance |
| HBOT | Hyperbaric oxygen therapy |
| HPLC | High-pressure Liquid Chromatography |
| HR | Heart Rate |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| ISSHL | Idiopathic Sudden Sensorineural Hearing Loss |
| NO | Nitric Oxide |
| NOx | Nitric Oxide metabolites |
| OxS | Oxidative Stress |
| SBP | Systolic Blood Pressure |
| ROS | Reactive Oxygen Species |
| TAC | Total Antioxidant Capacity |
| TQR | Total Quality of Recovery |
| TNFα | Tumor Necrosis Factor-alpha |
| VAS | Visual Analog Scale |
| 8-iso-PGF2α | 8-isoprostane-PGF2 alpha |
| 8-OH-dG | 8-Hydroxy-2′-deoxyguanosine |
References
- World Health Organization. Available online: http:who.int (accessed on 5 June 2023).
- Hernandez Acosta, R.A.; Garrigos, Z.E.; Marcelin, J.R.; Vijayvargiya, P. COVID-19 Pathogenesis and Clinical Manifestations. Infect. Dis. Clin. N. Am. 2022, 36, 231–249. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, A.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; Shen, Q.; Cintron, S.A.; Hiebert, J.B. Post-COVID-19 Syndrome. Nurs. Res. 2022, 71, 164–174. [Google Scholar] [CrossRef]
- Talwar, D.; Kumar, S.; Acharya, S.; Raisinghani, N.; Madaan, S.; Hulkoti, V.; Akhilesh, A.; Khanna, S.; Shah, D.; Nimkar, S. Interleukin 6 and Its Correlation with COVID-19 in Terms of Outcomes in an Intensive Care Unit of a Rural Hospital: A Cross-sectional Study. Indian J. Crit. Care Med. 2022, 26, 39–42. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regener. 2020, 40, 37. [Google Scholar] [CrossRef]
- Buicu, A.-L.; Cernea, S.; Benedek, I.; Buico, C.-F.; Benedek, T. Systemic Inflammation and COVID-19 Mortality in Patients with Major Noncommunicable Diseases: Chronic Coronary Syndromes, Diabetes and Obesity. J. Clin. Med. 2021, 10, 1545. [Google Scholar] [CrossRef]
- Saha, P.; Bose, S.; Srivastava, A.K.; Chaudhary, A.A.; Lall, R.; Prasad, S. Jeopardy of COVID-19: Rechecking the Perks of Phytotherapeutic Interventions. Molecules 2021, 26, 6783. [Google Scholar] [CrossRef]
- Guo, P.; Alvaro Benito Ballesteros, A.V.; Yeung, S.P.; Liu, R.; Saha, A.; Curtis, L.; Kaser, M.; Haggard, M.P.; Chekel, L.G. COVCOG 2: Cognitive and Memory Deficits in Long COVID: A Second Publication From the COVID and Cognition Study. Front. Aging Neurosci. 2022, 14, 804937. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Bonham, K.S.; Anam, F.A.; Walker, T.A.; Faliti, C.E.; Ishii, Y.; Kaminski, C.Y.; Ruunstrom, M.C.; Cooper, K.R.; Truong, A.D.; et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat. Commun. 2023, 14, 4201. [Google Scholar] [CrossRef]
- Grifoni, E.; Valoriani, A.; Cei, F.; Lamanna, R.; Gelli, A.M.G.; Ciambotti, B.; Vannucci, V.; Moroni, F.; Pelegatti, L.; Tarquini, R.; et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a Standardized ROS Production Profile in Humans by Electron Paramagnetic Resonance. Oxid. Med. Cell. Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Ancoraci, G.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Michael Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef]
- Bosco, G.; Vezzani, G.; Mrakic Sposta, S.; Rizzato, A.; Enten, G.; Abou-Samra, A.; Malacrida, S.; Quartesan, S.; Vezzoli, A.; Camporesi, E. Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress. J. Enzyme Inhib. Med. Chem. 2018, 33, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Hadanny, A.; Efrati, S. The hyperoxic-hypoxic paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- University of Hawaii at Manoa Honor Society. Hyperbaric Oxygen Therapy Indications, 14th ed.; Moon, R., Ed.; Best Publishing Company: North Palm Beach, FL, USA, 2019. [Google Scholar]
- Undersea and Hyperbaric Medical Society. UHMS Guidelines for credentialing, privileging and supervision of hyperbaric oxygen therapy in the U.S.A. Undersea Hyperb. Med. 2018, 45, 117–127. [Google Scholar] [CrossRef]
- Paganini, M.; Bosco, G.; Perozzo, F.A.G.; Kohlscheen, E.; Sonda, R.; Bassetto, F.; Garetto, G.; Camporesi, E.M.; Thom, S.R. The Role of Hyperbaric Oxygen Treatment for COVID-19: A Review. Adv. Exp. Med. Biol. 2021, 1289, 27–35. [Google Scholar] [CrossRef]
- Robbins, Y.T.; Gonevski, M.; Cain, C.; Baitule, S.; Sharma, K.; Magar, A.; Patel, K.; Sankar, S.; Kyrou, I.; Ali, A.; et al. Hyperbaric oxygen therapy for the treatment of long COVID: Early evaluation of a highly promising intervention. Clin. Med. 2021, 21, e629–e632. [Google Scholar] [CrossRef]
- Zilberman-Itskovich, S.; Catalogna, M.; Sasson, E.; Elman-Shina, K.; Amir Hadanny, A.; Lang, E.; Finci, S.; Polak, N.; Fishlev, G.; Korin, C.; et al. Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: Randomized controlled trial. Sci. Rep. 2022, 12, 11252. [Google Scholar] [CrossRef] [PubMed]
- Kjellberg, A.; Abdel-Halim, L.; Hassler, A.; El Gharbi, S.; Al-Ezerjawi, S.; Boström, E.; Sundberg, C.J.; Pernow, J.; Medson, K.; Kowalski, J.H.; et al. Hyperbaric oxygen for treatment of long COVID-19 syndrome (HOT-LoCO): Protocol for a randomised, placebo-controlled, double-blind, phase II clinical trial. BMJ Open 2022, 12, e061870. [Google Scholar] [CrossRef] [PubMed]
- Joli, J.; Buck, P.; Zipfel, S.; Stengel, A. Post-COVID-19 fatigue: A systematic review. Front. Psychiatry 2022, 13, 947973. [Google Scholar] [CrossRef] [PubMed]
- Bhaiyat, A.M.; Sasson, E.; Wang, Z.; Khairy, S.; Ginzarly, M.; Qureshi, U.; Fikree, M.; Efrati, S. Hyperbaric oxygen treatment for long coronavirus disease-19: A case report. J. Med. Case Rep. 2022, 16, 80. [Google Scholar] [CrossRef]
- Leitman, M.; Fuchs, S.; Tyomkin, V.; Hadanny, A.; Zilberman-Itskovich, S.; Efrati, S. The effect of hyperbaric oxygen therapy on myocardial function in post-COVID-19 syndrome patients: A randomized controlled trial. Sci. Rep. 2023, 13, 9473. [Google Scholar] [CrossRef]
- Oliaei, S.; Paranjkhoo, P.; SeyedAlinaghi, S.; Mehraeen, E.; Hackett, D. Is There a Role for Hyperbaric Oxygen Therapy in Reducing Long-Term COVID-19 Sequelae? J. Clin. Med. 2023, 12, 2270. [Google Scholar] [CrossRef]
- Kjellberg, A.; Hassler, A.; Boström, E.; El Gharbi, S.; Al-Ezerjawi, S.; Kowalski, J.; Rodriguez-Wallberg, K.A.; Bruchfeld, J.; Ståhlberg, M.; Nygren-Bonnier, M.; et al. Hyperbaric oxygen therapy for long COVID (HOT-LoCO), an interim safety report from a randomised controlled trial. BMC Infect. Dis. 2023, 23, 33. [Google Scholar] [CrossRef]
- Gorenstein, S.A.; Castellano, M.L.; Slone, E.S.; Gillette, B.; Liu, H.; Alsamarraie, C.; Jacobson, A.M.; Wall, S.P.; Adhikari, S.; Swartz, J.L.; et al. Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: Treated cases versus propensity-matched controls. Undersea Hyperb. Med. 2020, 47, 405–413. [Google Scholar] [CrossRef]
- Wilmshurst, P.; Bewley, S.; Murray, P. Hyperbaric oxygen therapy for the treatment of long COVID. Clin. Med. 2023, 23, 99–100. [Google Scholar] [CrossRef]
- Kitala, D.; Łabuś, W.; Kozielski, J.; Strzelec, P.; Nowak, M.; Knefel, G.; Dyjas, P.; Ma-terniak, K.; Kosmala, J.; Pająk, J.; et al. Preliminary Research on the Effect of Hyperbaric Oxygen Therapy in Patients with Post-COVID-19 Syndrome. J. Clin. Med. 2023, 12, 308. [Google Scholar] [CrossRef]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An overview. Diabetes Metab. Syndr. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Becker, R.C. COVID-19 and its sequelae: A platform for optimal patient care, discovery and training. J. Thromb. Thrombolysis 2021, 51, 587–594. [Google Scholar] [CrossRef]
- Chuang, L.-L.; Lin, K.-H.; Hsu, A.-L.; Wu, C.-Y.; Chang, K.-C.; Li, Y.-C.; Chen, Y. Reliability and validity of a vertical numerical rating scale supplemented with a faces rating scale in measuring fatigue after stroke. Health Qual. Life Outcomes 2015, 13, 91. [Google Scholar] [CrossRef]
- Van Campen, C.; Linda, M.C.; Rowe, P.C.; Verheugt, F.W.A.; Visser, F.C. Numeric Rating Scales Show Prolonged Post-exertional Symptoms After Orthostatic Testing of Adults With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Med. 2021, 7, 602894. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; D’Alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in Oxidative Stress Biomarkers During 30 Days in Saturation Dive: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef] [PubMed]
- Giacon, T.A.; Bosco, G.; Vezzoli, A.; Dellanoce, C.; Cialoni, D.; Paganini, M.; Mrakic-Sposta, S. Oxidative Stress and Motion Sickness in One Crew during Competitive Offshore Sailing. Sci. Rep. 2022, 12, 1142. [Google Scholar] [CrossRef] [PubMed]
- Brizzolari, A.; Bosco, G.; Vezzoli, A.; Dellanoce, C.; Barassi, A.; Paganini, M.; Cialoni, D.; Mrakic-Sposta, S. Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method. Int. J. Environ. Res. Public Health 2023, 20, 3157. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Giacon, T.A.; Paolocci, N.; Vezzoli, A.; Noce, C.D.; Paganini, M.; Agrimi, J.; Garetto, G.; Cialoni, D.; D’Alessandro, N.; et al. Dopamine/BDNF loss underscores narcosis cognitive impairment in divers: A proof of concept in a dry condition. Eur. J. Appl. Physiol. 2023, 123, 143–158. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Maderna, L.; Gregorini, F.; Montorsi, M.; Moretti, S.; Greco, F.; Cova, E.; Gussoni, M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxid. Med. Cell. Longev. 2018, 2018, 1767265. [Google Scholar] [CrossRef]
- Moretti, S.; Mrakic-Sposta, S.; Roncoroni, L.; Vezzoli, A.; Dellanoce, C.; Monguzzi, E.; Branchi, F.; Ferretti, F.; Lombardo, V.; Doneda, L.; et al. Oxidative stress as a biomarker for monitoring treated celiac disease. Clin. Transl. Gastroenterol. 2018, 9, 157. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Dellanoce, C.; Marzorati, M.; Montorsi, M.; Rasica, L.; Pratali, L.; D’Angelo, G.; Martinelli, M.; Bastiani, L.; et al. Effects of acute and sub-acute hypobaric hypoxia on oxidative stress: A field study in the Alps. Eur. J. Appl. Physiol. 2021, 121, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Mrakic Sposta, S.; Montorsi, M.; Porcelli, S.; Marzorati, M.; Healey, B.; Dellanoce, C.; Vezzoli, A. Effects of Prolonged Exposure to Hypobaric Hypoxia on Oxidative Stress: Overwintering in Antarctic Concordia Station. Oxid. Med. Cell. Longev. 2022, 2022, 4430032. [Google Scholar] [CrossRef] [PubMed]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Gussoni, M.; Levenez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different Levels (10% or 15%) of Normobaric Hypoxia Exposure. Int. J. Mol. Sci. 2023, 24, 10188. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Bosco, G.; Rizzato, A.; Quartesan, S.; Camporesi, E.; Mrakic-Sposta, S.; Moretti, S.; Balestra, C.; Rubini, A. Spirometry and oxidative stress after rebreather diving in warm water. Undersea Hyperb. Med. 2018, 45, 191–198. [Google Scholar] [CrossRef]
- Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J. Environ. Res. Public Health 2021, 18, 9755. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Napodano, C.; Callà, C.; Fiorita, A.; Marino, M.; Taddei, E.; Di Cesare, T.; Passali, G.C.; Di Santo, R.; Stefanile, A.; Fantoni, M.; et al. Salivary Biomarkers in COVID-19 Patients: Towards a Wide-Scale Test for Monitoring Disease Activity. J. Pers. Med. 2021, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Alwafi, H.A.; Ali, S.S.; Kotha, S.B.; Abuljadayel, L.W.; Ibrahim, M.; Noor Elahi, I.R.; Alwafi, H.A.; Almuhayawi, M.S.; Finkelman, M.D.; Nagla, A. El-Shitany Elevated Salivary Inflammatory Biomarkers are Associated with SARS-CoV-2 Infection Severity. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 1543918. [Google Scholar] [CrossRef] [PubMed]
- Nourin Shakeeb, N.; Varkey, P.; Hynse, A.; Ajit, A. Saliva as a Potential Specimen to Monitor IL-6, TNF-α and IL-10 in COVID-19 Patients. Inflammation 2022, 45, 2368–2374. [Google Scholar] [CrossRef]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production According to Duration of a Competitive Race. Oxid. Med. Cell. Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front. Pharmacol. 2022, 13, 899198. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Heimfarth, L.; Monteiro, B.S.; Corrêa, C.B.; Moura, T.R.; Araújo, A.A.S.; Martins-Filho, P.R.; Quintans-Júnior, L.J.; Quintans, J.S.S. Oxidative stress and inflammatory markers in patients with COVID-19: Potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. Int. Immunopharmacol. 2022, 104, 108502. [Google Scholar] [CrossRef]
- Ajčević, M.; Iscra, K.; Furlanis, G.; Michelutti, M.; Miladinović, A.; Buoite Stella, A.; Ukmar, M.; Cova, M.A.; Accardo, A.; Manganotti, P.; et al. Cerebral hypoperfusion in post-COVID-19 cognitively impaired subjects revealed by arterial spin labeling MRI. Sci. Rep. 2023, 13, 5808. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18 F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef]
- De Pace, N.L.; Colombo, J. Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems. Curr. Cardiol. Rep. 2022, 24, 1711–1726. [Google Scholar] [CrossRef]
- Halbach, J.L.; Prieto, J.M.; Wang, A.W.; Hawisher, D.; Cauvi, D.M.; Reyes, T.; Okerblom, J.; Ramirez-Sanchez, I.; Villarreal, F.; Patel, H.H.; et al. Early hyperbaric oxygen therapy improves survival in a model of severe sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R160–R168. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R. Oxidative stress is fundamental to hyperbaric oxygen therapy. J. Appl. Physiol. 2009, 106, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Salhanick, S.D.; Belikoff, B.; Orlow, D.; Holt, D.; Reenstra, W.; Buras, J.A. Hyperbaric oxygen reduces acetaminophen toxicity and increases HIF-1alpha expression. Acad. Emerg. Med. 2006, 13, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Sanja Novak, S.; Drenjancevic, I.; Vukovic, R.; Kellermayer, Z.; Cosic, A.; Levak, M.T.; Balogh, P.; Culo, F.; Mihalj, M. Anti-inflammatory effects of hyperbaric oxygenation during DSS-induced colitis in BALB/c mice include changes in gene expression of HIF-1alpha, proinflammatory cytokines, and antioxidative enzymes. Mediat. Inflamm. 2016, 2016, 7141430. [Google Scholar] [CrossRef]
- De Wolde, S.D.; Hulskes, R.H.; de Jonge, S.W.; Hollmann, M.W.; van Hulst, R.A.; Weenink, R.P.; Matthijs Kox, M. The Effect of Hyperbaric Oxygen Therapy on Markers of Oxidative Stress and the Immune Response in Healthy Volunteers. Front. Physiol. 2022, 13, 826163. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Glasauer, S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. From online data collection to identification of disease mechanisms: The IL-1ß, IL-6 and TNF-α cytokine triad is associated with post-acute sequelae of COVID-19 in a digital research cohort. SSRN Electron. J. 2021, 3, 100663. [Google Scholar] [CrossRef]
- Low, R.N.; Low, R.J.; Akrami, A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? J. Signal. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Chin, C.-S.; Lee, T.-Y.; Chen, Y.-W.; Wu, M.-F. Idiopathic Sudden Sensorineural Hearing Loss: Is Hyperbaric Oxygen Treatment the Sooner and Longer, the Better? J. Pers. Med. 2022, 12, 1652. [Google Scholar] [CrossRef]
- Kim, T.D.; Lee, S.; Yoon, S. Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants 2020, 9, 107. [Google Scholar] [CrossRef]
- Fonkoue, I.T.; Parvar, J.P.; Norrholm, S.; Li, Y.; Kankam, M.L.; Jones, T.N.; Vermulapalli, M.; Rothbaum, B.; Bremner, J.D.; Le, N.-H.; et al. Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD). Brain Behav. Immun. 2020, 83, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Beretta, S.; Cristillo, V.; Camera, G.; Morotti Colleoni, C.; Pellitteri, G.; Viti, B.; Bianchi, E.; Gipponi, S.; Grimoldi, M.; Valente, M.; et al. Incidence and Long-term Functional Outcome of Neurologic Disorders in Hospitalized Patients with COVID-19 Infected with Pre-Omicron Variants. Neurology 2023, 101, e892–e903. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef] [PubMed]
- Versace, A.; Sebastianelli, L.; Ferrazzoli, D.; Romanello, R.; Ortelli, P.; Saltuari, L.; D’Acunto, A.; Porrazzini, F.; Ajello, V.; Oliviero, A.; et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin. Neurophysiol. 2021, 132, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Ciarlone, G.E.; Hinojo, C.M.; Stavitzski, N.M.; Dean, J.B. CNS function and dysfunction during exposure to hyperbaric oxygen in operational and clinical settings. Redox Biol. 2019, 27, 101159. [Google Scholar] [CrossRef]
- Izquierdo-Alventosa, R.; Inglès, M.; Cortés-Amador, S.; Gimeno-Mallench, L.; Sempere-Rubio, N.; Chirivella, J.; Serra-Añó, P. Comparative study of the effectiveness of a low-pressure hyperbaric oxygen treatment and physical exercise in women with fi-bromyalgia: Randomized clinical trial. Ther. Adv. Musculoskelet Dis. 2020, 12, 1759720X20930493. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, A.B.; Mankowska, N.D.; Kot, J.; Winklewski, P.J. Impact of Hyperbaric Oxygen Therapy on Cognitive Functions: A Systematic Review. Neuropsychol. Rev. 2022, 32, 99–126. [Google Scholar] [CrossRef]
- Versace, V.; Ortelli, P.; Dezi, S.; Ferrazzoli, D.; Alibardi, A.; Bonini, I.; Engl, M.; Maestri, R.; Assogna, M.; Ajello, V.; et al. Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin. Neurophysiol. 2023, 145, 81–88. [Google Scholar] [CrossRef]
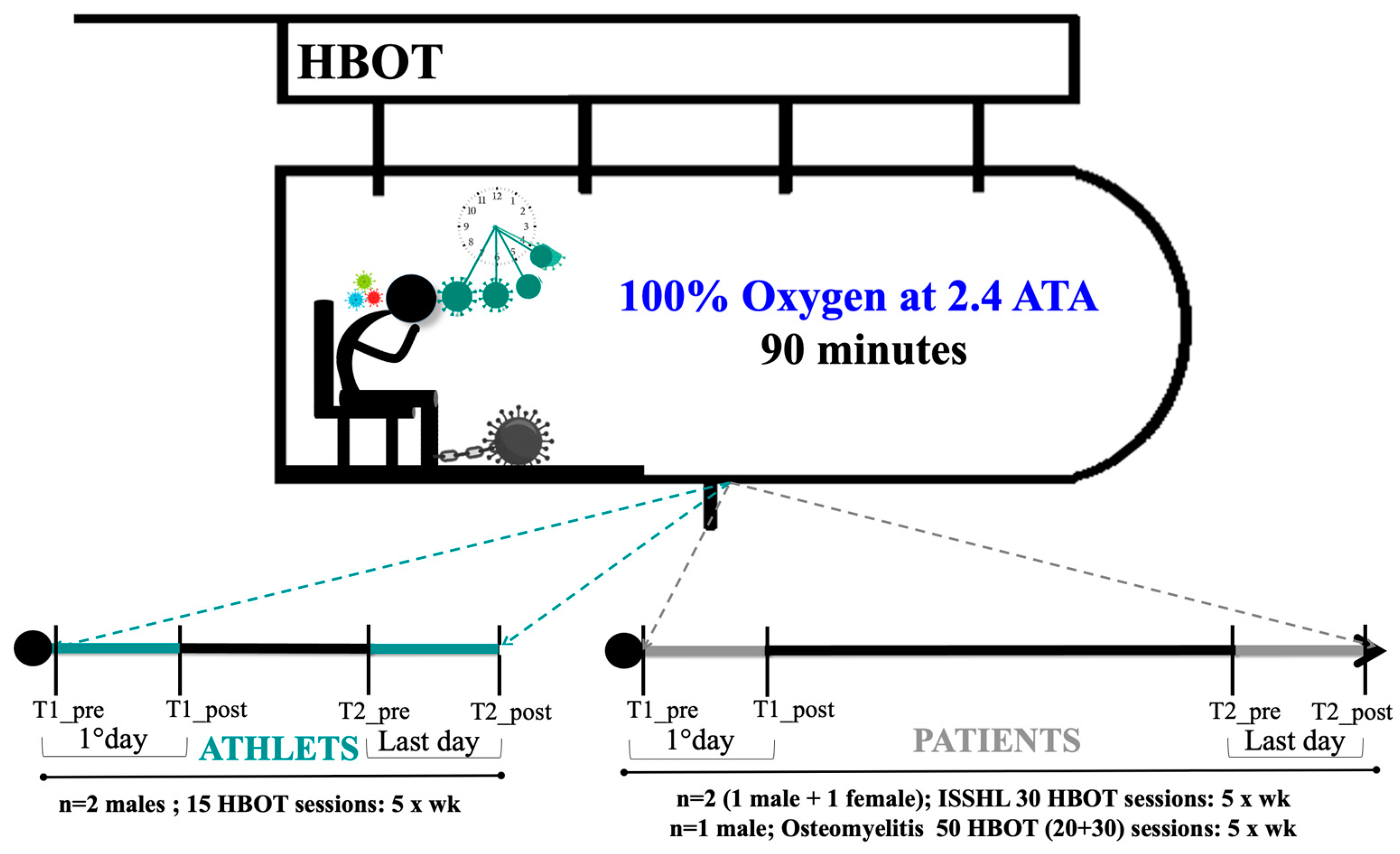

| Features of the Subjects | |||||
|---|---|---|---|---|---|
| Athlete 1 | Athlete 2 | Patient 1 ISSHL | Patient 2 ISSHL | Patient 3 Osteomyelitis | |
| Sex | Male | Male | Male | Female | Female |
| Age (years) | 28 | 28 | 48 | 55 | 47 |
| Weight (kg) | 74 | 76 | 82 | 53 | 54 |
| Height (cm) | 177 | 181 | 176 | 164 | 163 |
| BMI | 23.6 | 23.2 | 26.5 | 19.7 | 20.3 |
| HR (bpm) | 62 | 60 | 88 | 72 | 75 |
| SBP (mmHg) | 110 | 120 | 120 | 125 | 120 |
| DBP (mmHg) | 80 | 75 | 80 | 85 | 80 |
| T (°C) | 36.3 | 36.5 | 36.7 | 37.6 | 37.8 |
| Subject | Duration of Symptoms | Symptoms Long-COVID-19 | Categories by Type |
|---|---|---|---|
| 1—Athlete | 3 to >6 months | Extreme fatigue Dyspnea | Type 3B |
| 2—Athlete | 3 to >6 months | Fatigue Dyspnea | Type 3B |
| ISSHL | 3 to >6 months | Fatigue Dyspnea Dry Cough Fever | Type 3B |
| ISSHL | 1 to >3 months | Dyspnea | Type 4A |
| Osteomyelitis | 1 to >3 months | Fatigue Dyspnea Fever | Type 4A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrakic-Sposta, S.; Vezzoli, A.; Garetto, G.; Paganini, M.; Camporesi, E.; Giacon, T.A.; Dellanoce, C.; Agrimi, J.; Bosco, G. Hyperbaric Oxygen Therapy Counters Oxidative Stress/Inflammation-Driven Symptoms in Long COVID-19 Patients: Preliminary Outcomes. Metabolites 2023, 13, 1032. https://doi.org/10.3390/metabo13101032
Mrakic-Sposta S, Vezzoli A, Garetto G, Paganini M, Camporesi E, Giacon TA, Dellanoce C, Agrimi J, Bosco G. Hyperbaric Oxygen Therapy Counters Oxidative Stress/Inflammation-Driven Symptoms in Long COVID-19 Patients: Preliminary Outcomes. Metabolites. 2023; 13(10):1032. https://doi.org/10.3390/metabo13101032
Chicago/Turabian StyleMrakic-Sposta, Simona, Alessandra Vezzoli, Giacomo Garetto, Matteo Paganini, Enrico Camporesi, Tommaso Antonio Giacon, Cinzia Dellanoce, Jacopo Agrimi, and Gerardo Bosco. 2023. "Hyperbaric Oxygen Therapy Counters Oxidative Stress/Inflammation-Driven Symptoms in Long COVID-19 Patients: Preliminary Outcomes" Metabolites 13, no. 10: 1032. https://doi.org/10.3390/metabo13101032
APA StyleMrakic-Sposta, S., Vezzoli, A., Garetto, G., Paganini, M., Camporesi, E., Giacon, T. A., Dellanoce, C., Agrimi, J., & Bosco, G. (2023). Hyperbaric Oxygen Therapy Counters Oxidative Stress/Inflammation-Driven Symptoms in Long COVID-19 Patients: Preliminary Outcomes. Metabolites, 13(10), 1032. https://doi.org/10.3390/metabo13101032