Searching for New Biomarkers to Assess COVID-19 Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Patients
2.3. Sample Preparation and Biochemical Analysis
2.4. Von Willebrand Factor Analysis
2.5. Statistical Data Processing
3. Results
4. Discussion
5. Limitations of the Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ActWF | vWF activity; |
| ADAMTS13 | disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; |
| AGWF | vWF antigen; |
| ALB | albumin; |
| ALP | alkaline phosphatase; |
| ALT | alanine aminotransferase; |
| ARDS | acute respiratory distress syndrome; |
| AST | aspartate aminotransferase; |
| ATCh | acetylthiocholine; |
| AUC | area under the curve; |
| BChEa | BChE activity with ATCh; |
| BChEb | BChE activity with BTCh; |
| BTCh | butyrylthiocholine; |
| BChE | butyrylcholinesterase; |
| CIC | coronavirus-induced coagulopathy; |
| CT | computed tomography; |
| CK-NAC | total creatine phosphokinase; |
| CK-MB | muscle brain isoform of creatine phosphokinase; |
| CRP | C-reactive protein; |
| DIC | disseminated intravascular coagulation; |
| DLCO | carbon monoxide diffusion capacity of the lungs; |
| EC | endothelial cells; |
| Ferr | ferritin; |
| FEV1 | forced expiratory volume in the first second; |
| FVC | forced volume capacity; |
| GGT | gamma-glutamyltransferase; |
| HUS | hemolytic-uremic syndrome; |
| IL-6 | interleukin 6; |
| ICU | intensive care unit; |
| LDH | lactate dehydrogenase; |
| LR | likelihood ratio; |
| MAP | microangiopathy; |
| MDA | malondialdehyde; |
| MODS | multiple organ dysfunction syndrome; |
| NPA | nitrophenylacetate; |
| OR | odds ratio; |
| PE | pulmonary embolism; |
| PON1 | paraoxonase 1; |
| ROS | reactive oxygen species; |
| SO2 | oxygen saturation; |
| TTP | thrombotic thrombocytopenic purpura; |
| Urea | urea; |
| vWF | von Willebrand factor; |
| WPBs | Weibel–Palade bodies. |
References
- Kokkoris, S.; Kanavou, A.; Kremmydas, P.; Katsaros, D.; Karageorgiou, S.; Gkoufa, A.; Georgakopoulou, V.E.; Spandidos, D.A.; Giannopoulos, C.; Kardamitsi, M.; et al. Temporal evolution of laboratory characteristics in patients critically ill with COVID-19 admitted to the intensive care unit (Review). Med. Int. 2023, 3, 52. [Google Scholar] [CrossRef]
- Sozio, E.; Moore, N.A.; Fabris, M.; Ripoli, A.; Rumbolo, F.; Minieri, M.; Boverio, R.; Rodríguez Mulero, M.D.; Lainez-Martinez, S.; Martínez Martínez, M.; et al. Identification of COVID-19 patients at risk of hospital admission and mortality: A European multicentre retrospective analysis of mid-regional pro-adrenomedullin. Respir. Res. 2022, 23, 221. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, L.; He, W.; Shang, N.; Li, J.; Qin, Z.; Du, X. Circulating mid-regional proadrenomedullin is a predictor of mortality in patients with COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Alsuwaidi, L.; Al Heialy, S.; Shaikh, N.; Al Najjar, F.; Seliem, R.; Han, A.; Hachim, M. Monocyte distribution width as a novel sepsis indicator in COVID-19 patients. BMC Infect. Dis. 2022, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Ligi, D.; Lo Sasso, B.; Henry, B.M.; Ciaccio, M.; Lippi, G.; Plebani, M.; Mannello, F. Deciphering the role of monocyte and monocyte distribution width (MDW) in COVID-19: An updated systematic review and meta-analysis. Clin. Chem. Lab. Med. 2023, 61, 960–973. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.J.M.D.; Chia, A.Z.Q.; Tan, B.K.J.; Teo, C.B.; Lim, V.; Chua, H.R.; Samuel, M.; Kee, A. Electrolyte imbalances as poor prognostic markers in COVID-19: A systemic review and meta-analysis. J. Endocrinol. Investig. 2023, 46, 235–259. [Google Scholar] [CrossRef]
- Hopkins, F.R.; Nordgren, J.; Fernandez-Botran, R.; Enocsson, H.; Govender, M.; Svanberg, C.; Svensson, L.; Hagbom, M.; Nilsdotter-Augustinsson, Å.; Nyström, S.; et al. Pentameric C-reactive protein is a better prognostic biomarker and remains elevated for longer than monomeric CRP in hospitalized patients with COVID-19. Front. Immunol. 2023, 14, 1259005. [Google Scholar] [CrossRef]
- Capra, A.P.; Ardizzone, A.; Pantò, G.; Paterniti, I.; Campolo, M.; Crupi, L.; Squeri, R.; Esposito, E. The Prognostic Value of Pentraxin-3 in COVID-19 Patients: A Systematic Review and Meta-Analysis of Mortality Incidence. Int. J. Mol. Sci. 2023, 24, 3537. [Google Scholar] [CrossRef]
- Ke, Y.; Wu, K.; Shen, C.; Zhu, Y.; Xu, C.; Li, Q.; Hu, J.; Liu, S. Clinical Utility of Circulating Pentraxin 3 as a Prognostic Biomarker in Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Infect. Dis. Ther. 2023, 12, 67–80. [Google Scholar] [CrossRef]
- Larsson, A.; Lipcsey, M.; Hultström, M.; Frithiof, R.; Eriksson, M. Plasma Leptin Is Increased in Intensive Care Patients with COVID-19-An Investigation Performed in the PronMed-Cohort. Biomedicines 2021, 10, 4. [Google Scholar] [CrossRef]
- Grewal, T.; Buechler, C. Adipokines as Diagnostic and Prognostic Markers for the Severity of COVID-19. Biomedicines 2023, 11, 1302. [Google Scholar] [CrossRef]
- de Nooijer, A.H.; Pickkers, P.; Netea, M.G.; Kox, M. Inflammatory biomarkers to predict the prognosis of acute bacterial and viral infections. J. Crit. Care 2023, 78, 154360. [Google Scholar] [CrossRef]
- Liu, A.; Hammond, R.; Donnelly, P.D.; Kaski, J.C.; Coates, A.R.M. Effective prognostic and clinical risk stratification in COVID-19 using multimodality biomarkers. J. Intern. Med. 2023, 294, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Kettunen, J.; Würtz, P.; Haller, T.; Havulinna, A.S.; Kangas, A.J.; Soininen, P.; Esko, T.; Tammesoo, M.L.; Mägi, R.; et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons. PLoS Med. 2014, 11, e1001606. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yu, S.; Liu, H.; Suo, L.; Tang, K.; Hu, J.; Shi, Y.; Hu, K. Survival Analysis and Risk Factors in COVID-19 Patients. Disaster Med. Public Health Prep. 2022, 16, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, H.; Liao, X.; Qin, Z.; Xu, F.; Friedman, S.; Ma, G.; Ye, K.; Lin, S. Building a predictive model to identify clinical indicators for COVID-19 using machine learning method. Med. Biol. Eng. Comput. 2022, 60, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Saini, N.; Ram, S.; Soni, S.L.; Suri, V.; Malhotra, P.; Kaur, J.; Verma, I.; Sharma, S.; Zohmangaihi, D. COVID-19 associated variations in liver function parameters: A retrospective study. Postgrad. Med. J. 2022, 98, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Al-Shajlawi, M.; Alsayed, A.R.; Abazid, H.; Awajan, D.; Al-Imam, A.; Basheti, I. Using laboratory parameters as predictors for the severity and mortality of COVID-19 in hospitalized patients. Pharm. Pract. 2022, 20, 2721. [Google Scholar] [CrossRef]
- Wang, F.; Tessier, A.J.; Liang, L.; Wittenbecher, C.; Haslam, D.E.; Fernández-Duval, G.; Heather Eliassen, A.; Rexrode, K.M.; Tobias, D.K.; Li, J.; et al. Plasma metabolomic profiles associated with mortality and longevity in a prospective analysis of 13,512 individuals. Nat. Commun. 2023, 14, 5744. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Ukolov, A.I.; Orlova, T.I.; Migalovskaia, E.D.; Voitenko, N.G. Metabolomics: On the Way to Integration of Biochemistry, Analytical Chemistry, and Informatics. Usp. Sovr. Biol. 2015, 135, 3–17. (In Russian) [Google Scholar] [CrossRef]
- Voitenko, N.G.; Garniuk, V.V.; Prokofieva, D.S.; Gontcharov, N.V. On new screening biomarker to evaluate health state in personnel engaged into chemical weapons extinction. Med. Tr. I Promyshlennaya Ekol. 2015, 2015, 38–42. (In Russian) [Google Scholar]
- Ukolov, A.I.; Kessenikh, E.D.; Radilov, A.S.; Goncharov, N.V. Toxicometabolomics: Identification of markers of chronic exposure to low doses of aliphatic hydrocarbons. J. Evol. Biochem. Physiol. 2017, 53, 25–36. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Popova, P.I.; Voitenko, N.G.; Shmurak, V.I.; Vovk, M.A.; Baranova, T.I.; Batalova, A.A.; Korf, E.A.; Avdonin, P.V.; et al. Albumin Is a Component of the Esterase Status of Human Blood Plasma. Int. J. Mol. Sci. 2023, 24, 10383. [Google Scholar] [CrossRef] [PubMed]
- Sipahioglu, H.; Onuk, S. Lactate dehydrogenase/albumin ratio as a prognostic factor in severe acute respiratory distress syndrome cases associated with COVID-19. Medicine 2022, 101, 30759. [Google Scholar] [CrossRef] [PubMed]
- Sai, I.N.; Prasad, R.; Varsha, T. Assessing the Prognostic Value of Crp/Albumin Ratio and Lactate/Albumin Ratio in Critically Ill Patients. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar] [PubMed]
- Anzo, F.M.; Buan-Mayo, M. Nutritional biomarkers as predictors of clinical outcomes between COVID-19 severity groups in a tertiary government hospital. Clin. Nutr. ESPEN 2023, 53, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Torun, A.; Çakırca, T.D.; Çakırca, G.; Portakal, R.D. The value of C-reactive protein/albumin, fibrinogen/albumin, and neutrophil/lymphocyte ratios in predicting the severity of CoVID-19. Rev. Assoc. Med. Bras. (1992) 2021, 67, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Makkar, K.; Sharma, Y.P.; Batta, A.; Hatwal, J.; Panda, P.K. Role of fibrinogen, albumin and fibrinogen to albumin ratio in determining angiographic severity and outcomes in acute coronary syndrome. World J. Cardiol. 2023, 15, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Tosun, F.; Comert, E.; Duran, M.; Tuna, V.D. The Relationship of CRP/Albumin ratio level and prognosis in pregnant COVID-19 patients. Niger. J. Clin. Pract. 2022, 25, 1745–1750. [Google Scholar] [CrossRef]
- Kalyon, S.; Gültop, F.; Şimşek, F.; Adaş, M. Relationships of the neutrophil-lymphocyte and CRP-albumin ratios with the duration of hospitalization and fatality in geriatric patients with COVID-19. J. Int. Med. Res. 2021, 49, 3000605211046112. [Google Scholar] [CrossRef]
- Detsika, M.G.; Grigoriou, E.; Psarra, K.; Jahaj, E.; Tsipilis, S.; Athanassiou, N.; Zacharis, A.; Dimopoulou, I.; Orfanos, S.E.; Tsirogianni, A.; et al. Combination of the CD8+:B-cell and Neutrophil-to-Lymphocyte Ratio as a Novel Prediction Model for Intubation Need and Disease Severity in COVID-19 Patients. Vivo 2021, 35, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Tocoglu, A.; Dheir, H.; Bektas, M.; Acikgoz, S.B.; Karabay, O.; Sipahi, S. Predictors of Mortality in Patients with COVID-19 Infection-associated Acute Kidney Injury. J. Coll. Physicians Surg. Pak. 2021, 30, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Feketea, G.M.; Vlacha, V. The Diagnostic Significance of Usual Biochemical Parameters in Coronavirus Disease 19 (COVID-19): Albumin to Globulin Ratio and CRP to Albumin Ratio. Front. Med. 2020, 7, 566591. [Google Scholar] [CrossRef] [PubMed]
- Afsin, D.E.; Kerget, B. Evaluation of the Relationship between CRP/Albumin Ratio and Pulmonary Function Parameters in Patients with Post-Acute COVID-19. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef] [PubMed]
- Avdonin, P.P.; Tsvetaeva, N.V.; Goncharov, N.V.; Rybakova, E.Y.; Trufanov, S.K.; Tsitrina, A.A.; Avdonin, P.V. Von Willebrand Factor in Health and Disease. Biochem. Moscow Suppl. Ser. A 2021, 15, 201–218. [Google Scholar] [CrossRef]
- Naß, J.; Terglane, J.; Gerke, V. Weibel Palade Bodies: Unique Secretory Organelles of Endothelial Cells that Control Blood Vessel Homeostasis. Front. Cell. Dev. Biol. 2021, 9, 813995. [Google Scholar] [CrossRef]
- Furlan, M.; Robles, R.; Solenthaler, M.; Wassmer, M.; Sandoz, P.; Lämmle, B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood 1997, 89, 3097–3103. [Google Scholar] [CrossRef]
- Blasco, M.; Guillén-Olmos, E.; Diaz-Ricart, M.; Palomo, M. Complement Mediated Endothelial Damage in Thrombotic Microangiopathies. Front. Med. 2022, 9, 811504. [Google Scholar] [CrossRef]
- Kwaan, H.C.; Bennett, C.L. Thrombotic thrombocytopenic purpura—2005. Semin. Thromb. Hemost. 2005, 31, 611–614. [Google Scholar] [CrossRef]
- Nightingale, T.D.; McCormack, J.J.; Grimes, W.; Robinson, C.; Lopes da Silva, M.; White, I.J.; Vaughan, A.; Cramer, L.P.; Cutler, D.F. Tuning the endothelial response: Differential release of exocytic cargos from Weibel-Palade bodies. J. Thromb. Haemost. 2018, 16, 1873–1886. [Google Scholar] [CrossRef]
- Cramer, E.M.; Meyer, D.; le Menn, R.; Breton-Gorius, J. Eccentric localization of von Willebrand factor in an internal structure of platelet alpha-granule resembling that of Weibel-Palade bodies. Blood 1985, 66, 710–713. [Google Scholar] [CrossRef]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood 2015, 125, 2019–2028. [Google Scholar] [CrossRef]
- Torisu, T.; Torisu, K.; Lee, I.H.; Liu, J.; Malide, D.; Combs, C.A.; Wu, X.S.; Rovira, I.I.; Fergusson, M.M.; Weigert, R.; et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat. Med. 2013, 19, 1281–1287. [Google Scholar] [CrossRef]
- El-Mansi, S.; Nightingale, T.D. Emerging mechanisms to modulate VWF release from endothelial cells. Int. J. Biochem. Cell Biol. 2021, 131, 105900. [Google Scholar] [CrossRef]
- Antonova, O.A.; Loktionova, S.A.; Golubeva, N.V.; Romanov, Y.A.; Mazurov, A.V. Damage and activation of endothelial cells during in vitro hypoxia. Bull. Exp. Biol. Med. 2007, 144, 504–506. [Google Scholar] [CrossRef]
- Okhota, S.; Melnikov, I.; Avtaeva, Y.; Kozlov, S.; Gabbasov, Z. Shear Stress-Induced Activation of von Willebrand Factor and Cardiovascular Pathology. Int. J. Mol. Sci. 2020, 21, 7804. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.W.; van Wijk, X.M.R.; Pham, H.P.; Marin, M.J. Role of von Willebrand Factor in COVID-19 Associated Coagulopathy. J. Appl. Lab. Med. 2021, 6, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.; Baronciani, L.; Artoni, A.; Colpani, P.; Biganzoli, M.; Cozzi, G.; Novembrino, C.; Boscolo Anzoletti, M.; De Zan, V.; Pagliari, M.T.; et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J. Thromb. Haemost. 2021, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Marco, P. Von Willebrand factor and ADAMTS13 activity as clinical severity markers in patients with COVID-19. J. Thromb. Thrombolysis 2021, 52, 497–503. [Google Scholar] [CrossRef]
- Barnes, P.W.; McFadden, S.L.; Machin, S.J.; Simson, E.; international consensus group for hematology. The international consensus group for hematology review: Suggested criteria for action following automated CBC and WBC differential analysis. Lab. Hematol. 2005, 11, 83–90. [Google Scholar] [CrossRef]
- Kanduri, S.R.; Ramanand, A.; Varghese, V.; Wen, Y.; Mohamed, M.M.B.; Velez, J.C.Q. Refractoriness of Hyperkalemia and Hyperphosphatemia in Dialysis-Dependent AKI Associated with COVID-19. Kidney360 2022, 3, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Geeting, D.; Alibrahim, O.; Patel, M.; Kumar, R.; Mallory, P. COVID-19 and Severe Rhabdomyolysis Causing Acute Kidney Injury and Life-Threatening Hyperkalemia in a Pediatric Patient: A Case Report. SN Compr. Clin. Med. 2023, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Erfurt, S.; Lehmann, R.; Matyukhin, I.; Marahrens, B.; Patschan, S.; Patschan, D. Stratification of Acute Kidney Injury Risk, Disease Severity, and Outcomes by Electrolyte Disturbances. J. Clin. Med. Res. 2023, 15, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Al Qahtani, S.Y. Impact of hyperchloremia on inflammatory markers, serum creatinine, hemoglobin, and outcome in critically ill patients with COVID-19 infection. J. Med. Life. 2023, 16, 699–706. [Google Scholar] [CrossRef]
- Efat, A.; Shoeib, S.; ElKholy, A.; Hussein Aboelela, O.S.; Elshamy, D. Blood phenotype O and indirect bilirubin are associated with lower, early COVID-19-related mortality: A retrospective study. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221133952. [Google Scholar] [CrossRef]
- Asha, K.S.; Singh, V.; Singi, Y.; Ranjan, R. The Association of Hematological and Biochemical Parameters with Mortality Among COVID-19 Patients: A Retrospective Study From North India. Cureus 2022, 14, e29198. [Google Scholar] [CrossRef]
- Torabizadeh, C.; Iloonkashkooli, R.; Haghshenas, H.; Fararouei, M. Prevalence of Cardiovascular Complications in Coronavirus Disease 2019 adult Patients: A Systematic Review and Meta-Analysis. Iran J. Med. Sci. 2023, 48, 243–267. [Google Scholar] [CrossRef]
- Keykavousi, K.; Nourbakhsh, F.; Abdollahpour, N.; Fazeli, F.; Sedaghat, A.; Soheili, V.; Sahebkar, A. A Review of Routine Laboratory Biomarkers for the Detection of Severe COVID-19 Disease. Int. J. Anal. Chem. 2022, 2022, 9006487. [Google Scholar] [CrossRef]
- Semiz, S. COVID19 biomarkers: What did we learn from systematic reviews? Front. Cell Infect. Microbiol. 2022, 12, 1038908. [Google Scholar] [CrossRef]
- Arigondam, A.K.; Hakeem, A.R.; Reddy, M.S.; Rela, M. An Evidence-based Protocol for Minimizing Thromboembolic Events in SARS-CoV-2 Infection. Arch. Med. Res. 2021, 52, 252–260. [Google Scholar] [CrossRef]
- McGrath, R.T.; McRae, E.; Smith, O.P.; O’Donnell, J.S. Platelet von Willebrand factor--structure, function and biological importance. Br. J. Haematol. 2010, 148, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Schmugge, M.; Rand, M.L.; Freedman, J. Platelets and von Willebrand factor. Transfus. Apher. Sci. 2003, 28, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.W.; Tisdall, P.; Fremont-Smith, P.; Liu, Y.; Huang, X.P.; White, K.M.; Miorin, L.; Moreno, E.; Alon, A.; Delaforge, E.; et al. COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms. Front. Pharmacol. 2021, 12, 633680. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Vasilyev, K.A.; Kudryavtsev, I.V.; Avdonin, P.P.; Belinskaia, D.A.; Stukova, M.A.; Shamova, O.V.; Avdonin, P.V. Experimental Search for New Means of Pathogenetic Therapy COVID-19: Inhibitor of H2-Receptors Famotidine Increases the Effect of Oseltamivir on Survival and Immune Status of Mice Infected by A/PR/8/34 (H1N1). J. Evol. Biochem. Phys. 2022, 58, 230–246. [Google Scholar] [CrossRef]
- Miteva, K.T.; Pedicini, L.; Wilson, L.A.; Jayasinghe, I.; Slip, R.G.; Marszalek, K.; Gaunt, H.J.; Bartoli, F.; Deivasigamani, S.; Sobradillo, D.; et al. Rab46 integrates Ca2+ and histamine signaling to regulate selective cargo release from Weibel-Palade bodies. J. Cell. Biol. 2019, 218, 2232–2246. [Google Scholar] [CrossRef] [PubMed]
- De Ceunynck, K.; De Meyer, S.F.; Vanhoorelbeke, K. Unwinding the von Willebrand factor strings puzzle. Blood 2013, 121, 270–277. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Garnyuk, V.V.; Nadeev, A.D.; Goncharov, N.V. Hydrogen peroxide modulates expression of surface antigens by human umbilical vein endothelial cells in vitro. Biochem. Moscow Suppl. Ser. A 2014, 8, 97–102. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, Y.; Hou, X. Lipoxin A4 restores oxidative stress-induced vascular endothelial cell injury and thrombosis-related factor expression by its receptor-mediated activation of Nrf2-HO-1 axis. Cell Signal 2019, 60, 146–153. [Google Scholar] [CrossRef]
- Avdonin, P.V.; Tsitrina, A.A.; Mironova, G.Y.; Avdonin, P.P.; Zharkikh, I.L.; Nadeev, A.D.; Goncharov, N.V. Hydrogen Peroxide Stimulates Exocytosis of Von Willebrand Factor in Human Umbilical Vein Endothelial Cells. Biol. Bull. 2017, 44, 531–537. [Google Scholar] [CrossRef]
- Avdonin, P.P.; Trufanov, S.K.; Rybakova, E.Y.; Tsitrina, A.A.; Goncharov, N.V.; Avdonin, P.V. The use of fluorescently labeled ARC1779 aptamer for assessimg the effect of H2O2 on von Willebrand factor exocytosis. Biochemistry 2021, 86, 1–9. [Google Scholar] [CrossRef]
- Bernardo, A.; Ball, C.; Nolasco, L.; Moake, J.F.; Dong, J.F. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood 2004, 104, 100–106. [Google Scholar] [CrossRef]
- Mo, S.J.; Son, E.W.; Rhee, D.K.; Pyo, S. Modulation of TNF-alpha-induced ICAM-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch. Pharm. Res. 2003, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Yamakuchi, M.; Morrell, C.N.; Ozaki, M.; O’Rourke, B.; Irani, K.; Lowenstein, C.J. Vascular endothelial growth factor regulation of Weibel-Palade-body exocytosis. Blood 2005, 105, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.M.; Thompson, M.D.; Bolotina, V.M.; Tong, X.; Cohen, R.A. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic. Biol. Med. 2012, 53, 2327–2334. [Google Scholar] [CrossRef]
- Galstyan, G.M. Coagulopathy in COVID-19. Pulmonologiya 2020, 30, 645–657. [Google Scholar] [CrossRef]
- Chang, J.C. COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on “Two-Path Unifying Theory” of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy. Vasc. Health Risk Manag. 2021, 17, 273–298. [Google Scholar] [CrossRef]
- Ward, S.; O’Sullivan, J.M.; O’Donnell, J.S. von Willebrand factor sialylation-A critical regulator of biological function. J. Thromb. Haemost. 2019, 17, 1018–1029. [Google Scholar] [CrossRef]
- Ducastel, M.; Chenevier-Gobeaux, C.; Ballaa, Y.; Meritet, J.F.; Brack, M.; Chapuis, N.; Pene, F.; Carlier, N.; Szwebel, T.A.; Roche, N.; et al. Oxidative Stress and Inflammatory Biomarkers for the Prediction of Severity and ICU Admission in Unselected Patients Hospitalized with COVID-19. Int. J. Mol. Sci. 2021, 22, 7462. [Google Scholar] [CrossRef]
- Kaya Gök, A.; Turkmen, A.; Köse, E.; Çengel, F.; Şehirlioglu, S. Correlation of important prognostic factors and CT scores in invasive and non-invasive ventilation of COVID-19 patients. Ulus. Travma Acil Cerrahi Derg. 2023, 29, 163–168. [Google Scholar] [CrossRef]
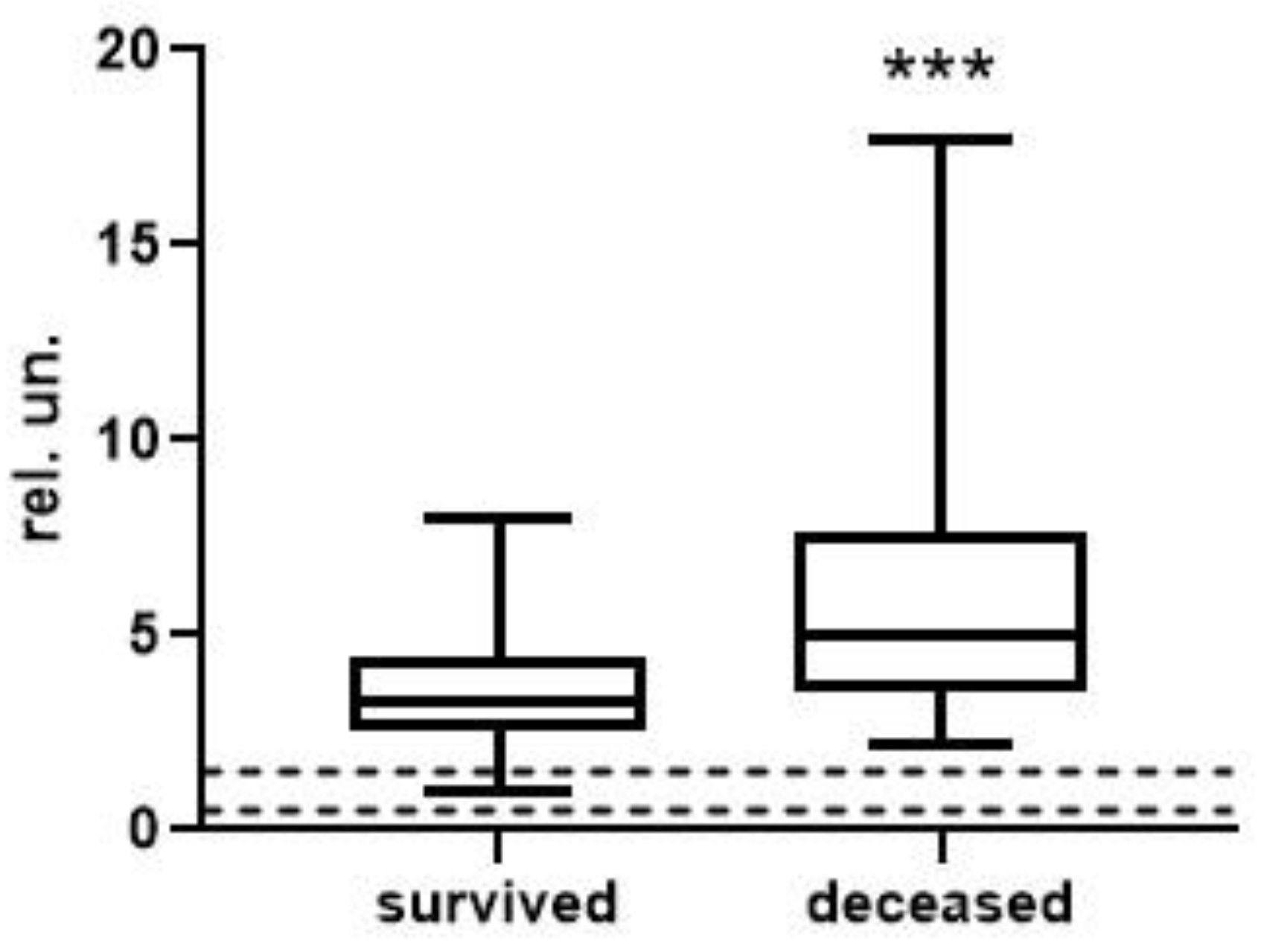
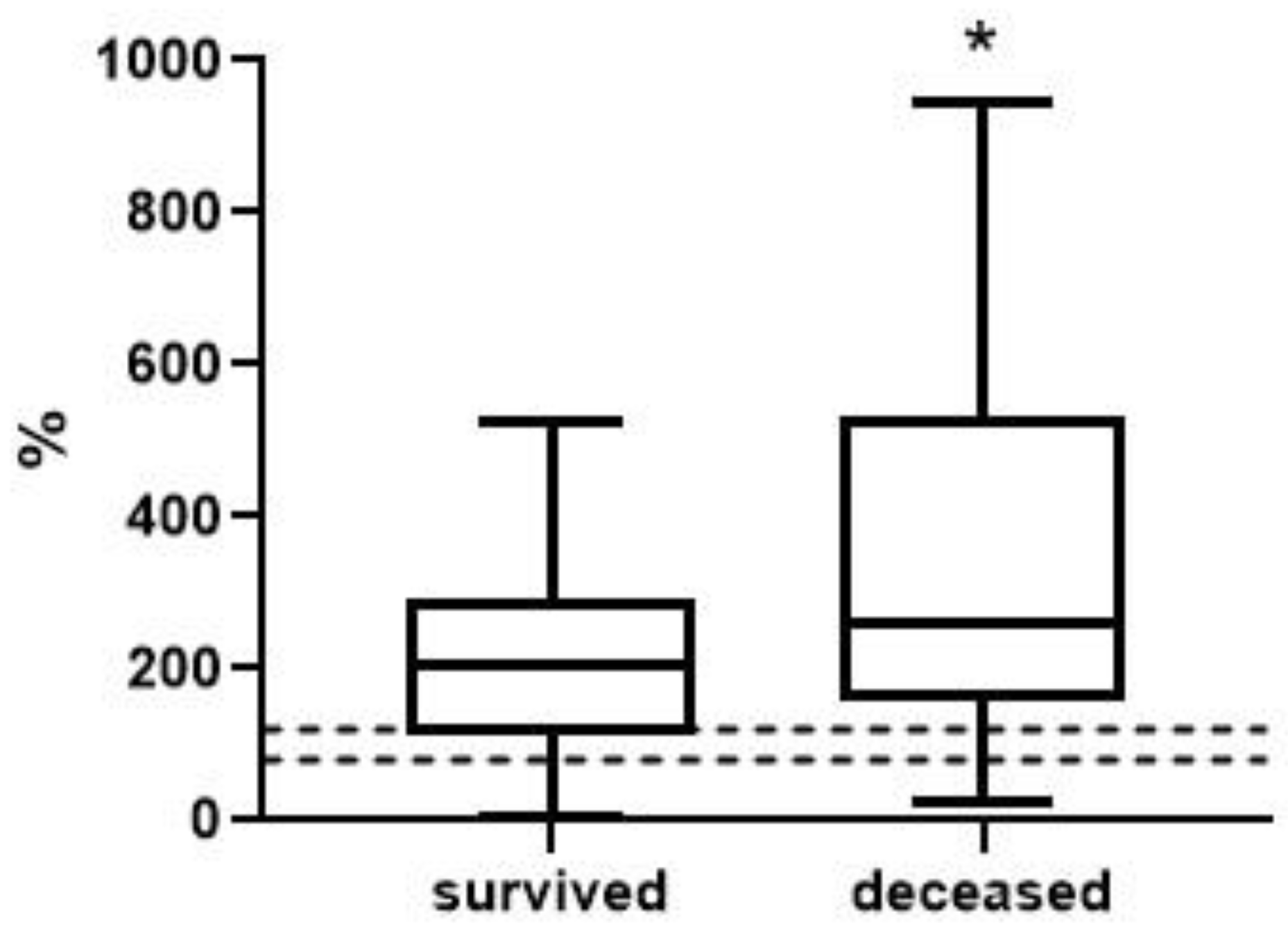



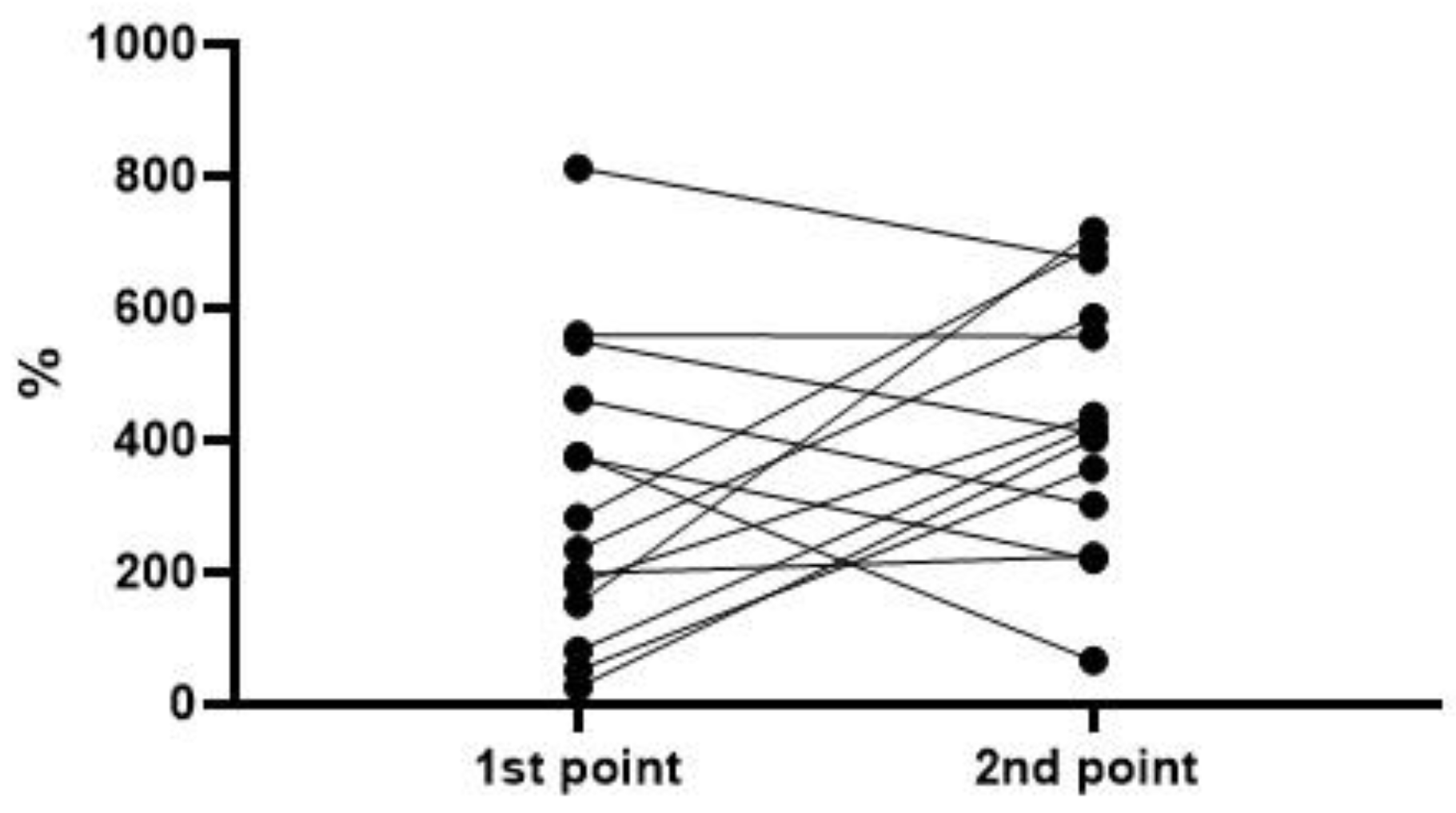
| Survivors | Deceased | |
|---|---|---|
| Total patients (101) | 77 | 24 |
| Age (M ± SD) | 49.6 ± 10.9 | 53.8 ± 7.9 |
| Age Me (min, max) | 55 (19, 60) | 57.5 (35, 62) |
| CT results, % lung lesion on admission | 36 ± 15 | 56 ± 19 |
| Men (63) | 44 | 19 |
| Age of men (M ± SD) | 48 ± 11.8 | 53.3 ± 7.7 |
| Age of men Me (min, max) | 55 (19, 60) | 56 (35, 62) |
| CT results, % lung lesion on admission | 36 ± 15 | 56 ± 17 |
| Women (38) | 33 | 5 |
| Age of women (M ± SD) | 51.7 ± 9.2 | 55.6 ± 9.3 |
| Age of women Me (min, max) | 56 (25, 60) | 60 (39, 60) |
| CT results, % lung lesion on admission | 36 ± 16 | 57 ± 26 |
| Biochemical Values, Normal Ranges | Outcome | n | Median | Range (Min to Max) | Interquartile Range (1–3 Quartiles) | p Value |
|---|---|---|---|---|---|---|
| Potassium, 3.5–5.5 mmol/L | S | 68 (88%) | 3.88 | 2.40–5.60 | 3.50–4.22 | 0.0087 |
| D | 22 (92%) | 4.25 ** | 3.40–6.30 | 3.80–4.70 | ||
| Sodium, 130–150 mmol/L | S | 66 (86%) | 137.1 | 105–145.1 | 135–139 | 0.0008 |
| D | 22 (92%) | 140.0 *** | 131–148 | 138.7–143.1 | ||
| Chloride, 95–110 mmol/L | S | 66 (86%) | 103.6 | 79.4–137.0 | 101–105 | 0.0449 |
| D | 20 (83%) | 105.9 * | 91.0–119.0 | 101.7–110.1 | ||
| Calcium, 2.20–2.65 mmol/L | S | 5 (6%) | 2.29 | 2.15–2.35 | 2.20–2.32 | |
| D | 0 (0%) | - | - | - | ||
| Direct bilirubin, 0–3.4 µmol/L | S | 7 (9%) | 2.10 | 1.50–3.00 | 1.70–2.90 | 0.0105 |
| D | 10 (42%) | 8.70 * | 1.30–76.80 | 2.88–26.58 | ||
| Total bilirubin, 8.5–20.5 µmol/L | S | 60 (78%) | 10.00 | 4.80–23.40 | 8.13–12.33 | 0.0769 |
| D | 16 (67%) | 12.55 | 6.4–45.0 | 8.5–25.23 | ||
| Albumin, 35–52 g/L | S | 39 (51%) | 37.4 | 29.0–46.5 | 34.4–41.2 | <0.0001 |
| D | 15 (63%) | 30.8 **** | 19.9–38.9 | 28.3–34.4 | ||
| Total protein, 66–87 g/L | S | 65 (84%) | 70.6 | 45.3–88.2 | 66.6–74.3 | 0.0057 |
| D | 19 (79%) | 61.6 ** | 44–83 | 57.6–70.5 | ||
| Glucose, 3.5–6.10 mmol/L | S | 68 (88%) | 5.36 | 3.40–18.32 | 4.81–6.64 | 0.3383 |
| D | 19 (79%) | 6.20 | 1.00–15.30 | 4.20–9.10 | ||
| Iron, 10–32 µmol/L | S | 25 (32%) | 7.6 | 2.3–28.6 | 3.3–13.0 | 0.5165 |
| D | 4 (17%) | 12.8 | 2.7–58.7 | 3.4–49.1 | ||
| Ferritin, 20–250 µg/L | S | 53 (69%) | 482 | 7.8–3306 | 190.4–656 | 0.1311 |
| D | 11 (46%) | 616 | 134–1212 | 330–963 | ||
| Creatinine, 72–127 µmol/L | S | 70 (91%) | 90.3 | 61.1–203.8 | 76.9–104.4 | 0.5848 |
| D | 23 (96%) | 93.1 | 7.9–279.5 | 71.5–142.8 | ||
| Urea, 2.80–7.20 mmol/L | S | 73 (95%) | 4.90 | 2.30–21.0 | 4.15–7.05 | <0.0001 |
| D | 23 (96%) | 11.90 **** | 3.20–50.50 | 8.10–24.10 | ||
| ALT, 0–50 U/L | S | 75 (97%) | 30.5 | 10.7–191 | 20.6–56.9 | 0.0553 |
| D | 23 (96%) | 42.1 | 13.0–2504 | 32.8–50.2 | ||
| AST, 0–50 U/L | S | 74 (96%) | 37.3 | 16.7–168.6 | 28.1–55.9 | 0.1301 |
| D | 23 (96%) | 43.7 | 21.3–4045 | 33.7–80.0 | ||
| GGT, 0–55 U/L | S | 26 (34%) | 75.5 | 11.4–429.3 | 36.75–139.6 | 0.9630 |
| D | 2 (8%) | - | 14.5–178.6 | - | ||
| ALP, 30–120 U/L | S | 54 (70%) | 71.5 | 31.7–346.8 | 55.3–103.0 | 0.0478 |
| D | 10 (42%) | 89.6 | 61.2–198.1 | 80.7–117.8 | ||
| Amylase, 28–100 U/L | S | 65 (84%) | 54.9 | 14.4–157.8 | 40.3–77.0 | 0.1819 |
| D | 21 (88%) | 87.9 | 15.2–494.2 | 38.1–119.0 | ||
| LDH, 0–248 U/L | S | 31 (40%) | 262 | 113–595 | 208–406 | 0.0758 |
| D | 2 (8%) | - | 455.4–1193 | - | ||
| CK-NAC, 0–171 U/L | S | 71 (92%) | 105.4 | 22.5–2567 | 57.7–252.8 | <0.0001 |
| D | 14 (58%) | 636.9 **** | 85.6–2408 | 166.3–1061 | ||
| CK-MB, 0–24 U/L | S | 72 (94%) | 12.7 | 3.5–101.1 | 9.1–17.2 | <0.0001 |
| D | 15 (63%) | 29.8 **** | 10.8–201.5 | 19.1–64.3 | ||
| Troponin, 0–342 pg/mL | S | 40 (52%) | 3.05 | 0.10–20,019 | 1.65–7.35 | 0.0030 |
| D | 7 (29%) | 152.1 ** | 1.6–50,000 | 9.9–2317 | ||
| Procalcitonin (PCT), 0–0.046 ng/mL # | S | 7 (9%) | 0.060 | 0.020–0.580 | 0.020–0.130 | 0.0208 |
| D | 14 (58%) | 0.210 * | 0.050–3.690 | 0.108–0.725 | ||
| CRP 0–5 mg/mL | S | 77 (100%) | 49.5 | 0.67–292.7 | 17.5–107.5 | 0.3189 |
| D | 24 (100%) | 59.6 | 2.2–204.4 | 20.2–143.5 |
| Biochemical Values, Normal Ranges | Outcome | n | Median | Range (Min to Max) | Interquartile Range (1–3 Quartiles) | p Value |
|---|---|---|---|---|---|---|
| BChE activity with ATCh, µmol min−1 L−1 | S | 63 (82%) | 1145 | 307–2172 | 836–1286 | 0.0083 |
| D | 22 (92%) | 832 ** | 249–1796 | 505–1168 | ||
| BChE activity with BTCh, µmol min−1 L−1 | S | 63 (82%) | 2317 | 664–4775 | 1761–2564 | 0.0002 |
| D | 22 (92%) | 1471 *** | 422–3266 | 944–2000 | ||
| PON1 activity, mmol min−1 L−1 | S | 63 (82%) | 26.80 | 1.28–55.20 | 22.84–32.90 | 0.0924 |
| D | 22 (92%) | 24.28 | 4.40–36.60 | 17.78–31.12 | ||
| Esterase activity of albumin with NPA, µmol min−1 L−1 | S | 63 (82%) | 13.60 | 6.00–523.50 | 6.00–59.70 | >0.9999 |
| D | 22 (92%) | 13.47 | 6.00–246.80 | 6.00–54.65 | ||
| MDA, µmol/L | S | 63 (82%) | 2.00 | 0.10–7.87 | 1.30–3.20 | 0.0053 |
| D | 22 (92%) | 3.05 ** | 0.90–8.37 | 2.18–5.80 |
| Index | Survived | Deceased | p Value |
|---|---|---|---|
| [Urea] × [AGWF] × 1000/(BchEb × [ALB]) | 0.21 (0.04; 1.70) n = 37 | 1.54 (0.21; 23.63) **** n = 15 | <0.0001 |
| [Urea] × ActWF × 1000/(BchEb × [ALB]) | 11.35 (0.47; 81.57) n = 37 | 71.85 (2.30; 1435.00) *** n = 15 | 0.0001 |
| [AGWF] × 10,000/(BchEb × [ALB]) | 0.39 (0.10; 1.37) n = 38 | 1.35 (0.26; 4.69) **** n = 15 | <0.0001 |
| ActWF × 1000/(BchEb × [ALB]) | 2.31 (0.09; 8.72) n = 38 | 7.93 (0.28; 33.06) ** n = 15 | 0.0036 |
| [AGWF] × [Ferr] × 1000/(BchEb × [ALB]) | 16.78 (0.20; 127.80) n = 34 | 95.51 (6.26; 459.60) * n = 8 | 0.0286 |
| ActWF × [Ferr] × 10/(BchEb × [ALB]) | 11.65 (0.12; 78.61) n = 34 | 74.39 (0.68; 279.00) n = 8 | 0.0600 |
| Indices without data on vWF activity and concentration | |||
| [Urea] × 10/[ALB] | 1.41 (0.49; 5.98) n = 38 | 4.26 (1.62; 20.20) **** n = 15 | <0.0001 |
| [MDA] × 100/[ALB] | 5.54 (0.62; 25.56) n = 38 | 12.20 (6.98; 27.14) ** n = 15 | 0.0010 |
| BChEa/[ALB] | 30.18 (13.92; 46.85) n = 38 | 24.93 (12.38; 39.47) n = 15 | 0.0916 |
| BChEb/[ALB] | 60.21 (21.45; 89.35) n = 38 | 47.09 (25.51; 65.38) ** n = 15 | 0.0012 |
| [Creatinine] × [MDA]/[ALB] | 5.06 (0.47; 26.25) n = 38 | 8.45 (2.14; 53.08) * n = 15 | 0.0255 |
| [Urea] × [MDA]/[ALB] | 0.28 (0.01; 2.08) n = 38 | 1.38 (0.54; 13.71) **** n = 15 | <0.0001 |
| BChEa/[MDA] | 514 (101; 9098) n = 63 | 195 (59; 898) *** n = 21 | 0.0001 |
| BChEb/[MDA] | 1021 (251; 17,648) n = 63 | 382 (99; 1705) **** n = 21 | <0.0001 |
| [Urea] × [MDA] × 1000/(BChEb × [ALB]) | 0.14 (0.01; 0.96) n = 37 | 1.24 (0.32; 10.36) **** n = 15 | <0.0001 |
| [Urea] × 1000/(BChEb × [ALB]) | 0.06 (0.02; 0.27) n = 37 | 0.24 (0.09; 1.59) **** n = 15 | <0.0001 |
| Biochemical Values or Index, n | AUC, (95% CI) | p Value | Critical Value | Likelihood Ratio | OR, (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Troponin, 47 | 0.84 (0.64–1.00) | 0.0045 | >51.45 | 14.3 | 47.5 (5.4–416) | 0.0005 |
| Procalcitonin, 21 | 0.81 (0.60–1.00) | 0.02 | >0.145 | 5.0 | 15.0 (1.3–168) | 0.028 |
| CRP, 101 | 0.57 (0.43–0.70) | 0.32 | >167.0 | 3.2 | 3.7 (0.8–15.9) | 0.085 |
| [Urea] × [AGWF] × 1000/ (BChEb × [ALB]), 52 | 0.91 (0.83–1.00) | <0.0001 | >0.96 | 24.7 | 72.0 (7.5–689) | 0.0002 |
| [Urea] × ActWF × 1000/ (BChEb × [ALB]), 52 | 0.83 (0.68–0.97) | 0.0002 | >63.23 | 22.2 | 72.0 (7.5–689) | 0.0002 |
| [AGWF] × 10,000/(BChEb × [ALB]), 53 | 0.86 (0.73–0.98) | <0.0001 | >1.20 | 22.8 | 55.50 (5.9–521) | 0.0004 |
| ActWF × 1000/ (BChEb × [ALB]), 53 | 0.75 (0.56–0.93) | 0.0059 | >7.77 | 11.4 | 27.00 (4.7–157) | 0.0002 |
| [Urea] × 10/[ALB], 53 | 0.94 (0.87–1.00) | <0.0001 | >3.85 | 20.3 | 42.3 (4.6–393) | 0.001 |
| [Urea] × [MDA]/[ALB], 52 | 0.92 (0.84–0.99) | <0.0001 | >2.00 | 14.8 | 24.0 (2.6–225) | 0.0054 |
| [Urea] × [MDA] × 1000/ (BChEb × [ALB]), 52 | 0.94 (0.89–1.00) | <0.0001 | >0.945 | 19.7 | 41.1 (4.4–383) | 0.0011 |
| [Urea] × 1000/(BChEb × [ALB]), 52 | 0.95 (0.89–1.00) | <0.0001 | >0.265 | 17.3 | 31.5 (3.4–293) | 0.0024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncharov, N.V.; Avdonin, P.P.; Voitenko, N.G.; Voronina, P.A.; Popova, P.I.; Novozhilov, A.V.; Blinova, M.S.; Popkova, V.S.; Belinskaia, D.A.; Avdonin, P.V. Searching for New Biomarkers to Assess COVID-19 Patients: A Pilot Study. Metabolites 2023, 13, 1194. https://doi.org/10.3390/metabo13121194
Goncharov NV, Avdonin PP, Voitenko NG, Voronina PA, Popova PI, Novozhilov AV, Blinova MS, Popkova VS, Belinskaia DA, Avdonin PV. Searching for New Biomarkers to Assess COVID-19 Patients: A Pilot Study. Metabolites. 2023; 13(12):1194. https://doi.org/10.3390/metabo13121194
Chicago/Turabian StyleGoncharov, Nikolay V., Piotr P. Avdonin, Natalia G. Voitenko, Polina A. Voronina, Polina I. Popova, Artemy V. Novozhilov, Maria S. Blinova, Victoria S. Popkova, Daria A. Belinskaia, and Pavel V. Avdonin. 2023. "Searching for New Biomarkers to Assess COVID-19 Patients: A Pilot Study" Metabolites 13, no. 12: 1194. https://doi.org/10.3390/metabo13121194
APA StyleGoncharov, N. V., Avdonin, P. P., Voitenko, N. G., Voronina, P. A., Popova, P. I., Novozhilov, A. V., Blinova, M. S., Popkova, V. S., Belinskaia, D. A., & Avdonin, P. V. (2023). Searching for New Biomarkers to Assess COVID-19 Patients: A Pilot Study. Metabolites, 13(12), 1194. https://doi.org/10.3390/metabo13121194







