Metabolic Deficits in the Retina of a Familial Dysautonomia Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Retina Sample Collection for NMR and MS Metabolite Extractions
2.3. Metabolite Extraction for 1H NMR Analysis
2.4. Acquisition and Processing of 1H NMR Spectra
2.5. Chenomx Spectral Signal Annotation and Quantitation
2.6. NMR and Statistical Analyses
2.7. Extraction of Mouse Retinae for Dopamine Isolation
2.8. UHPLC/Q-TOF-MS Analysis for Relative Dopamine Quantitation
2.9. Retinal Histology for Flat Mount Preparations
3. Results
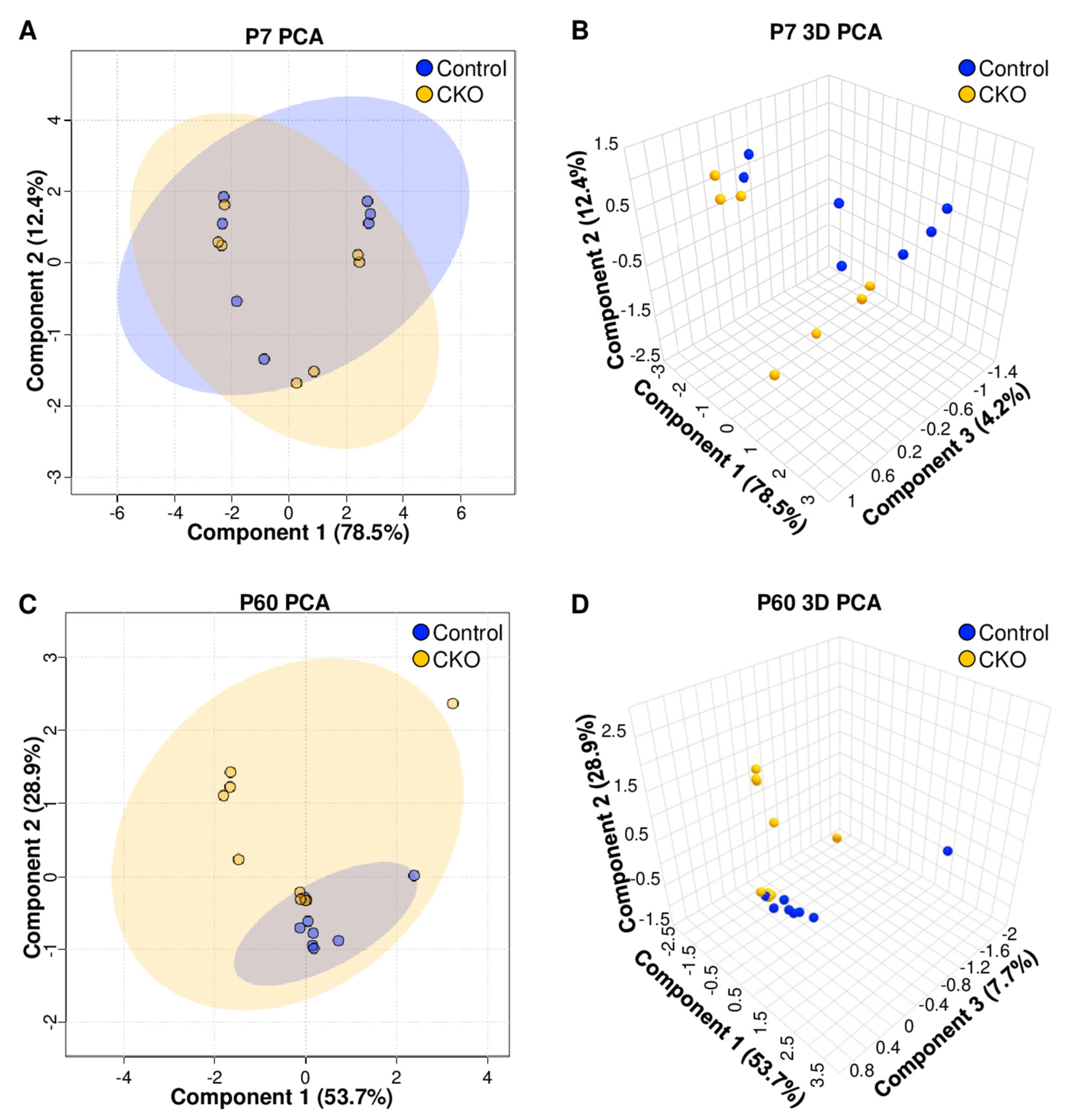
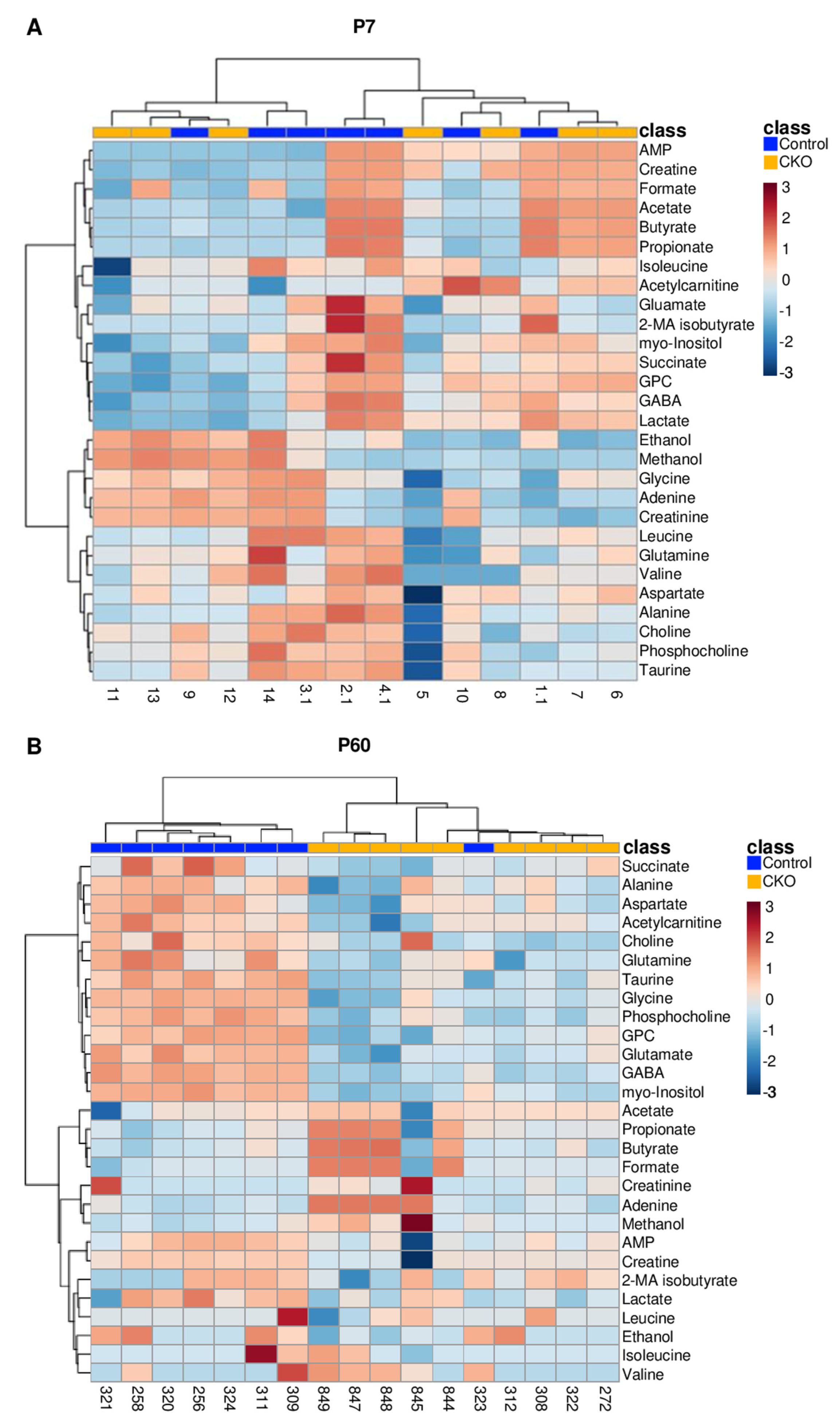
3.1. Retina Metabolomic Differences Emerge by 2 Months of Age
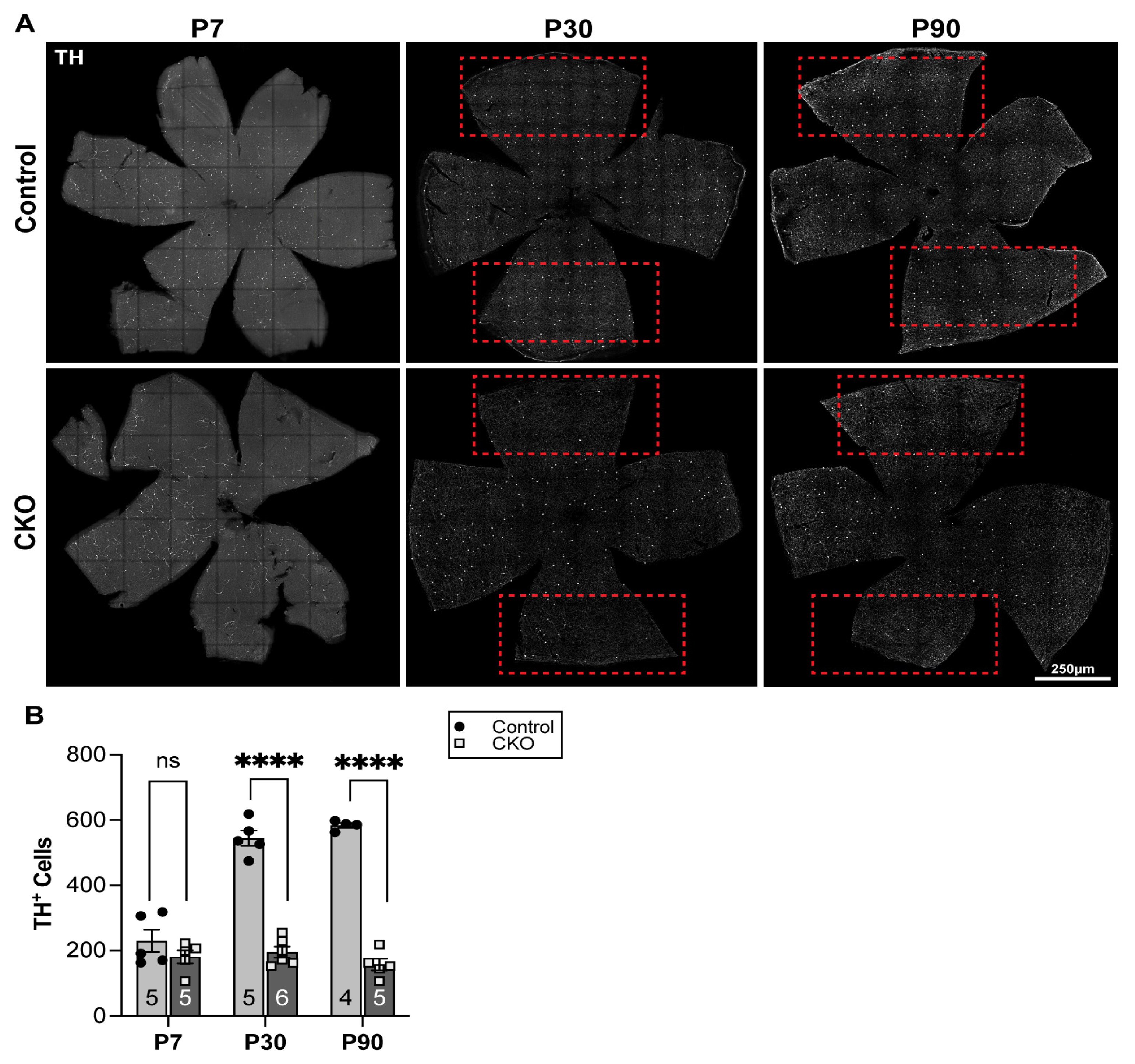
3.2. Alterations in Dopaminergic Amacrine Cell Population in CKO Retinae
3.3. Reduced Dopamine Levels at P30 in CKO Retina
4. Discussion
4.1. 1H NMR Data Indicate That Metabolic Disturbances Correlate Temporally with Elp1-Induced RGC Degeneration
4.2. Taurine Is Fundamental for Mitochondrial Electron Transport Chain (ETC) Complex 1 Function and RGC Health
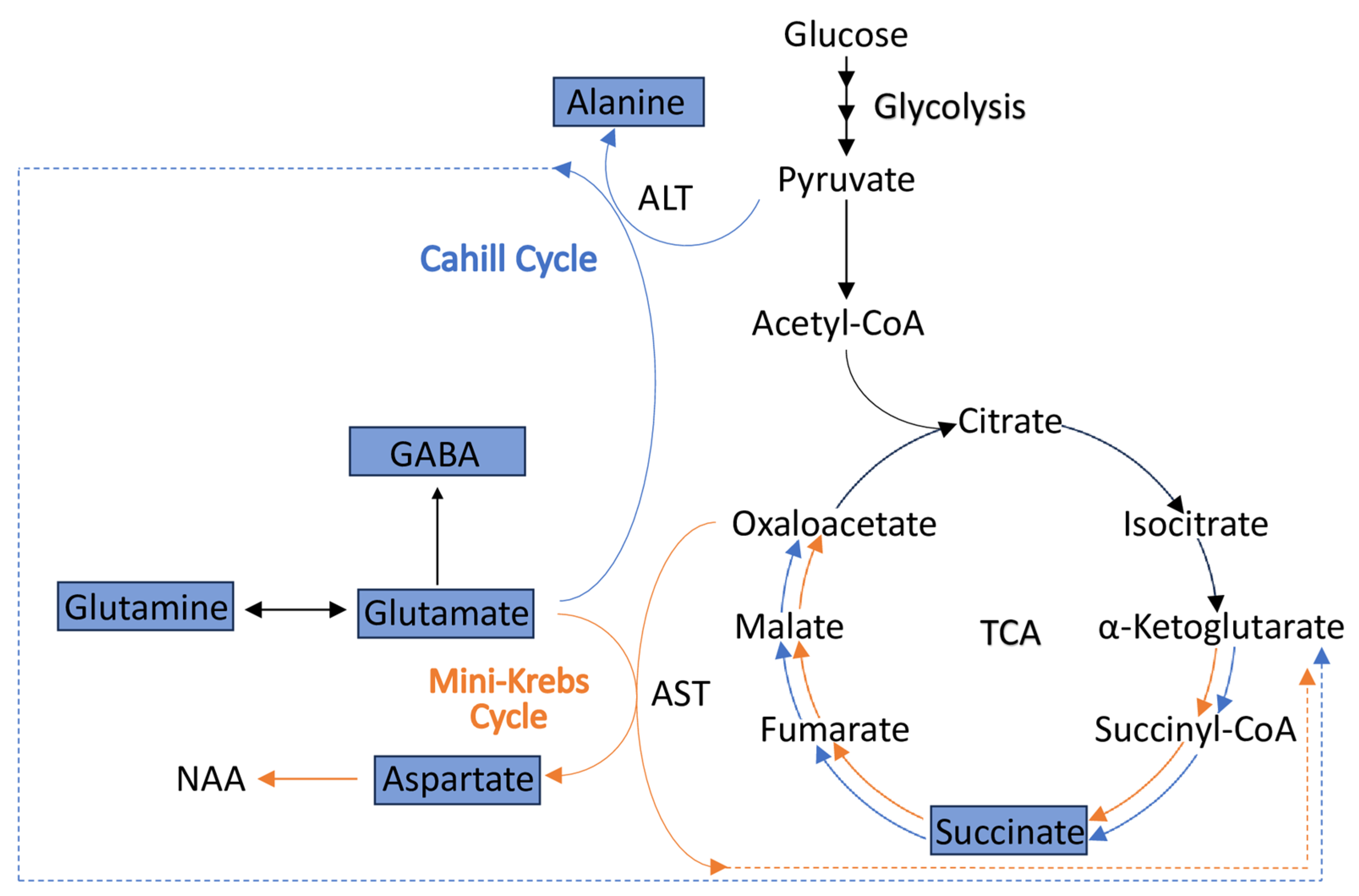
4.3. Alterations in the Levels of Metabolites Involved in the Cahill (Alanine-Glucose) and Mini-Krebs Cycles Point to Energetic (ATP) Deficits in CKO Retinae
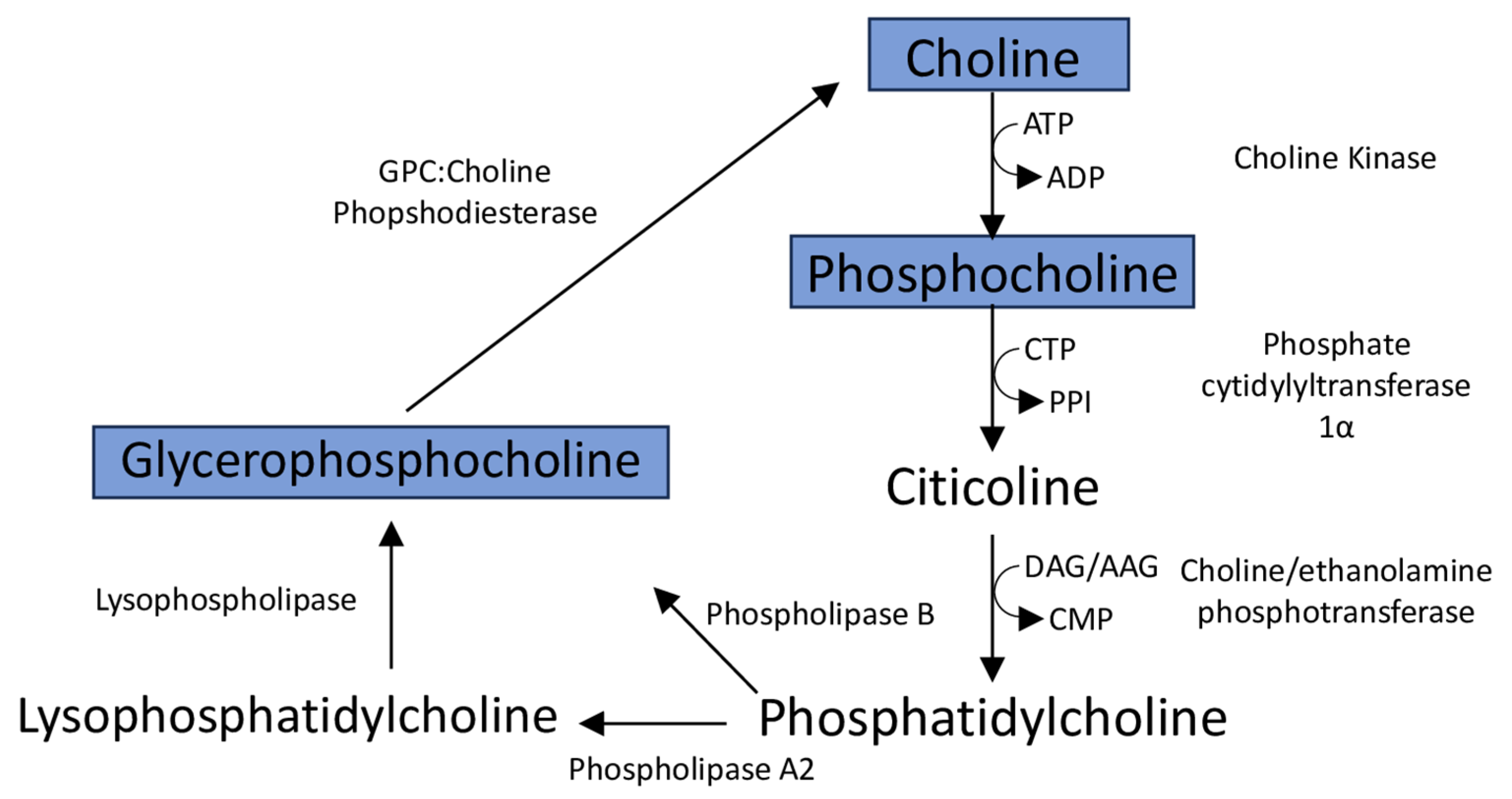
4.4. Reduced Levels of Membrane Phospholipid-Associated Metabolites Myo-Inositol and Choline Are Associated with Retinopathy
4.5. Alterations in Dopaminergic Cell Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietrich, P.; Dragatsis, I. Familial Dysautonomia: Mechanisms and Models. Genet. Mol. Biol. 2016, 39, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Norcliffe-Kaufmann, L.; Slaugenhaupt, S.A.; Kaufmann, H. Familial dysautonomia: History, genotype, phenotype and translational research. Prog. Progress. Neurobiol. 2017, 152, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Slaugenhaupt, S.A.; Blumenfeld, A.; Gill, S.P.; Leyne, M.; Mull, J.; Cuajungco, M.P.; Liebert, C.B.; Chadwick, B.; Idelson, M.; Reznik, L.; et al. Tissue-Specific Expression of a Splicing Mutation in the IKBKAP Gene Causes Familial Dysautonomia. Am. J. Hum. Genet. 2001, 68, 598–605. [Google Scholar] [CrossRef]
- Chekuri, A.; Logan, E.M.; Krauson, A.J.; Salani, M.; Ackerman, S.; Kirchner, E.G.; Bolduc, J.M.; Wang, X.; Dietrich, P.; Dragatsis, I.; et al. Selective retinal ganglion cell loss and optic neuropathy in a humanized mouse model of familial dysautonomia. Hum. Mol. Genet. 2022, 31, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Cotrina, M.L.; Morgenstein, B.; Perez, M.; Norcliffe-Kaufmann, L.; Palma, J.-A.; Kaufmann, H. Height, weight, and body mass index in patients with familial dysautonomia. PLoS ONE 2023, 18, e0293800. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Santiesteban, C.E.; Palma, J.-A.; Hedges, T.R.; Laver, N.V.; Farhat, N.; Norcliffe-Kaufmann, L.; Kaufmann, H. Pathological Confirmation of Optic Neuropathy in Familial Dysautonomia. J. Neuropathol. Exp. Neurol. 2017, 76, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Santiesteban, C.E.; Hedges, T.R., III; Norcliffe-Kaufmann, L.; Axelrod, F.; Kaufmann, H. Selective retinal ganglion cell loss in familial dysautonomia. J. Neurol. 2014, 261, 702–709. [Google Scholar] [CrossRef]
- Mendoza-Santiesteban, C.E.; Hedges, T.R.; Norcliffe-Kaufmann, L.; Warren, F.; Reddy, S.; Axelrod, F.B.; Kaufmann, H. Clinical Neuro-ophthalmic Findings in Familial Dysautonomia. J. Neuroophthalmol. 2012, 32, 23–26. [Google Scholar] [CrossRef]
- Ueki, Y.; Ramirez, G.; Salcedo, E.; Stabio, M.E.; Lefcort, F. Loss of Ikbkap Causes Slow, Progressive Retinal Degeneration in a Mouse Model of Familial Dysautonomia. eNeuro 2016, 3. [Google Scholar] [CrossRef]
- Ueki, Y.; Shchepetkina, V.; Lefcort, F. Retina-specific loss of Ikbkap/Elp1 causes mitochondrial dysfunction that leads to selective retinal ganglion cell degeneration in a mouse model of familial dysautonomia. Dis. Models Mech. 2018, 11, dmm033746. [Google Scholar] [CrossRef]
- Potilinski, M.C.; Lorenc, V.; Perisset, S.; Gallo, J.E. Mechanisms behind Retinal Ganglion Cell Loss in Diabetes and Therapeutic Approach. Int. J. Mol. Sci. 2020, 21, 2351. [Google Scholar] [CrossRef]
- Gastinger, M.J.; Singh, R.S.J.; Barber, A.J. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3143–3150. [Google Scholar] [CrossRef]
- Kolb, H. Anatomical pathways for color vision in the human retina. Vis. Neurosci. 1991, 7, 61–74. [Google Scholar] [CrossRef]
- Kolb, H. Roles of Amacrine Cells. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. 2004, 108, 17–39. [Google Scholar] [CrossRef]
- Indrieri, A.; Pizzarelli, R.; Franco, B.; De Leonibus, E. Dopamine, Alpha-Synuclein, and Mitochondrial Dysfunctions in Parkinsonian Eyes. Front. Neurosci. 2020, 14, 567129. [Google Scholar] [CrossRef]
- Ortuno-Lizaran, I.; Sanchez-Saez, X.; Lax, P.; Serrano, G.E.; Beach, T.G.; Adler, C.H.; Cuenca, N. Dopaminergic Retinal Cell LOss and Visual Dysfunction in Parkinson Disease. Ann. Neurol. 2020, 88, 893–906. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Pu, J.-L.; Zheng, R.; Yan, Y.-Q.; Liu, K.-Y.; Liu, Y.; Zheng, R.; Chen, Y.; Lin, Z.-H.; Xue, N.-J.; et al. Mitochondrial morphology and synaptic structure altered in the retina of parkin-deficient mice. Neurosci. Lett. 2022, 790, 136888. [Google Scholar] [CrossRef]
- La Morgia, C.; Barboni, P.; Rizzo, G.; Carbonelli, M.; Savini, G.; Scaglione, C.; Capellari, S.; Bonazza, S.; Giannoccaro, M.P.; Calandra-Buonaura, G.; et al. Loss of temporal retinal nerve fibers in Parkinson disease: A mitochondrial pattern? Eur. J. Neurol. 2013, 20, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Cheney, A.M.; Costello, S.M.; Pinkham, N.V.; Waldum, A.; Broadaway, S.C.; Cotrina-Vidal, M.; Mergy, M.; Tripet, B.; Kominsky, D.J.; Grifka-Walk, H.M.; et al. Gut microbiome dysbiosis drives metabolic dysfunction in Familial dysautonomia. Nat. Commun. 2023, 14, 218. [Google Scholar] [CrossRef]
- Costello, S.M.; Cheney, A.M.; Waldum, A.; Tripet, B.; Cotrina-Vidal, M.; Kaufmann, H.; Norcliffe-Kaufmann, L.; Lefcort, F.; Copié, V. A Comprehensive NMR Analysis of Serum and Fecal Metabolites in Familial Dysautonomia Patients Reveals Significant Metabolic Perturbations. Metabolites 2023, 13, 433. [Google Scholar] [CrossRef]
- Chen, Y.; Zizmare, L.; Calbiague, V.; Yu, S.; Herberg, F.W.; Schmachtenberg, O.; Paquet-Durand, F.; Trautwein, C. Retinal metabolism: Evidence for uncoupling of glycolysis and oxidative phosphorylation via Cori-, Cahill-, and mini-Krebs-cycle. eLife 2023, 12, RP91141. [Google Scholar] [CrossRef] [PubMed]
- Zanna, C.; Ghelli, A.; Porcelli, A.M.; Karbowski, M.; Youle, R.J.; Schimpf, S.; Wissinger, B.; Pinti, M.; Cossarizza, A.; Vidoni, S.; et al. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain 2008, 131, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Sadun, A.A.; Morgia, C.L.; Carelli, V. Leber’s Hereditary Optic Neuropathy. Curr. Treat. Options Neurol. 2011, 13, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Prokosch, V. Energy Metabolism in the Inner Retina in Health and Glaucoma. Int. J. Mol. Sci. 2021, 22, 3689. [Google Scholar] [CrossRef]
- Country, M.W. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017, 1672, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Cheng, S.-Y.; Kirchner, E.; Costello, S.; Miettinen, H.; Chaverra, M.; King, C.; George, L.; Zhao, X.; Narasimhan, J.; et al. Reduction of retinal ganglion cell death in mouse models of familial dysautonomia using AAV-mediated gene therapy and splicing modulators. Sci. Rep. 2023, 13, 18600. [Google Scholar] [CrossRef] [PubMed]
- Midelfart, A.; Dybdahl, A.; Gribbestad, I.S. Detection of different metabolites in the rabbit lens by high resolution 1H NMR spectroscopy. Curr. Eye Res. 1996, 15, 1175–1181. [Google Scholar] [CrossRef]
- Santiago, A.R.; Garrido, M.J.; Cristóvão, A.J.; Duarte, J.M.N.; Carvalho, R.A.; Ambrósio, A.F. Evaluation of the Impact of Diabetes on Retinal Metabolites by NMR Spectroscopy. Curr. Eye Res. 2010, 35, 992–1001. [Google Scholar] [CrossRef]
- Fuchs, A.L.; Miller, I.R.; Schiller, S.M.; Ammons, M.C.B.; Eilers, B.; Tripet, B.; Copié, V. Pseudomonas aeruginosa Planktonic- and Biofilm-Conditioned Media Elicit Discrete Metabolic Responses in Human Macrophages. Cells 2020, 9, 2260. [Google Scholar] [CrossRef]
- Fuchs, A.L.; Schiller, S.M.; Keegan, W.J.; Ammons, M.C.B.; Eilers, B.; Tripet, B.; Copié, V. Quantitative 1H NMR Metabolomics Reveal Distinct Metabolic Adaptations in Human Macrophages Following Differential Activation. Metabolites 2019, 9, 248. [Google Scholar] [CrossRef]
- O’Shea-Stone, G.; Lambert, R.; Tripet, B.; Berardinelli, J.; Thomson, J.; Copié, V.; Garrott, R. 1H NMR based metabolic profiling distinguishes the differential impact of capture techniques on wild bighorn sheep. Sci. Rep. 2021, 11, 11308. [Google Scholar] [CrossRef]
- Fuchs, A.L.; Weaver, A.J.; Tripet, B.P.; Ammons, M.C.B.; Teintze, M.; Copié, V. Characterization of the antibacterial activity of Bald’s eyesalve against drug resistant Staphylococcus aureus and Pseudomonas aeruginosa. PLoS ONE 2018, 13, e0208108. [Google Scholar] [CrossRef]
- Mercier, P.; Lewis, M.J.; Chang, D.; Baker, D.; Wishart, D.S. Towards automatic metabolomic profiling of high-resolution one-dimensional proton NMR spectra. J. Biomol. NMR 2011, 49, 307–323. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2021, 50, D622–D631. [Google Scholar] [CrossRef]
- Wishart, D.S. Quantitative metabolomics using NMR. TrAC Trends Anal. Chem. 2008, 27, 228–237. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr. Protoc. Bioinform. 2011, 34, 14.10.1–14.10.48. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; Harman, D.G.; Morley, J.W.; Cameron, M.A. Optimized Method to Quantify Dopamine Turnover in the Mammalian Retina. Anal. Chem. 2017, 89, 12276–12283. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Keeley, P.W.; Reese, B.E. Role of Afferents in the Differentiation of Bipolar Cells in the Mouse Retina. J. Neurosci. 2010, 30, 1677–1685. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tolman, Z.; Chaverra, M.; George, L.; Lefcort, F. Elp1 is required for development of visceral sensory peripheral and central circuitry. Dis. Models Mech. 2022, 15, dmm049274. [Google Scholar] [CrossRef]
- George, L.; Chaverra, M.; Wolfe, L.; Thorne, J.; Close-Davis, M.; Eibs, A.; Riojas, V.; Grindeland, A.; Orr, M.; Carlson, G.A.; et al. Familial dysautonomia model reveals Ikbkap deletion causes apoptosis of Pax3+ progenitors and peripheral neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 18698–18703. [Google Scholar] [CrossRef]
- Kaushik, P.; Gorin, F.; Vali, S. Dynamics of tyrosine hydroxylase mediated regulation of dopamine synthesis. J. Comput. Neurosci. 2007, 22, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-Y.; Cringle, S.J.; Yu, P.K.; Su, E.-N. Retinal energetics: Its critical role in retinal physiology and pathology. Expert. Rev. Ophthalmol. 2011, 6, 395–399. [Google Scholar] [CrossRef]
- Castelli, V.; Paladini, A.; d’Angelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrassi, G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Ansar, M.; Ranza, E.; Shetty, M.; Paracha, S.A.; Azam, M.; Kern, I.; Iwaszkiewicz, J.; Farooq, O.; Pournaras, C.J.; Malcles, A.; et al. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum. Mol. Genet. 2020, 29, 618–623. [Google Scholar] [CrossRef]
- Bonelli, R.; Woods, S.M.; Lockwood, S.; Bishop, P.N.; Khan, K.N.; Bahlo, M.; Ansell, B.R.E.; Fruttiger, M. Spatial distribution of metabolites in the retina and its relevance to studies of metabolic retinal disorders. Metabolomics 2023, 19, 10. [Google Scholar] [CrossRef]
- García-Ayuso, D.; Di Pierdomenico, J.; Hadj-Said, W.; Marie, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-Pérez, M.P. Taurine Depletion Causes ipRGC Loss and Increases Light-Induced Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1396–1409. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef] [PubMed]
- Goffena, J.; Lefcort, F.; Zhang, Y.; Lehrmann, E.; Chaverra, M.; Felig, J.; Walters, J.; Buksch, R.; Becker, K.G.; George, L. Elongator and codon bias regulate protein levels in mammalian peripheral neurons. Nat. Commun. 2018, 9, 889. [Google Scholar] [CrossRef]
- Merckx, C.; De Paepe, B. The Role of Taurine in Skeletal Muscle Functioning and Its Potential as a Supportive Treatment for Duchenne Muscular Dystrophy. Metabolites 2022, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Ohlen, S.B.; Russell, M.L.; Brownstein, M.J.; Lefcort, F. BGP-15 prevents the death of neurons in a mouse model of familial dysautonomia. Proc. Natl. Acad. Sci. USA 2017, 114, 5035–5040. [Google Scholar] [CrossRef]
- Masino, S.A.; Rho, J.M. Mechanisms of Ketogenic Diet Action. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Vogel, S. Myo-Inositol Supplementation Augments Visual System Maturation in Mice. Ph.D. Thesis, Tufts University-Graduate School of Biomedical Sciences, Boston, MA, USA, 2021. [Google Scholar]
- Best, J.G.; Stagg, C.J.; Dennis, A. Other Significant Metabolites. In Magnetic Resonance Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 122–138. [Google Scholar]
- MacGregor, L.C.; Rosecan, L.R.; Laties, A.M.; Matschinsky, F.M. Altered retinal metabolism in diabetes. I. Microanalysis of lipid, glucose, sorbitol, and myo-inositol in the choroid and in the individual layers of the rabbit retina. J. Biol. Chem. 1986, 261, 4046–4051. [Google Scholar] [CrossRef]
- Wensel, T.G. Phosphoinositides in Retinal Function and Disease. Cells 2020, 9, 866. [Google Scholar] [CrossRef]
- Bar-Shai, A.; Maayan, C.; Vromen, A.; Udassin, R.; Nissan, A.; Freund, H.R.; Hanani, M. Decreased density of ganglia and neurons in the myenteric plexus of familial dysautonomia patients. J. Neurol. Sci. 2004, 220, 89–94. [Google Scholar] [CrossRef]
- Chaverra, M.; George, L.; Mergy, M.; Waller, H.; Kujawa, K.; Murnion, C.; Sharples, E.; Thorne, J.; Podgajny, N.; Grindeland, A.; et al. The familial dysautonomia disease gene IKBKAP is required in the developing and adult mouse central nervous system. Dis. Models Mech. 2017, 10, 605–618. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Hodakoski, C.; Barrows, D.; Mense, S.; Parsons, R.E. PTEN function, the long and the short of it. Trends Biochem. Sci. 2014, 39, 183–190. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Liscovitch, M.; Richardson, U.I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 5474–5477. [Google Scholar] [CrossRef]
- Wurtman, R.J. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci. 1992, 15, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-S.; Shin, Y.-J. Role of Choline in Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 4733. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Oddone, F.; Ziccardi, L.; Roberti, G.; Coppola, G.; Manni, G. Citicoline and Retinal Ganglion Cells: Effects on Morphology and Function. Curr. Neuropharmacol. 2018, 16, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Jiang, H.; Xiao, D.; Zou, M.; Zhu, J.; Xiang, M. Tfap2a and 2b act downstream of Ptf1a to promote amacrine cell differentiation during retinogenesis. Mol. Brain 2015, 8, 28. [Google Scholar] [CrossRef]
- Voinescu, P.E.; Kay, J.N.; Sanes, J.R. Birthdays of retinal amacrine cell subtypes are systematically related to their molecular identity and soma position. J. Comp. Neurol. 2009, 517, 737–750. [Google Scholar] [CrossRef]
- Yan, W.; Laboulaye, M.A.; Tran, N.M.; Whitney, I.E.; Benhar, I.; Sanes, J.R. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 2020, 40, 5177–5195. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costello, S.M.; Schultz, A.; Smith, D.; Horan, D.; Chaverra, M.; Tripet, B.; George, L.; Bothner, B.; Lefcort, F.; Copié, V. Metabolic Deficits in the Retina of a Familial Dysautonomia Mouse Model. Metabolites 2024, 14, 423. https://doi.org/10.3390/metabo14080423
Costello SM, Schultz A, Smith D, Horan D, Chaverra M, Tripet B, George L, Bothner B, Lefcort F, Copié V. Metabolic Deficits in the Retina of a Familial Dysautonomia Mouse Model. Metabolites. 2024; 14(8):423. https://doi.org/10.3390/metabo14080423
Chicago/Turabian StyleCostello, Stephanann M., Anastasia Schultz, Donald Smith, Danielle Horan, Martha Chaverra, Brian Tripet, Lynn George, Brian Bothner, Frances Lefcort, and Valérie Copié. 2024. "Metabolic Deficits in the Retina of a Familial Dysautonomia Mouse Model" Metabolites 14, no. 8: 423. https://doi.org/10.3390/metabo14080423
APA StyleCostello, S. M., Schultz, A., Smith, D., Horan, D., Chaverra, M., Tripet, B., George, L., Bothner, B., Lefcort, F., & Copié, V. (2024). Metabolic Deficits in the Retina of a Familial Dysautonomia Mouse Model. Metabolites, 14(8), 423. https://doi.org/10.3390/metabo14080423






