Effects of Different Drying Methods on Amino Acid Metabolite Content and Quality of Ophiocordyceps sinensis by LC-MS/MS Combined with Multivariate Statistical Methods
Abstract
:1. Introduction
2. Materials and Procedures
2.1. Source of Materials
2.2. Metabolite Extraction
2.3. Analysis Using LC-MS/MS
2.4. Data Processing
2.5. Preparation and Calculation of Amino Acid Metabolites Content Standard Curve
3. Results
3.1. Characterization of Amino Acid Metabolic Profiles
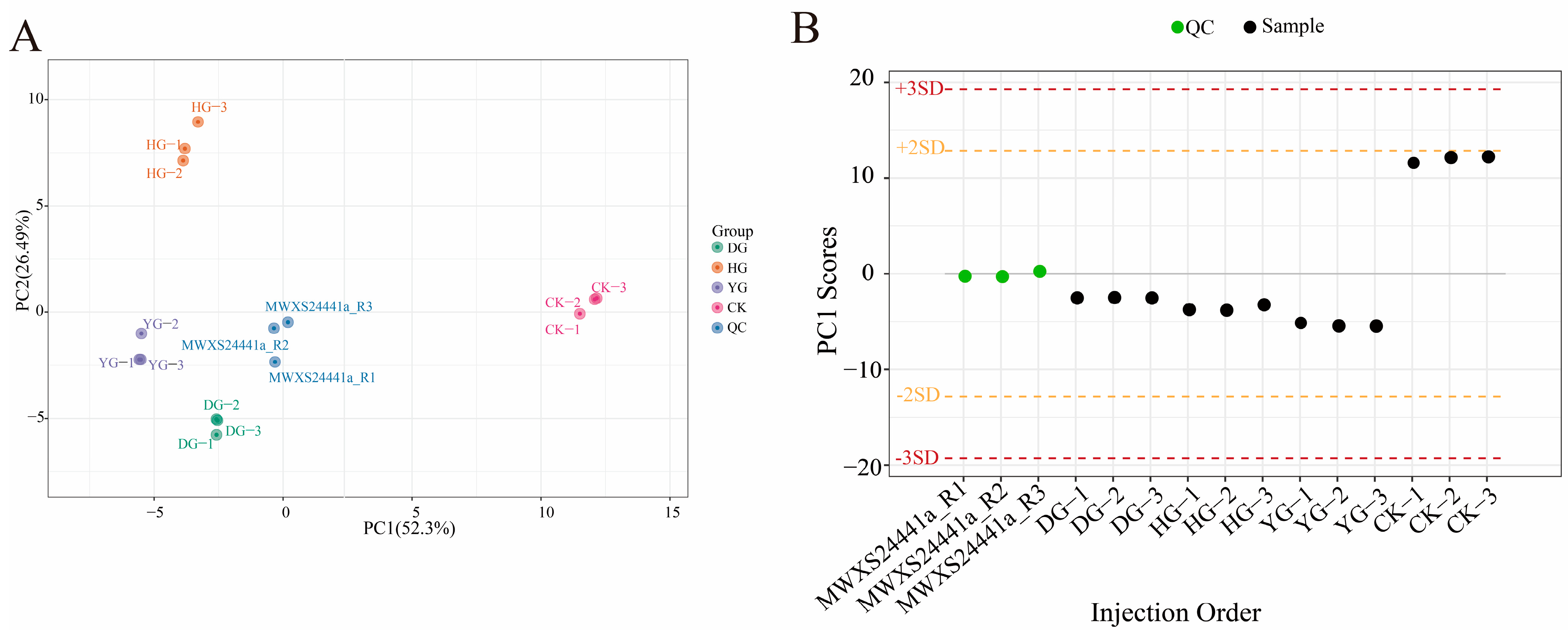
3.2. Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
3.3. Calculation of Amino Acid Metabolites
Difference Analysis of Essential Amino Acid Metabolites and Delicious Amino Acids Metabolites Content
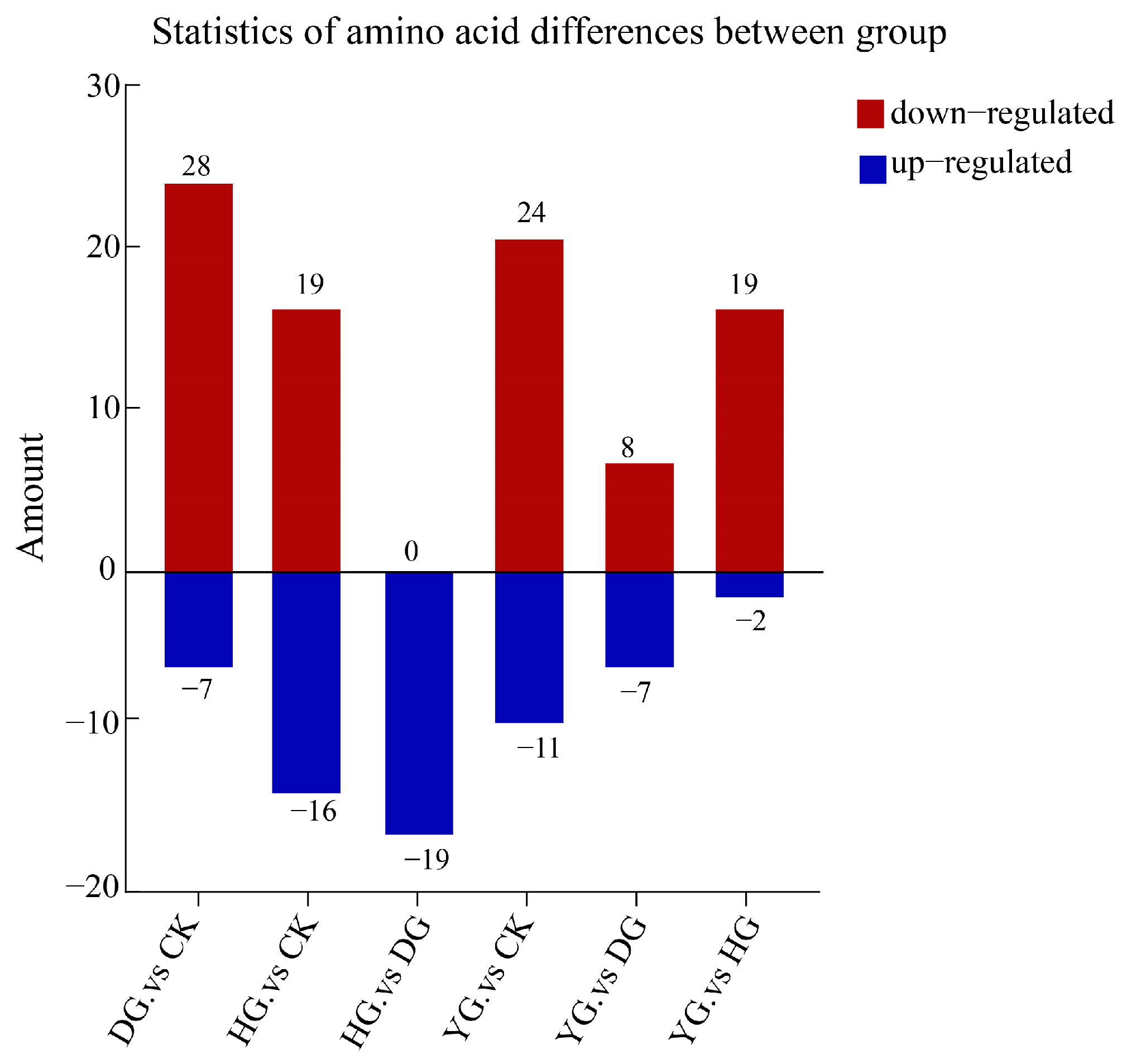
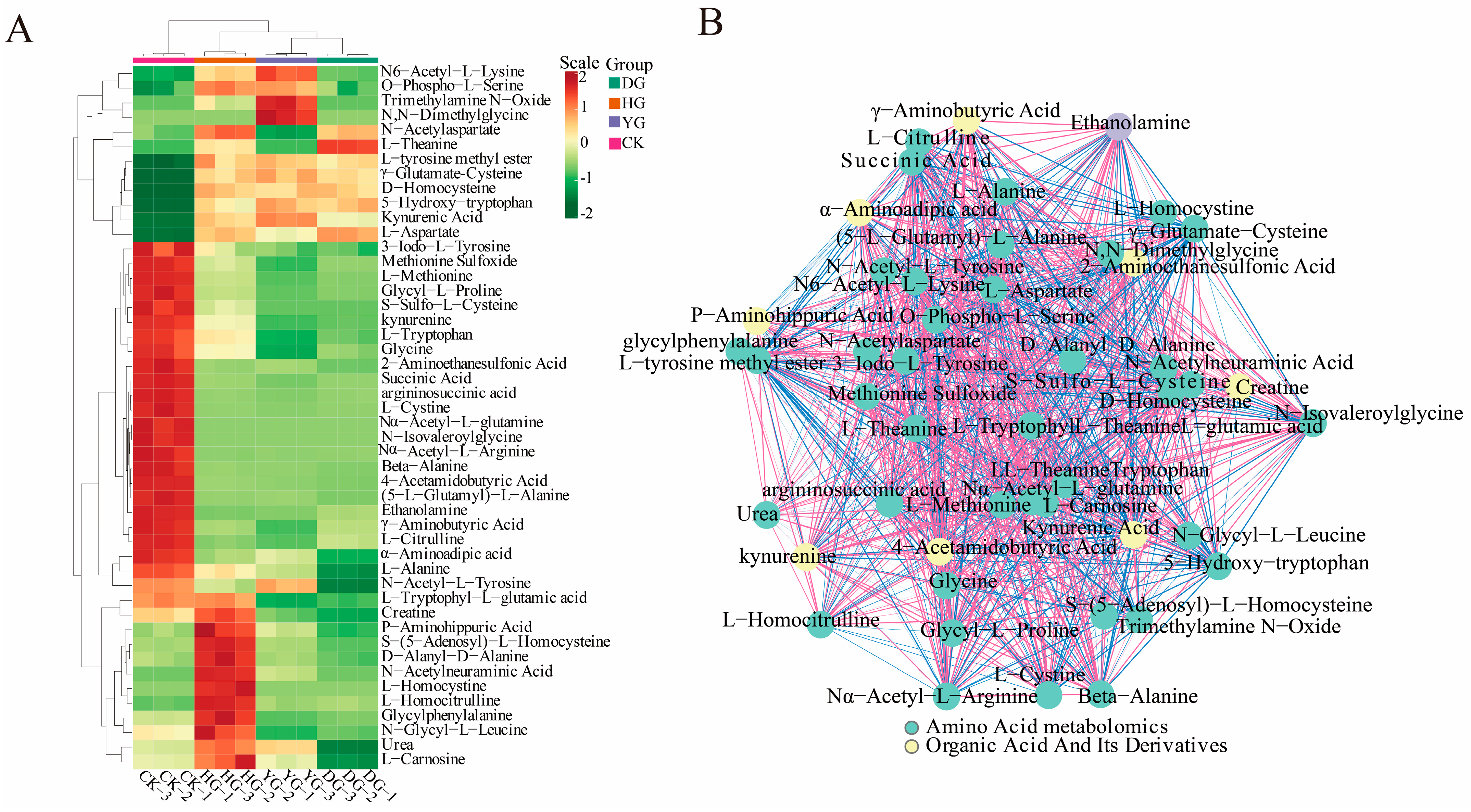
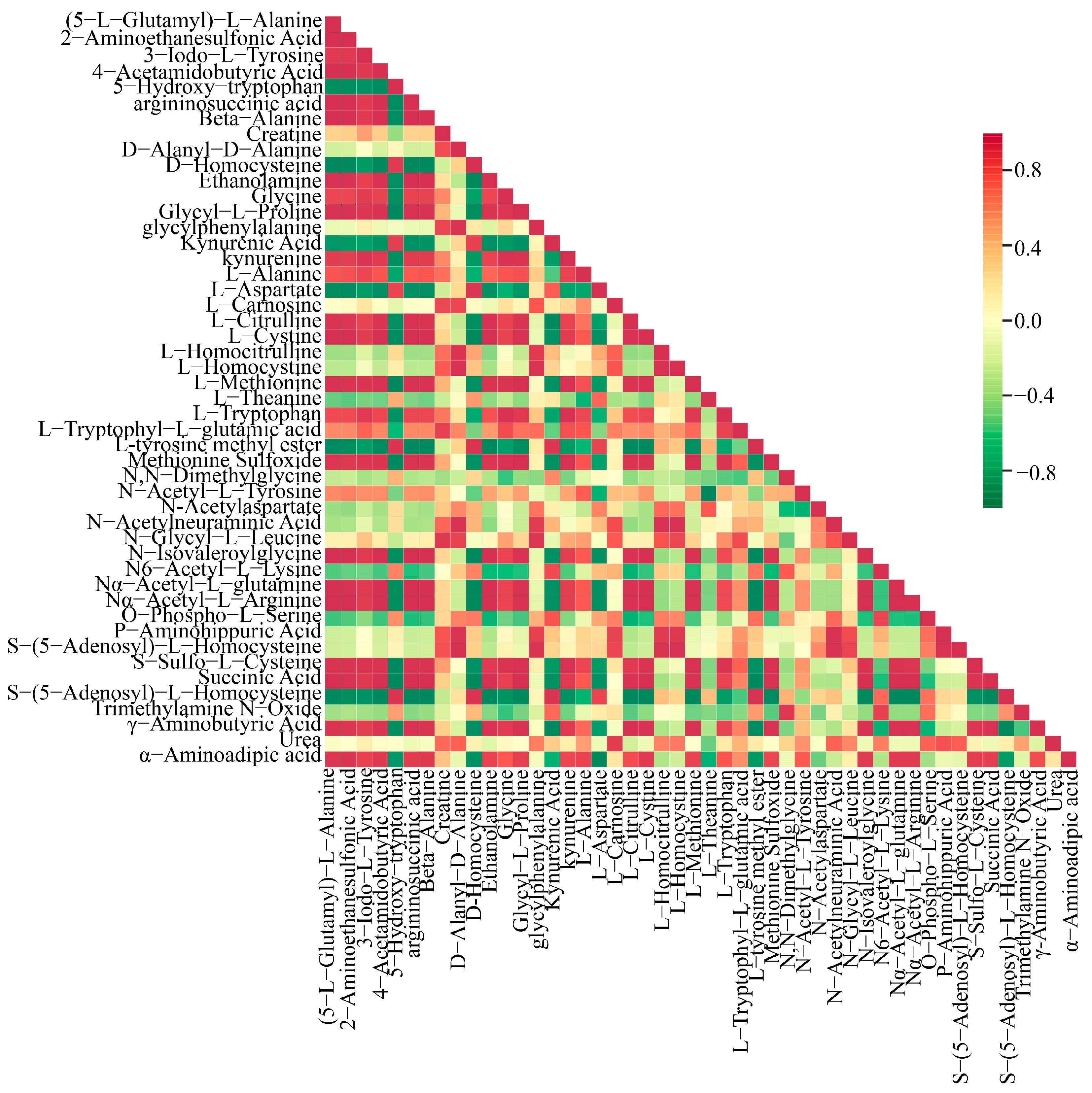
3.4. Screening of Differential Amino Acid Metabolites (DAMs)
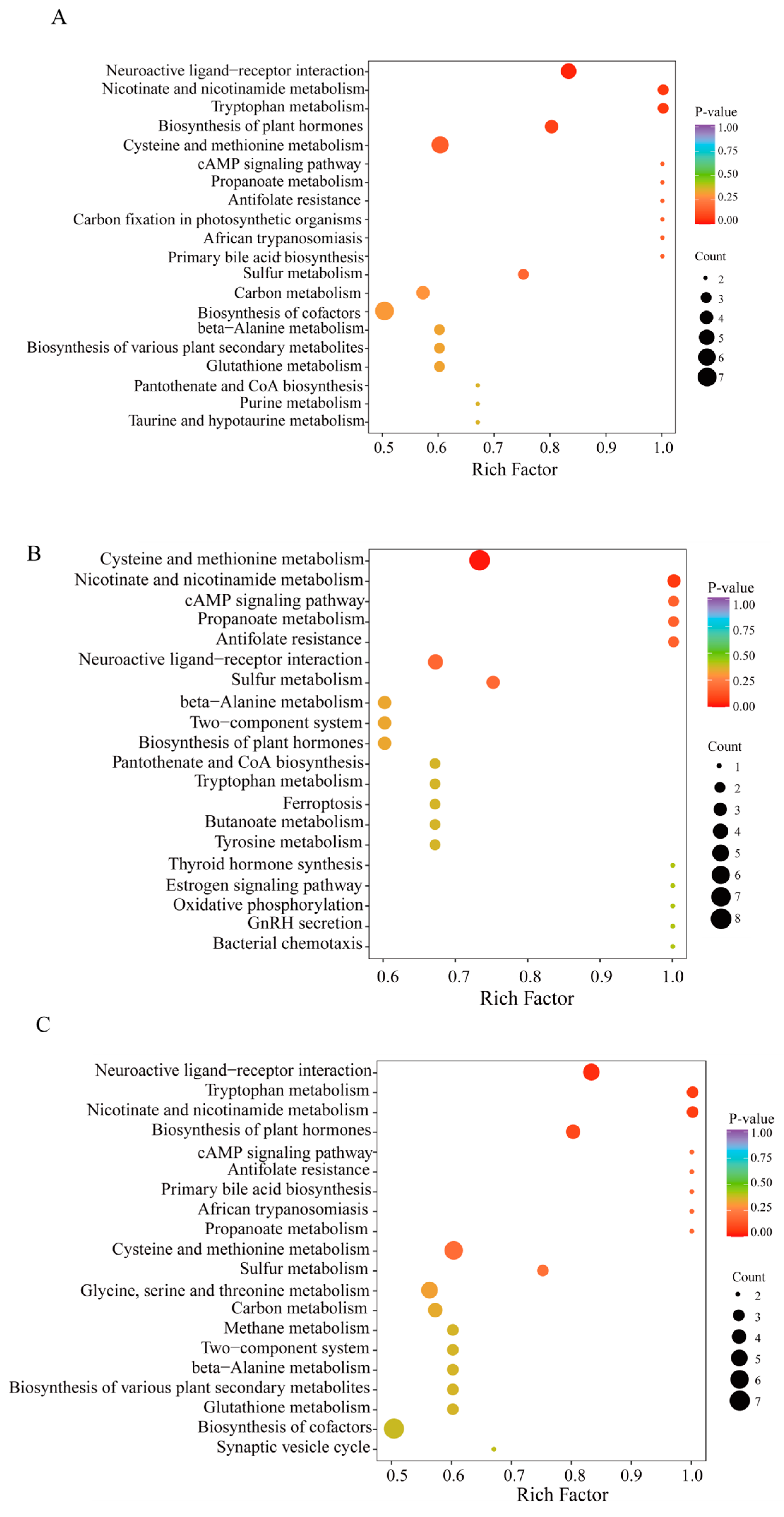
3.5. KEGG Analysis
3.6. DAMs Analysis in Critical Paths
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, X.; Cao, L.; Han, R. Analysis of volatile components in different Ophiocordyceps sinensis and insect host products. Molecules 2020, 25, 1603. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, D.; Luo, W.; Zhu, Z.; Li, W.; Qian, Z.; Li, G.; Sun, D.W. Rapid freezing using atomized liquid nitrogen spray followed by frozen storage below glass transition temperature for Cordyceps sinensis preservation: Quality attributes and storage stability. LWT 2020, 123, 109066. [Google Scholar] [CrossRef]
- Li, F.; Wu, Z.; Sui, X. Biotransformation of ginsenoside Rb1 with wild Cordyceps sinensis and Ascomycota sp. and its antihyperlipidemic effects on the diet-induced cholesterol of zebrafish. J. Food Biochem. 2020, 44, e13192. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gao, Y.; Zhou, Y.; Yu, F.; Li, X.; Zhang, N. Mechanism of Cordyceps sinensis and its Extracts in the Treatment of Diabetic Kidney Disease: A Review. Front. Pharmacol. 2022, 13, 881835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Luo, L.; Dressel, W.; Shadier, G.; Krumbiegel, D.; Schmidtke, P.; Zepp, F.; Meyer, C.U. Cordycepin is an immunoregulatory active ingredient of Cordyceps sinensis. Am. J. Chin. Med. 2008, 36, 67–80. [Google Scholar] [CrossRef]
- Wang, J.; Kan, L.; Nie, S.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT 2015, 63, 2–7. [Google Scholar] [CrossRef]
- Wu, D.T.; Lv, G.P.; Zheng, J.; Li, Q.; Ma, S.C.; Li, S.P.; Zhao, J. Cordyceps collected from Bhutan, an appropriate alternative of Cordyceps sinensis. Sci. Rep. 2016, 6, 37668. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Tan, J.; Yuan, Y.; Mei, H.; Zheng, Y.; Sheng, R. Extraction, structure and pharmacological effects of the polysaccharides from Cordyceps sinensis: A review. J. Funct. Foods 2022, 89, 104909. [Google Scholar] [CrossRef]
- Wang, T.; Tang, C.; Xiao, M.; Cao, Z.; He, M.; Qi, J.; Li, Y.; Li, X. Effect of Air Drying on the Metabolic Profile of Fresh Wild and Artificial Cordyceps sinensis. Foods 2023, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.j.; Pan, J.; Zhao, B.; Liang, J.; Ze Yu, W.; Yang, J.j. Comparisons on enhancing the immunity of fresh and dry Cordyceps militaris in vivo and in vitro. J. Ethnopharmacol. 2013, 149, 713–719. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.; Pérez-Coello, M.; Cabezudo, M. Effect of different drying methods on the volatile components of parsley (Petroselinum crispum L.). Eur. Food Res. Technol. 2002, 215, 227–230. [Google Scholar]
- Rodríguez, Ó.; Eim, V.; Rosselló, C.; Femenia, A.; Cárcel, J.A.; Simal, S. Application of power ultrasound on the convective drying of fruits and vegetables: Effects on quality. J. Sci. Food Agric. 2018, 98, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
- Bourdoux, S.; Li, D.; Rajkovic, A.; Devlieghere, F.; Uyttendaele, M. Performance of Drying Technologies to Ensure Microbial Safety of Dried Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1056–1066. [Google Scholar] [CrossRef]
- Maurya, V.K.; Gothandam, K.M.; Ranjan, V.; Shakya, A.; Pareek, S. Effect of drying methods (microwave vacuum, freeze, hot air and sun drying) on physical, chemical and nutritional attributes of five pepper (Capsicum annuum var. annuum) cultivars. J. Sci. Food Agric. 2018, 98, 3492–3500. [Google Scholar] [CrossRef]
- Mediani, A.; Hamezah, H.S.; Jam, F.A.; Mahadi, N.F.; Chan, S.X.Y.; Rohani, E.R.; Che Lah, N.H.; Azlan, U.K.; Khairul Annuar, N.A.; Azman, N.A.F.; et al. A comprehensive review of drying meat products and the associated effects and changes. Front. Nutr. 2022, 9, 1057366. [Google Scholar] [CrossRef]
- Wu, X.F.; Zhang, M.; Bhandari, B.; Li, Z. Effects of microwave-assisted pulse-spouted bed freeze-drying (MPSFD) on volatile compounds and structural aspects of Cordyceps militaris. J. Sci. Food Agric. 2018, 98, 4634–4643. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.; Bucalá, V. A Review on Influence of Spray Drying Process Parameters on the Production of Medicinal Plant Powders. Curr. Drug Discov. Technol. 2019, 16, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xing, S.; Fu, C.; Fang, F.; Liu, J.; Kan, J.; Qian, C.; Chai, Q.; Jin, C. Effects of Drying Methods on Taste Components and Flavor Characterization of Cordyceps militaris. Foods 2022, 11, 3933. [Google Scholar] [CrossRef]
- Du, C.; Zhou, J.; Liu, J. Identification of Chinese medicinal fungus Cordyceps sinensis by depth-profiling mid-infrared photoacoustic spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 489–494. [Google Scholar] [CrossRef]
- Wei, Y.; Li, L.; Liu, Y.; Xiang, S.; Zhang, H.; Yi, L.; Shang, Y.; Xu, W. Identification techniques and detection methods of edible fungi species. Food Chem. 2022, 374, 131803. [Google Scholar] [CrossRef]
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.A.; Tadmor, Y.a.; Gepstein, S.; Giovannoni, J.J.; Katzir, N. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Zhou, W.; Li, P.; Alolga, R.N.; Qi, L.-W.; Yin, X. A comparative proteomic characterization and nutritional assessment of naturally-and artificially cultivated Cordyceps sinensis. J. Proteom. 2018, 181, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.J.; Abel, T. The role of protein synthesis in memory consolidation: Progress amid decades of debate. Neurobiol. Learn. Mem. 2008, 89, 293–311. [Google Scholar] [CrossRef]
- Zhou, X.; Xiong, H.; Lu, Y.; Geng, M.; Lu, Y.; Zhang, L.; Zhu, X. PKD2 deficiency suppresses amino acid biosynthesis in ADPKD by impairing the PERK-TBL2-eIF2ɑ-ATF4 pathway. Biochem. Biophys. Res. Commun. 2021, 561, 73–79. [Google Scholar] [CrossRef]
- Sun, L.b.; Zhang, Z.y.; Xin, G.; Sun, B.x.; Bao, X.j.; Wei, Y.y.; Zhao, X.m.; Xu, H.r. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, Y.; Sun, C.; Li, W.; Qiu, J.; Qiao, Y.; Wu, F.; Huo, X.; An, Y.; et al. Nucleosides and amino acids, isolated from Cordyceps sinensis, protected against cyclophosphamide-induced myelosuppression in mice. Nat. Prod. Res. 2022, 36, 6056–6059. [Google Scholar] [CrossRef]
- Andries, A.; Rozenski, J.; Vermeersch, P.; Mekahli, D.; Van Schepdael, A. Recent progress in the LC-MS/MS analysis of oxidative stress biomarkers. Electrophoresis 2021, 42, 402–428. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Taniguchi, M.; Umakoshi, Y.; Watai, D.; Fukusaki, E. High-Throughput LC-MS/MS Method for Chiral Amino Acid Analysis Without Derivatization. Methods Mol. Biol. 2019, 2030, 253–261. [Google Scholar]
- Galili, G.; Amir, R.; Fernie, A.R. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of Amino Acid Taste and Appetite. Adv. Nutr. 2016, 7, 806s–822s. [Google Scholar] [CrossRef]
- Chacko, S.; Haseeb, Y.B.; Haseeb, S. Metabolomics Work Flow and Analytics in Systems Biology. Curr. Mol. Med. 2022, 22, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Tateoka, Y.; Miyaguchi, Y.; Takahashi, M.; Ogita, H. Associations Between Habitual Dietary Behaviors and Glutamic Acid Levels in Human Milk. J. Hum. Lact. 2023, 39, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Boerboom, G.; Busink, R.; Smits, C.; van Harn, J.; Bikker, P. Effect of L-glutamic acid N, N-diacetic acid on the availability of dietary zinc in broiler chickens. Poult. Sci. 2021, 100, 100913. [Google Scholar] [CrossRef] [PubMed]
- Servant, G.; Frerot, E. Pharmacology of the Umami Taste Receptor. Handb. Exp. Pharmacol. 2022, 275, 109–136. [Google Scholar] [PubMed]
- Chapel-Crespo, C.C.; Diaz, G.A.; Oishi, K. Efficacy of N-carbamoyl-L-glutamic acid for the treatment of inherited metabolic disorders. Expert. Rev. Endocrinol. Metab. 2016, 11, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhao, Z.; Yin, N.; Xu, Y.; Yin, T.; Gou, J.; He, H.; Wang, Y.; Zhang, Y.; Tang, X. Co-delivery of gemcitabine and cisplatin via Poly (L-glutamic acid)-g-methoxy poly (ethylene glycol) micelle to improve the in vivo stability and antitumor effect. Pharm. Res. 2021, 38, 2091–2108. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, V.Y.; Malchenko, L.A.; Butorina, N.N.; Lazarev Konchenko, D.S.; Zvezdina, N.D.; Dubovaya, T.K. Glutamic Acid as Enhancer of Protein Synthesis Kinetics in Hepatocytes from Old Rats. Biochemistry 2017, 82, 957–961. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, B.; Yu, C.; Li, J.; Zhang, L.; Sun, H.; Gao, F.; Zhou, G. L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS ONE 2014, 9, e111950. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Diniz, V.L.S.; Dodd, G.T.; Cruzat, V. Glutamine metabolism and optimal immune and CNS function. Proc. Nutr. Soc. 2023, 82, 22–31. [Google Scholar] [CrossRef]
- Durthi, C.P.; Pola, M.; Rajulapati, S.B.; Kola, A.K.; Kamal, M.A. Versatile and Valuable Utilization of Amidohydrolase L-glutaminase in Pharma and Food industries: A Review. Curr. Drug Metab. 2020, 21, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Y.B.; Ferreira, F.V.; Musumeci, M.A. L-Glutamine-, peptidyl- and protein-glutaminases: Structural features and applications in the food industry. World J. Microbiol. Biotechnol. 2022, 38, 204. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Jorge, J.M.P.; Pérez-García, F.; Sgobba, E. Updates on industrial production of amino acids using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2016, 32, 105. [Google Scholar] [CrossRef]
- Cysewski, P.; Szefler, B. Environment influences on the aromatic character of nucleobases and amino acids. J. Mol. Model. 2010, 16, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kollemparembil, A.M.; Zettel, V.; Claßen, T.; Gatternig, B.; Delgado, A.; Hitzmann, B. Experimental investigation of CO2 uptake in CO2 hydrates formation with amino acids as kinetic promoters and its dissociation at high temperature. Sci. Rep. 2022, 12, 8359. [Google Scholar] [CrossRef] [PubMed]
- Somjai, C.; Siriwoharn, T.; Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Wiriyacharee, P. Utilization of Maillard reaction in moist-dry-heating system to enhance physicochemical and antioxidative properties of dried whole longan fruit. Heliyon 2021, 7, e07094. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, F.B. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef]
- Dao, H.M.; Sahakijpijarn, S.; Chrostowski, R.; Peng, H.H.; Moon, C.; Xu, H.; Mangolini, F.; Do, H.H.; Cui, Z.; Williams, R.O., 3rd. Entrapment of air microbubbles by ice crystals during freezing exacerbates freeze-induced denaturation of proteins. Int. J. Pharm. 2022, 628, 122306. [Google Scholar] [CrossRef]
- Nishi, H.; Uchida, K.; Saito, M.; Yamanaka, D.; Nagata, H.; Tomoshige, H.; Miyata, I.; Ito, K.; Toyoshima, Y.; Takahashi, S.I.; et al. Essential Amino Acid Intake Is Required for Sustaining Serum Insulin-like Growth Factor-I Levels but Is Not Necessarily Needed for Body Growth. Cells 2022, 11, 1523. [Google Scholar] [CrossRef]
- Öztürk, F.; Gündüz, H. The effect of different drying methods on chemical composition, fatty acid, and amino acid profiles of sea cucumber (Holothuria tubulosa Gmelin, 1791). J. Food Process. Preserv. 2018, 42, e13723. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, H.; Du, Z. Effect of drying methods on aroma, taste and antioxidant activity of Dendrobium officinale flower tea: A sensomic and metabolomic study. Food Res. Int. 2024, 187, 114455. [Google Scholar] [CrossRef] [PubMed]



| No. | Compounds | Q1 (Da) | Q3 (Da) | Molecular Weight | Formula | RT | DG | HG | YG | CK |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ethanolamine | 62 | 44 | 61.08 | C2H7NO | 4.73 | 52,263.13 | 30,905.25 | 27,724.37 | 34,209.60 |
| 2 | Phosphorylethanolamine | 142.026 | 44.049 | 141.06 | C2H8NO4P | N/A | N/A | N/A | N/A | N/A |
| 3 | Anserine | 241 | 170 | 240.26 | C10H16N4O3 | 10.47 | 2791.09 | 3269.22 | 2023.59 | 3102.44 |
| 4 | (5-L-Glutamyl)-L-Alanine | 219.09 | 202 | 218.09 | C8H14N2O5 | 9.84 | 59,690.84 | 152,090.55 | 70,597.08 | 174,067.24 |
| 5 | O-Phospho-L-Serine | 186 | 88.2 | 185.01 | C3H8NO6P | 10.92 | 5014.20 | 10,415.13 | 5146.75 | 9795.23 |
| 6 | N-Acetylaspartate | 176 | 134 | 175.05 | C6H9NO5 | 4.76 | 18,063.90 | 20,873.08 | 12,980.58 | 8152.68 |
| 7 | N-Acetyl-L-Tyrosine | 224 | 136 | 223.09 | C11H13NO4 | 1.74 | 680.43 | 1210.69 | 630.95 | 1665.39 |
| 8 | L-tyrosine methyl ester | 196.2 | 136.3 | 195.09 | C10H13NO3 | 1.52 | 409.23 | 434.79 | 281.85 | 437.05 |
| 9 | L-Cystathionine | 223.07 | 134 | 222.07 | C7H14N2O4S | 10.86 | 409,853.38 | 386,011.47 | 265,291.90 | 383,347.89 |
| 10 | Homoserine | 120.065 | 74.06 | 119.12 | C4H9NO3 | 8.57 | 963,95.82 | 160,750.02 | 85,718.14 | 181,130.92 |
| 11 | D-Alanyl-D-Alanine | 161.09 | 44.04 | 160.09 | C6H12N2O3 | 8.76 | 3157.84 | 7888.17 | 3684.92 | 3824.47 |
| 12 | S-(5-Adenosyl)-L-Homocysteine | 385 | 136.06 | 384.12 | C14H20N6O5S | 9.37 | 115,970.38 | 835,272.49 | 317,084.08 | 224,819.03 |
| 13 | 3-Iodo-L-Tyrosine | 308.2 | 261.97 | 306.97 | C9H10INO3 | 4.75 | 37.26 | 66.00 | 36.00 | 42.47 |
| 14 | glycylphenylalanine | 223.2 | 120.2 | 222.1 | C11H14N2O3 | 7.17 | 881.62 | 7363.57 | 2750.79 | 417.58 |
| 15 | L-Valine | 118.1 | 72.1 | 117.08 | C5H11NO2 | 6.14 | 405,663.10 | 512,162.35 | 305,943.86 | 389,962.93 |
| 16 | L-Tyrosine | 182.08 | 136.07 | 181.19 | C9H11NO3 | 6.67 | 808,353.61 | 869,834.54 | 559,398.27 | 872,333.69 |
| 17 | L-Tryptophan | 205.1 | 118.06 | 204.09 | C11H12N2O2 | 4.46 | 101,179.10 | 151,314.67 | 84,166.08 | 86,786.14 |
| 18 | Trans-4-Hydroxy-L-Proline | 132.058 | 86 | 131.06 | C5H9NO3 | 8.05 | 98,744.37 | 156,291.27 | 85,014.56 | 191,836.09 |
| 19 | Glutathione Oxidized | 613.152 | 484 | 612.15 | C20H32N6O12S2 | 11.31 | 7,287,906.55 | 8,895,298.80 | 5,394,405.55 | 8,784,995.30 |
| 20 | argininosuccinic acid | 291.5 | 176.1 | 290.27 | C10H18N4O6 | 10.99 | 2,964,334.61 | 3,027,278.70 | 1,997,208.10 | 1,930,567.70 |
| 21 | D-Homocysteine | 136 | 90.036 | 135.04 | C4H9NO2S | 6.14 | 464.30 | 486.99 | 319.14 | 452.44 |
| 22 | L-Cystine | 239 | 120 | 240.02 | C6H12N2O4S2 | 10.91 | 401.06 | 594.09 | 335.35 | N/A |
| 23 | Nicotinuric Acid | 181.1 | 135 | 180.05 | C8H8N2O3 | 3.55 | N/A | N/A | N/A | N/A |
| 24 | N8-Acetylspermidine | 190.18 | 173.15 | 189.18 | C9H23N3O | 9.72 | N/A | N/A | N/A | N/A |
| 25 | N-Propionylglycine | 130.1 | 74 | 131.06 | C5H9NO3 | 2.14 | N/A | N/A | N/A | N/A |
| 26 | L-Cysteine | 122.02 | 59 | 121.02 | C3H7NO2S | 7.13 | N/A | N/A | N/A | N/A |
| 27 | 3-Chloro-L-Tyrosine | 216.1 | 170.1 | 215.04 | C9H10ClNO3 | 5.33 | N/A | N/A | N/A | N/A |
| 28 | 5-Hydroxy-Tryptamine | 177.1 | 160 | 176.21 | C10H12N20 | 2.55 | N/A | N/A | N/A | N/A |
| 29 | Nα-Acetyl-L-glutamine | 189.1 | 130.1 | 188.18 | C7H12N2O4 | 6.39 | N/A | N/A | N/A | N/A |
| 30 | L-Threonine | 120.06 | 74 | 119.06 | C4H9NO3 | 8.38 | 1,590,252.44 | 1,797,878.87 | 1,129,379.90 | 1,226,147.51 |
| 31 | N,N-Dimethylglycine | 104 | 58 | 103.06 | C4H9NO2 | 5.91 | N/A | N/A | N/A | 566.57 |
| 32 | L-Homocystine | 269 | 136 | 268.06 | C8H16N2O4S2 | 10.50 | N/A | 3648.26 | N/A | N/A |
| 33 | Trimethylamine N-Oxide | 76 | 58.1 | 75.07 | C3H9NO | 2.94 | N/A | 3.87 | N/A | 13.15 |
| 34 | N-Isovaleroylglycine | 158.1 | 74 | 159.09 | C7H13NO3 | 1.32 | N/A | N/A | N/A | N/A |
| 35 | S-Sulfo-L-Cysteine | 201.98 | 120 | 200.98 | C3H7NO5S2 | 7.86 | N/A | 1428.78 | N/A | N/A |
| 36 | N-Acetylneuraminic Acid | 310.11 | 274.09 | 309.11 | C11H19NO9 | 9.57 | N/A | 130,727.88 | N/A | 22,726.06 |
| 37 | γ-Glutamate-Cysteine | 251.07 | 130.05 | 250.06 | C8H14N2O5S | 9.84 | 207,439.45 | 215,940.92 | 141,130.07 | 244,866.08 |
| 38 | 5-Hydroxy-tryptophan | 219.1 | 74 | 220.22 | C11H12N2O3 | 6.69 | 15,855.02 | 13,153.19 | 9671.64 | 16,623.56 |
| 39 | L-Theanine | 175 | 158 | 174.1 | C7H14N2O3 | 7.14 | 2049.75 | 1054.52 | 1037.14 | N/A |
| 40 | L-Tryptophyl-L-glutamic acid | 334 | 159 | 333.13 | C16H19N3O5 | 8.19 | 52,085.37 | 235,428.36 | 95,840.64 | 34,439.12 |
| 41 | Urea | 61 | 44 | 60.03 | CH4N2O | 1.46 | 73,727.18 | 380,087.66 | 151,272.10 | 304,614.48 |
| 42 | L-Serine | 106.04 | 60 | 105.04 | C3H7NO3 | 9.03 | 590,199.33 | 684,869.12 | 425,025.83 | 474,754.60 |
| 43 | L-Histidine | 156.07 | 110.07 | 155.07 | C6H9N3O2 | 10.06 | 1,405,379.02 | 1,404,476.98 | 936,622.02 | 1,071,662.56 |
| 44 | Glycyl-L-Proline | 173.1 | 116.071 | 172.09 | C7H12N2O3 | 9.10 | 788.76 | 1743.52 | 847.13 | 285.07 |
| 45 | Glycine | 76.03 | 30 | 75.03 | C2H5NO2 | 8.6 | 403,027.10 | 553,800.33 | 318,945.34 | 282,386.73 |
| 46 | Succinic Acid | 117.03 | 99 | 118.03 | C4H6O4 | 1.36 | 1,299,153.87 | 1,491,250.31 | 930,135.18 | 730,942.74 |
| 47 | L-Glutamine | 147.069 | 84 | 146.07 | C5H10N2O3 | 8.98 | 9,799,945.11 | 11,662,924.50 | 7,154,292.86 | 11,198,431.33 |
| 48 | Beta-Alanine | 90.2 | 30 | 89.05 | C3H7NO2 | 8.05 | 43,449.96 | 60,525.95 | 34,661.32 | 54,251.24 |
| 49 | L-α-Aspartyl-L-phenylalanine | 281.3 | 166 | 280.11 | C13H16N2O5 | 8.73 | 3981.51 | 5021.82 | 3004.02 | 3711.77 |
| 50 | L-Aspartate | 134.04 | 74 | 133.1 | C4H7NO4 | 9.93 | 3,544,943.12 | 3,319,674.22 | 2,288,209.09 | 2,792,831.29 |
| 51 | L-Asparagine Anhydrous | 133.06 | 74 | 132.05 | C4H8N2O3 | 9.13 | 895,965.13 | 974,125.64 | 623,366.63 | 815,367.47 |
| 52 | L-Arginine | 175.1 | 70.06 | 174.11 | C6H14N4O2 | 10.28 | 1,231,313.96 | 1,537,085.89 | 922,803.38 | 1,047,209.36 |
| 53 | L-Alanine | 90.05 | 44.05 | 89.05 | C3H7NO2 | 8.07 | 866,190.62 | 1,556,109.93 | 807,436.21 | 1,313,825.59 |
| 54 | 5-Hydroxylysine | 163.1 | 128.07 | 162.18 | C6H14N2O3 | 10.76 | 13,266.38 | 14,017.87 | 9098.34 | 12,117.00 |
| 55 | 3-N-Methyl-L-Histidine | 170 | 96 | 169.18 | C7H11N3O2 | 10.19 | 31,543.58 | 32,713.75 | 21,422.51 | 30,344.09 |
| 56 | 3-Aminoisobutanoic Acid | 104.07 | 86.05 | 103.06 | C4H9NO2 | 6.92 | 165,603.26 | 257,073.67 | 140,894.62 | 232,608.79 |
| 57 | 1-Methylhistidine | 170.1 | 124 | 169.09 | C7H11N3O2 | 9.40 | 224,915.30 | 237,642.61 | 154,189.10 | 220,762.47 |
| 58 | Homo-L-arginine | 189 | 144 | 188.13 | C7H16N4O2 | 10.14 | 399,822.88 | 407,488.07 | 269,107.03 | 329,249.02 |
| 59 | L-Isoleucine | 132.1 | 86.1 | 131.1 | C6H13NO2 | 4.67 | 220,177.26 | 229,017.66 | 149,733.20 | 167,765.33 |
| 60 | L-Glutamic acid | 148.06 | 84 | 147.05 | C5H9NO4 | 9.39 | 14,736,617.03 | 17,017,140.07 | 10,584,588.83 | 17,347,695.43 |
| 61 | L-Citrulline | 176.1 | 113 | 175.1 | C6H13N3O3 | 9.37 | 210,279.70 | 144,586.95 | 118,292.00 | 103,110.97 |
| 62 | L-Proline | 116.1 | 70.1 | 115.06 | C5H9NO2 | 6.20 | 1,026,738.00 | 1,117,680.18 | 714,808.13 | 1,002,877.35 |
| 63 | L-Phenylalanine | 166.1 | 120.08 | 165.08 | C9H11NO2 | 4.29 | 212,228.29 | 147,826.34 | 120,019.64 | 139,718.17 |
| 64 | Nα-Acetyl-L-Arginine | 217.1 | 158 | 216.12 | C8H16N4O3 | 8.40 | 4080.90 | 3937.66 | 2675.65 | 4013.49 |
| 65 | L-Carnosine | 227.2 | 156 | 226.11 | C9H14N4O3 | 10.00 | 7058.33 | 16,999.45 | 8022.59 | 11,899.32 |
| 66 | N-Glycyl-L-Leucine | 189.1 | 86 | 188.12 | C8H16N2O3 | 7.12 | 3348.98 | 23,136.08 | 8830.73 | 1577.20 |
| 67 | Methionine Sulfoxide | 166 | 74 | 165.05 | C5H11NO3S | 8.95 | 2511.90 | 3535.30 | 2018.72 | 1485.36 |
| 68 | L-Methionine | 150.05 | 104 | 149.05 | C5H11NO2S | 5.49 | 12,821.99 | 21,771.01 | 11,532.83 | 7682.36 |
| 69 | L-Lysine | 147.069 | 84 | 146.11 | C6H14N2O2 | 10.45 | 1,016,825.72 | 1,230,450.69 | 749,095.62 | 1,073,002.26 |
| 70 | N6-Acetyl-L-Lysine | 189.1 | 126 | 188.12 | C8H16N2O3 | 8.32 | 142,014.00 | 207,737.40 | 116,586.57 | 252,095.63 |
| 71 | Sarcosine | 90.2 | 44.1 | 89.05 | C3H7NO2 | 7.51 | N/A | N/A | N/A | N/A |
| 72 | L-Leucine | 132.1 | 86.1 | 131.1 | C6H13NO2 | 4.37 | 371,341.70 | 383,117.21 | 251,487.76 | 307,224.27 |
| 73 | L-Pipecolic Acid | 130 | 84 | 129.08 | C6H11NO2 | 6.58 | 10,013.19 | 13,560.04 | 7859.94 | 13,645.17 |
| 74 | L-Ornithine | 133.09 | 70 | 132.16 | C5H13ClN2O2 | 10.50 | 6,438,468.41 | 5,370,492.12 | 3,936,323.68 | 6,452,913.39 |
| 75 | L-Homocitrulline | 190.1 | 127 | 189.11 | C7H15N3O3 | 9.14 | 8630.02 | 20,657.64 | 9765.60 | 8303.07 |
| 76 | (S)-β-Aminoisobutyric Acid | 104.07 | 57.03 | 103.06 | C4H9NO2 | 6.92 | 142,049.24 | 232,551.66 | 124,869.27 | 207,825.77 |
| 77 | 1,3-Dimethyluric Acid | 195 | 180 | 196.06 | C7H8N4O3 | 1.53 | 945.92 | 1515.58 | 821.01 | 1196.77 |
| 78 | Kynurenic Acid | 190.043 | 144 | 189.04 | C10H7NO3 | 3.02 | 70.73 | 86.35 | 53.37 | 102.69 |
| 79 | N’-Formylkynurenine | 237.1 | 146.06 | 236.08 | C11H12N2O4 | 5.47 | N/A | N/A | N/A | N/A |
| 80 | Guanidinoethyl Sulfonate | 168.04 | 151.01 | 167.04 | C3H9N3O3S | 6.37 | N/A | N/A | N/A | N/A |
| 81 | 2-Aminobutyric acid | 104.1 | 58.14 | 103.12 | C4H9NO2 | 7.25 | 217,013.31 | 413,898.45 | 210,306.34 | 403,754.53 |
| 82 | Creatine Phosphate | 212.04 | 114.06 | 211.11 | C4H8N3Na2O5P | N/A | N/A | N/A | N/A | N/A |
| 83 | 6-Aminocaproic Acid | 132.095 | 69 | 131.1 | C6H13NO2 | 3.76 | N/A | N/A | N/A | N/A |
| 84 | 5-Aminovaleric Acid | 118.2 | 55 | 117.08 | C5H11NO2 | 4.68 | 29,796.26 | 40,011.34 | 23,270.76 | 35,215.87 |
| 85 | 3,7-Dimethyluric Acid | 195 | 180 | 196.06 | C7H8N4O3 | 1.65 | N/A | N/A | N/A | N/A |
| 86 | Creatine | 132.1 | 90 | 131.07 | C4H9N3O2 | 8.09 | 2174.93 | 4947.67 | 2376.90 | 2622.21 |
| 87 | 3-Hydroxyhippuric Acid | 196.05 | 121 | 195.05 | C9H9NO4 | 1.98 | N/A | N/A | N/A | N/A |
| 88 | P-Aminohippuric Acid | 195.069 | 120 | 194.07 | C9H10N2O3 | 1.93 | 97.15 | 460.48 | 186.52 | 200.29 |
| 89 | 4-Acetamidobutyric Acid | 146.074 | 86 | 145.07 | C6H11NO3 | 1.17 | 1560.37 | 3167.75 | 1576.43 | 2300.27 |
| 90 | γ-Aminobutyric Acid | 104.06 | 68.8 | 103.06 | C4H9NO2 | 6.56 | 3,866,168.03 | 3,234,742.41 | 2,366,972.33 | 484,156.29 |
| 91 | kynurenine | 209.1 | 146 | 208.09 | C10H12N2O3 | 4.48 | 148.87 | 595.04 | 249.46 | 80.48 |
| 92 | α-Aminoadipic acid | 162.07 | 98.06 | 161.16 | C6H11NO4 | 8.97 | 360,464.64 | 460,812.56 | 273,762.06 | 508,061.11 |
| 93 | 2-Aminoethanesulfonic Acid | 124 | 80 | 125.15 | C2H7NO3S | 6.49 | 26,317.34 | 37,667.76 | 21,330.53 | 41,370.20 |
| 94 | 1,3,7-Trimethyluric Acid | 211.08 | 154.06 | 210.08 | C8H10N4O3 | 1.09 | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Wang, T.; Tang, C.; He, M.; Li, Y.; Li, X. Effects of Different Drying Methods on Amino Acid Metabolite Content and Quality of Ophiocordyceps sinensis by LC-MS/MS Combined with Multivariate Statistical Methods. Metabolites 2024, 14, 459. https://doi.org/10.3390/metabo14080459
Xiao M, Wang T, Tang C, He M, Li Y, Li X. Effects of Different Drying Methods on Amino Acid Metabolite Content and Quality of Ophiocordyceps sinensis by LC-MS/MS Combined with Multivariate Statistical Methods. Metabolites. 2024; 14(8):459. https://doi.org/10.3390/metabo14080459
Chicago/Turabian StyleXiao, Mengjun, Tao Wang, Chuyu Tang, Min He, Yuling Li, and Xiuzhang Li. 2024. "Effects of Different Drying Methods on Amino Acid Metabolite Content and Quality of Ophiocordyceps sinensis by LC-MS/MS Combined with Multivariate Statistical Methods" Metabolites 14, no. 8: 459. https://doi.org/10.3390/metabo14080459





