Xanthoceras sorbifolium Oil Attenuates Hyperlipidemia Through Dual Modulation of Gut Microbiota and Lipid Metabolites: Mechanistic Insights from Lipidomics and 16S rRNA Sequencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Research
2.2.1. Experimental Animals
2.2.2. Experimental Design

2.3. Body Weight Change and Organ Index in Rats
2.4. Serum Biochemical Detection
2.5. Detection of SCFAs in Feces
2.6. 16S rRNA Gene Sequencing Analysis
2.7. Serum Lipid Metabolomics Analysis
2.8. Statistical Analysis
3. Results
3.1. Body Weight and Tissue Weight
3.2. Serum Biochemical Parameters and Cardiovascular Indices

3.3. Fecal SCFAs
3.4. Effects of XSO on Gut Microbial Diversity
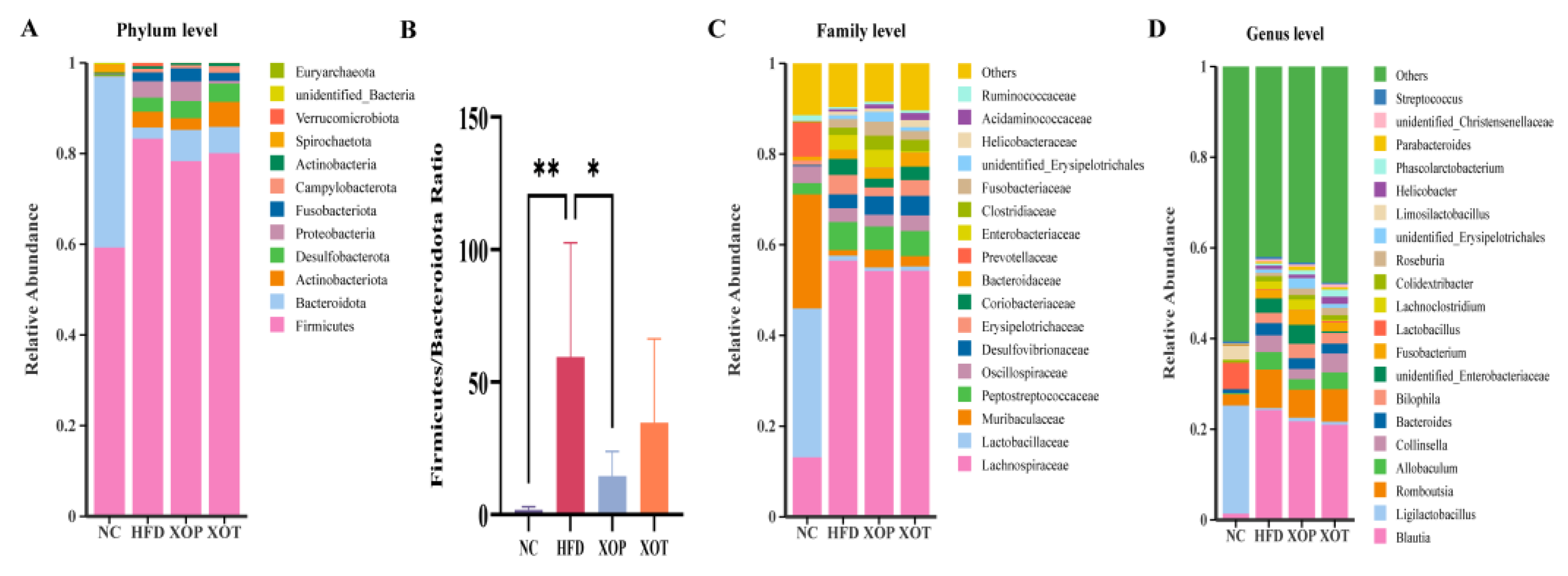
3.5. Effects of XSO on Intestinal Microbial Composition
3.6. Changes in Genus Microorganisms and Related Parameters
3.7. Enrichment Analysis of Microbial Metabolic Pathways
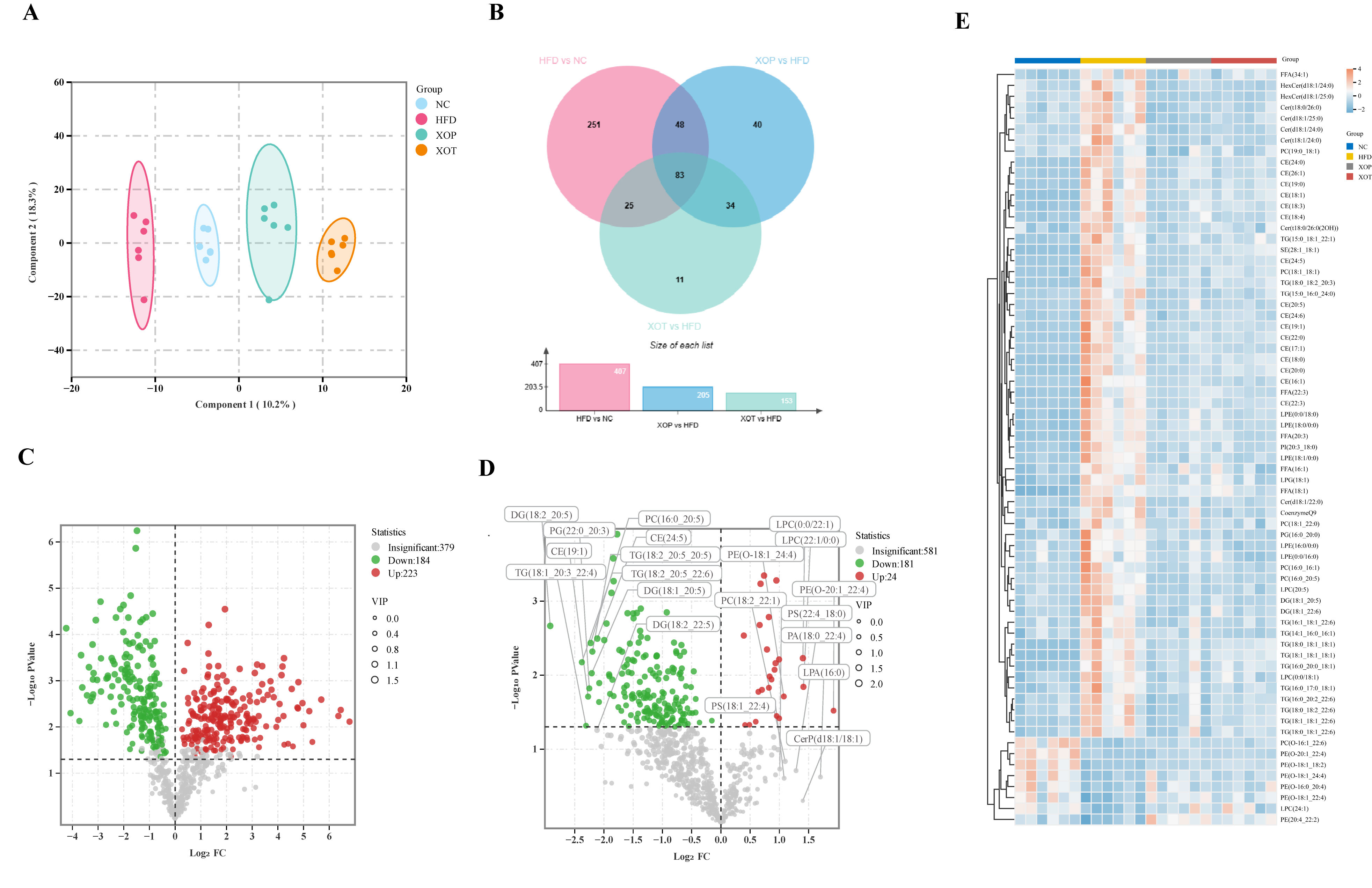
3.8. Serum Lipid Metabolism
3.9. Enrichment Pathway Analysis of Serum Lipid Metabolites
3.10. Changes in Serum Lipid Metabolites and Related Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XSO | Xanthoceras sorbifolium Oil |
| CVDs | Cardiovascular diseases |
| ASCVD | Atherosclerotic cardiovascular disease |
| TMA | Trimethylamine |
| SD | Sprague Dawley |
| HFD | High-fat diet |
| TC | Total Cholesterol |
| TG | Triglyceride |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| AI | Atherogenic index |
| AC | Atherogenic coefficient |
| HCY | Homocysteine |
| AST | Aspartate amino transferase |
| ALT | Alanine aminotransferase |
| BSTFA | N,O-Bis(trimethylsilyl) trifluoroacetamide |
| LPS | Lipopolysaccharides |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| CE | Cholesterol ester |
| PC | Phosphatidylcholine |
| DG | Diglyceride |
| FFA | Fatty acid |
| PUFAs | Polyunsaturated fatty acids |
| SCFAs | Short-chain fatty acids |
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [PubMed]
- Salami, J.A.; Valero-Elizondo, J.; Ogunmoroti, O.; Spatz, E.S.; Rana, J.S.; Virani, S.S.; Blankstein, R.; Younus, A.; Arrieta, A.; Blaha, M.J.; et al. Association between Modifiable Risk Factors and Pharmaceutical Expenditures among Adults with Atherosclerotic Cardiovascular Disease in the United States: 2012–2013 Medical Expenditures Panel Survey. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2017, 6, e004996. [Google Scholar] [CrossRef]
- Karr, S. Epidemiology and Management of Hyperlipidemia. Am. J. Manag. Care 2017, 23, S139–S148. [Google Scholar] [PubMed]
- Goh, R.S.J.; Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Kueh, M.T.W.; Shankar, K.; Li, H.; Chin, Y.H.; Kong, G.; et al. The Burden of Cardiovascular Disease in Asia from 2025 to 2050: A Forecast Analysis for East Asia, South Asia, South-East Asia, Central Asia, and High-Income Asia Pacific Regions. Lancet Reg. Health West. Pac. 2024, 49, 101138. [Google Scholar] [CrossRef]
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global Burden of Cardiovascular Diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, zwae281. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, S.; Yuan, M.; Zhang, M.; Tang, L.; Wang, P.; Liu, Y.; Xu, C.; Luo, P.; Gao, X. Fermented Rosa Roxburghii Tratt Juice Alleviates High-Fat Diet-Induced Hyperlipidemia in Rats by Modulating Gut Microbiota and Metabolites. Front. Pharmacol. 2022, 13, 883629. [Google Scholar] [CrossRef]
- Tabassum, R.; Rämö, J.T.; Ripatti, P.; Koskela, J.T.; Kurki, M.; Karjalainen, J.; Palta, P.; Hassan, S.; Nunez-Fontarnau, J.; Kiiskinen, T.T.J.; et al. Genetic Architecture of Human Plasma Lipidome and Its Link to Cardiovascular Disease. Nat. Commun. 2019, 10, 4329. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.K.; Chew, N.W.S.; Mehta, A. Beyond Cholesterol: Unraveling Residual Lipidomic Risk in Cardiovascular Health. Curr. Atheroscler. Rep. 2025, 27, 37. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención Con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef]
- Cirigliano, A.; Amelina, A.; Biferali, B.; Macone, A.; Mozzetta, C.; Bianchi, M.M.; Mori, M.; Botta, B.; Pick, E.; Negri, R.; et al. Statins Interfere with the Attachment of S. Cerevisiae mtDNA to the Inner Mitochondrial Membrane. J. Enzyme Inhib. Med. Chem. 2020, 35, 129–137. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating Ceramides Predict Cardiovascular Outcomes in the Population-Based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Wang, X.; Tian, Z.; Qi, D.; Li, Y.; Jiang, H. Antihypertensive Activity of Oleanolic Acid Is Mediated via Downregulation of Secretory Phospholipase A2 and Fatty Acid Synthase in Spontaneously Hypertensive Rats. Int. J. Mol. Med. 2020, 46, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-J.; Li, M.-Z.; Chen, C.-H.; Hong, T.; Yang, J.-R.; Huang, X.-J.; Geng, F.; Hu, J.-L.; Nie, S.-P. Tea Polyphenol and Epigallocatechin Gallate Ameliorate Hyperlipidemia via Regulating Liver Metabolism and Remodeling Gut Microbiota. Food Chem. 2023, 404, 134591. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Liu, J.; Yang, H.; Hu, Y.; Zhang, M.; Bai, T.; Chang, F. Lysophosphatidylcholine Promotes Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule-1 Expression in Human Umbilical Vein Endothelial Cells via an Orphan G Protein Receptor 2-Mediated Signaling Pathway. Bioengineered 2021, 12, 4520–4535. [Google Scholar] [CrossRef]
- Jiang, L.; Lang, S.; Duan, Y.; Zhang, X.; Gao, B.; Chopyk, J.; Schwanemann, L.K.; Ventura-Cots, M.; Bataller, R.; Bosques-Padilla, F.; et al. Intestinal Virome in Patients with Alcoholic Hepatitis. Hepatology 2020, 72, 2182–2196. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.; Ryu, J.; Lee, K.; Park, S.Y.; Hwang, K.T. Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets. Nutrients 2020, 12, 2448. [Google Scholar] [CrossRef]
- Yuan, X.; Bhat, O.M.; Zou, Y.; Li, X.; Zhang, Y.; Li, P.-L. Endothelial Acid Sphingomyelinase Promotes NLRP3 Inflammasome and Neointima Formation during Hypercholesterolemia. J. Lipid Res. 2022, 63, 100298. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Xia, M.; Chen, Y.; Bhat, O.M.; Yuan, X.; Boini, K.M.; Li, P.-L. Endothelial NLRP3 Inflammasome Activation and Arterial Neointima Formation Associated with Acid Sphingomyelinase during Hypercholesterolemia. Redox Biol. 2017, 13, 336–344. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Gut Microbiota Modulation through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Liu, G.S. The Embryology of Xanthoceras and Its Phylogenetic Implications. Plant Syst. Evol. 2012, 298, 457–468. [Google Scholar] [CrossRef]
- Chen, X.; Lei, Z.; Cao, J.; Zhang, W.; Wu, R.; Cao, F.; Guo, Q.; Wang, J. Traditional Uses, Phytochemistry, Pharmacology and Current Uses of Underutilized Xanthoceras sorbifolium Bunge: A Review. J. Ethnopharmacol. 2022, 283, 114747. [Google Scholar] [CrossRef]
- Zhou, W.; Lyu, S.-B.; Li, H.; Li, S.-X.; Yao, W.-H.; Shan, S.-L.; Tang, H.; Zhang, J.; Sun, C.-H.; Wen, C.-L.; et al. Toxic Effects and Safety Assessment of Xanthoceras sorbifolium Bunge Seed Kernels. J. Ethnopharmacol. 2025, 340, 119242. [Google Scholar] [CrossRef]
- Hu, D.; Cui, Y.; Zhang, J. Analysis of the Improvement Effect of Nervonic Acid Extracted from Xanthoceras sorbifolium Bunge Oil on Antioxidant Response and Inflammatory Response in Parkinson’s Disease. J. Integr. Neurosci. 2023, 22, 161. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Hu, W.; Jiang, A. Research Progress on the Development and Application of Xanthoceras sorbifolium Seed Oil. J. Food Ind. Sci. Technol. 2016, 37, 393–396+400. [Google Scholar]
- Qu, X.-J.; Fu, Y.-J.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Li, C.-Y.; Wang, W.; Li, J.; Wei, Z.-F. Acidic pH Based Microwave-Assisted Aqueous Extraction of Seed Oil from Yellow Horn (Xanthoceras sorbifolia Bunge.). Ind. Crops Prod. 2013, 43, 420–426. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Ao, Y.; Saunders, M.R.; Wang, X. Diversity of Seed and Seed Oil Physicochemical Traits of Xanthoceras sorbifolium Bunge. J. Food Compos. Anal. 2021, 96, 103705. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Jin, Q.; Wang, X. Effects of Dietary Linoleic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of 40 Randomized Controlled Trials. Foods 2023, 12, 2129. [Google Scholar] [CrossRef]
- Li, J.; Fu, Y.-J.; Qu, X.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G. Biodiesel Production from Yellow Horn (Xanthoceras sorbifolia Bunge.) Seed Oil Using Ion Exchange Resin as Heterogeneous Catalyst. Bioresour. Technol. 2012, 108, 112–118. [Google Scholar] [CrossRef]
- Kwek, E.; Zhu, H.; Ding, H.; He, Z.; Hao, W.; Liu, J.; Ma, K.Y.; Chen, Z.-Y. Peony Seed Oil Decreases Plasma Cholesterol and Favorably Modulates Gut Microbiota in Hypercholesterolemic Hamsters. Eur. J. Nutr. 2022, 61, 2341–2356. [Google Scholar] [CrossRef]
- Qiang, X.; Guo, C.; Gu, W.; Song, Y.; Zhang, Y.; Gong, X.; Wang, L.; Wang, G. The Complex of Phycobiliproteins, Fucoxanthin, and Krill Oil Ameliorates Obesity through Modulation of Lipid Metabolism and Antioxidants in Obese Rats. Nutrients 2022, 14, 4815. [Google Scholar] [CrossRef]
- Nenadovic, A.; Kovacevic, S.; Stankovic, A.; Popovic, T.; Debeljak Martacic, J.; Rankovic, S.; De Luka, S.R.; Milasin, J.; Nesovic Ostojic, J. Organ-Specific Responses to Chronic High-Fat Diets in Mice: Insights into Phospholipid Fatty Acid Distribution. Nutrients 2025, 17, 821. [Google Scholar] [CrossRef]
- Lemas, D.J.; Young, B.E.; Baker, P.R.; Tomczik, A.C.; Soderborg, T.K.; Hernandez, T.L.; de la Houssaye, B.A.; Robertson, C.E.; Rudolph, M.C.; Ir, D.; et al. Alterations in Human Milk Leptin and Insulin Are Associated with Early Changes in the Infant Intestinal Microbiome12. Am. J. Clin. Nutr. 2016, 103, 1291–1300. [Google Scholar] [CrossRef]
- Czarnowski, P.; Mikula, M.; Ostrowski, J.; Żeber-Lubecka, N. Gas Chromatography-Mass Spectrometry-Based Analyses of Fecal Short-Chain Fatty Acids (SCFAs): A Summary Review and Own Experience. Biomedicines 2024, 12, 1904. [Google Scholar] [CrossRef]
- Parker, B.L.; Calkin, A.C.; Seldin, M.M.; Keating, M.F.; Tarling, E.J.; Yang, P.; Moody, S.C.; Liu, Y.; Zerenturk, E.J.; Needham, E.J.; et al. An Integrative Systems Genetic Analysis of Mammalian Lipid Metabolism. Nature 2019, 567, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Modesto dos Santos, V.; Aparecida Soares, L.; de Carvalho Santos, S. Cardiovascular risk index and adequate nutrition. Rev. Med. Chil. 2010, 138, 652–653; author reply 654–655. [Google Scholar] [CrossRef] [PubMed]
- López-Almela, I.; Romaní-Pérez, M.; Bullich-Vilarrubias, C.; Benítez-Páez, A.; Gómez Del Pulgar, E.M.; Francés, R.; Liebisch, G.; Sanz, Y. Bacteroides Uniformis Combined with Fiber Amplifies Metabolic and Immune Benefits in Obese Mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Merscher, S.; Fornoni, A. Kidney Lipid Dysmetabolism and Lipid Droplet Accumulation in Chronic Kidney Disease. Nat. Rev. Nephrol. 2023, 19, 629–645. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa Roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic Db/Db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated Fatty Acids and Their Effects on Cardiovascular Disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Opoku, S.; Gan, Y.; Fu, W.; Chen, D.; Addo-Yobo, E.; Trofimovitch, D.; Yue, W.; Yan, F.; Wang, Z.; Lu, Z. Prevalence and Risk Factors for Dyslipidemia among Adults in Rural and Urban China: Findings from the China National Stroke Screening and Prevention Project (CNSSPP). BMC Public Health 2019, 19, 1500. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, J.; Gao, Z.; Zhao, H.; Sun, G.; Wang, X.; Jia, L. Characterization and Anti-Hyperlipidemia Effects of Enzymatic Residue Polysaccharides from Pleurotus Ostreatus. Int. J. Biol. Macromol. 2019, 129, 316–325. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Hu, X.; Bian, X.; Nian, S. Gastrodin Prevents Homocysteine-induced Human Umbilical Vein Endothelial Cells Injury via PI3K/Akt/eNOS and Nrf2/ARE Pathway. J. Cell Mol. Med. 2021, 25, 345–357. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, X.; Fang, P.; Yan, Y.; Song, J.; Gupta, S.; Schafer, A.I.; Durante, W.; Kruger, W.D.; Yang, X.; et al. Hyperhomocysteinemia Promotes Inflammatory Monocyte Generation and Accelerates Atherosclerosis in Transgenic Cystathionine β-Synthase Deficient Mice. Circulation 2009, 120, 1893–1902. [Google Scholar] [CrossRef]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-Evoked Activation of PKC and PKD Isoforms in Regulation of Glucose and Lipid Metabolism: A Review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Yu, J.S.; Youn, G.S.; Choi, J.; Kim, C.-H.; Kim, B.Y.; Yang, S.-J.; Lee, J.H.; Park, T.-S.; Kim, B.K.; Kim, Y.B.; et al. Lactobacillus Lactis and Pediococcus Pentosaceus-Driven Reprogramming of Gut Microbiome and Metabolome Ameliorates the Progression of Non-Alcoholic Fatty Liver Disease. Clin. Transl. Med. 2021, 11, e634. [Google Scholar] [CrossRef]
- Huang, Y.; Stinson, S.E.; Thodberg, M.; Holm, L.A.; Thielemann, R.; Sulek, K.; Lund, M.A.V.; Fonvig, C.E.; Kim, M.; Trost, K.; et al. Genetic Factors Shaping the Plasma Lipidome and the Relations to Cardiometabolic Risk in Children and Adolescents. Ebiomedicine 2025, 112, 105537. [Google Scholar] [CrossRef]
- Huynh, K.; Barlow, C.K.; Jayawardana, K.S.; Weir, J.M.; Mellett, N.A.; Cinel, M.; Magliano, D.J.; Shaw, J.E.; Drew, B.G.; Meikle, P.J. High-Throughput Plasma Lipidomics: Detailed Mapping of the Associations with Cardiometabolic Risk Factors. Cell Chem. Biol. 2019, 26, 71–84.e4. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Fogelson, K.A.; Dorrestein, P.C.; Zarrinpar, A.; Knight, R. The Gut Microbial Bile Acid Modulation and Its Relevance to Digestive Health and Diseases. Gastroenterology 2023, 164, 1069–1085. [Google Scholar] [CrossRef]
- Martínez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Moriyama, E.N.; Walter, J. Diet-Induced Metabolic Improvements in a Hamster Model of Hypercholesterolemia Are Strongly Linked to Alterations of the Gut Microbiota. Appl. Environ. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef]
- Martínez, I.; Perdicaro, D.J.; Brown, A.W.; Hammons, S.; Carden, T.J.; Carr, T.P.; Eskridge, K.M.; Walter, J. Diet-Induced Alterations of Host Cholesterol Metabolism Are Likely to Affect the Gut Microbiota Composition in Hamsters. Appl. Environ. Microbiol. 2013, 79, 516–524. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between Composition of the Human Gastrointestinal Microbiome and Development of Fatty Liver with Choline Deficiency. Gastroenterology 2011, 140, 976–986. [Google Scholar] [CrossRef]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of High-Fat Diet on the Intestinal Microbiota and Small Intestinal Physiology before and after the Onset of Obesity. Biochimie 2017, 141, 97–106. [Google Scholar] [CrossRef]
- Kleiboeker, B.; Lodhi, I.J. Peroxisomal Regulation of Energy Homeostasis: Effect on Obesity and Related Metabolic Disorders. Mol. Metab. 2022, 65, 101577. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut Microbiota and Cardiovascular Disease: Opportunities and Challenges. Microbiome 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Feng, M.; Chu, Y.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared with Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.Y.H.; Chew, W.S.; Torta, F.; Khoo, C.M.; Wenk, M.R.; Herr, D.R.; Tai, E.S.; van Dam, R.M. Dietary Fat and Protein Intake in Relation to Plasma Sphingolipids as Determined by a Large-Scale Lipidomic Analysis. Metabolites 2021, 11, 93. [Google Scholar] [CrossRef]
- Tindall, A.M.; McLimans, C.J.; Petersen, K.S.; Kris-Etherton, P.M.; Lamendella, R. Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease. J. Nutr. 2020, 150, 806–817. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]






| Group | NC | HFD | XOP | XOT |
|---|---|---|---|---|
| weight/g | 505.42 ± 63.66 | 583.92 ± 74.2 * | 597.83 ± 63.49 | 566.58 ± 78.06 |
| Liver Weight (g) | 12.15 ± 2.17 | 20.1 ± 3.93 **** | 20.3 ± 3.71 | 19.21 ± 3.26 |
| Perirenal Adipose (g) | 8.4 ± 4.07 | 21.99 ± 9.22 *** | 20.5 ± 5.37 | 17.46 ± 9.06 |
| Epididymal Adipose (g) | 9.08 ± 3.22 | 15.74 ± 6.36 * | 18.01 ± 5.46 | 16.55 ± 8.14 |
| Liver Index (%) | 2.4 ± 0.43 | 3.44 ± 0.67 *** | 3.4 ± 0.62 | 3.39 ± 0.57 |
| Perirenal Adipose Index (%) | 1.66 ± 0.8 | 3.77 ± 1.58 *** | 3.43 ± 0.9 | 3.08 ± 1.6 |
| Epididymal Adipose Index (%) | 1.76 ± 0.44 | 2.62 ± 0.74 * | 2.96 ± 0.66 | 2.81 ± 1.02 |
| Group | NC | HFD | XOP | XOT |
|---|---|---|---|---|
| Kidney (g) | 3.21 ± 0.44 | 3.1 ± 0.49 | 3.21 ± 0.44 | 3.1 ± 0.49 |
| Epididymis (g) | 0.79 ± 0.06 | 0.84 ± 0.18 | 0.76 ± 0.11 | 0.83 ± 0.05 |
| Testis (g) | 3.85 ± 0.51 | 3.85 ± 0.28 | 3.63 ± 0.47 | 3.43 ± 0.3 # |
| Brain (g) | 1.92 ± 0.09 | 1.81 ± 0.18 | 1.78 ± 0.3 | 1.9 ± 0.2 |
| Spleen (g) | 0.95 ± 0.16 | 1.25 ± 0.41 * | 1 ± 0.18 | 0.98 ± 0.24 # |
| Pancreas (g) | 1.06 ± 0.58 | 2.12 ± 0.63 *** | 2.02 ± 0.54 | 1.89 ± 0.85 |
| Aorta(heart) (g) | 1.91 ± 0.26 | 1.64 ± 0.34 * | 1.67 ± 0.22 | 1.55 ± 0.18 |
| Thymus (g) | 0.42 ± 0.12 | 0.55 ± 0.44 | 0.35 ± 0.11 | 0.32 ± 0.07 |
| Group | AI | AC | CRI-I | CRI-II |
|---|---|---|---|---|
| NC | −0.20 ± 0.15 | 1.14 ± 0.14 | 2.14 ± 0.14 | 0.23 ± 0.08 |
| HFD | 0.15 ± 0.15 #### | 4.12 ± 1.9 #### | 5.12 ± 1.90 #### | 1.83 ± 1.26 #### |
| XOP | −0.02 ± 0.09 * | 2.91 ± 0.78 * | 3.91 ± 0.78 * | 1.03 ± 0.43 * |
| XOT | −0.03 ± 0.15 ** | 2.59 ± 1.01 ** | 3.59 ± 1.01 ** | 0.90 ± 0.50 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Yao, M.; He, Q.; Kang, X.; Shi, F.; Hu, X.; Meng, Z.; Gan, H.; Gu, R.; Sun, Y.; et al. Xanthoceras sorbifolium Oil Attenuates Hyperlipidemia Through Dual Modulation of Gut Microbiota and Lipid Metabolites: Mechanistic Insights from Lipidomics and 16S rRNA Sequencing. Metabolites 2025, 15, 291. https://doi.org/10.3390/metabo15050291
Tao Y, Yao M, He Q, Kang X, Shi F, Hu X, Meng Z, Gan H, Gu R, Sun Y, et al. Xanthoceras sorbifolium Oil Attenuates Hyperlipidemia Through Dual Modulation of Gut Microbiota and Lipid Metabolites: Mechanistic Insights from Lipidomics and 16S rRNA Sequencing. Metabolites. 2025; 15(5):291. https://doi.org/10.3390/metabo15050291
Chicago/Turabian StyleTao, Yameng, Miaomiao Yao, Qi He, Xiaoyang Kang, Fangkai Shi, Xuan Hu, Zhiyun Meng, Hui Gan, Ruolan Gu, Yunbo Sun, and et al. 2025. "Xanthoceras sorbifolium Oil Attenuates Hyperlipidemia Through Dual Modulation of Gut Microbiota and Lipid Metabolites: Mechanistic Insights from Lipidomics and 16S rRNA Sequencing" Metabolites 15, no. 5: 291. https://doi.org/10.3390/metabo15050291
APA StyleTao, Y., Yao, M., He, Q., Kang, X., Shi, F., Hu, X., Meng, Z., Gan, H., Gu, R., Sun, Y., Dou, G., & Liu, S. (2025). Xanthoceras sorbifolium Oil Attenuates Hyperlipidemia Through Dual Modulation of Gut Microbiota and Lipid Metabolites: Mechanistic Insights from Lipidomics and 16S rRNA Sequencing. Metabolites, 15(5), 291. https://doi.org/10.3390/metabo15050291






