Antiparasitic Effect of Polyphenols and Terpenes from Natural Products Against Trypanosoma cruzi and Leishmania mexicana
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biological Assays
2.2.1. Anti-Trypanosoma cruzi Activity
2.2.2. Anti-Leishmania mexicana Activity
2.2.3. Cytotoxic Analysis Against J774.2 Macrophages
2.3. ADMET In Silico
3. Results
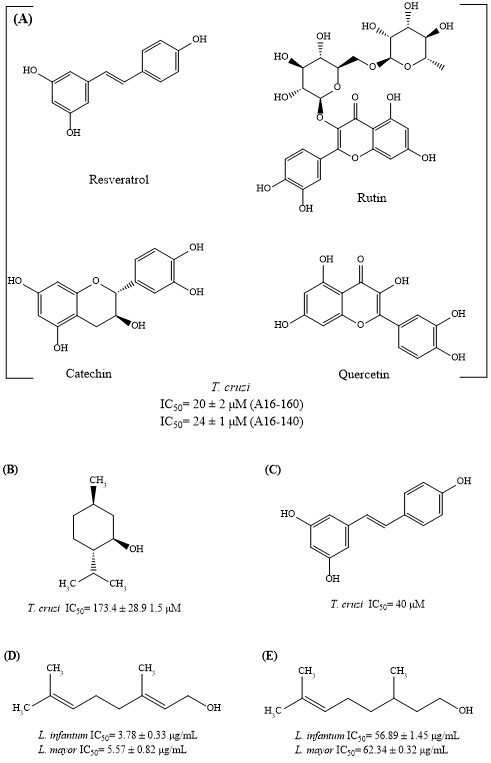
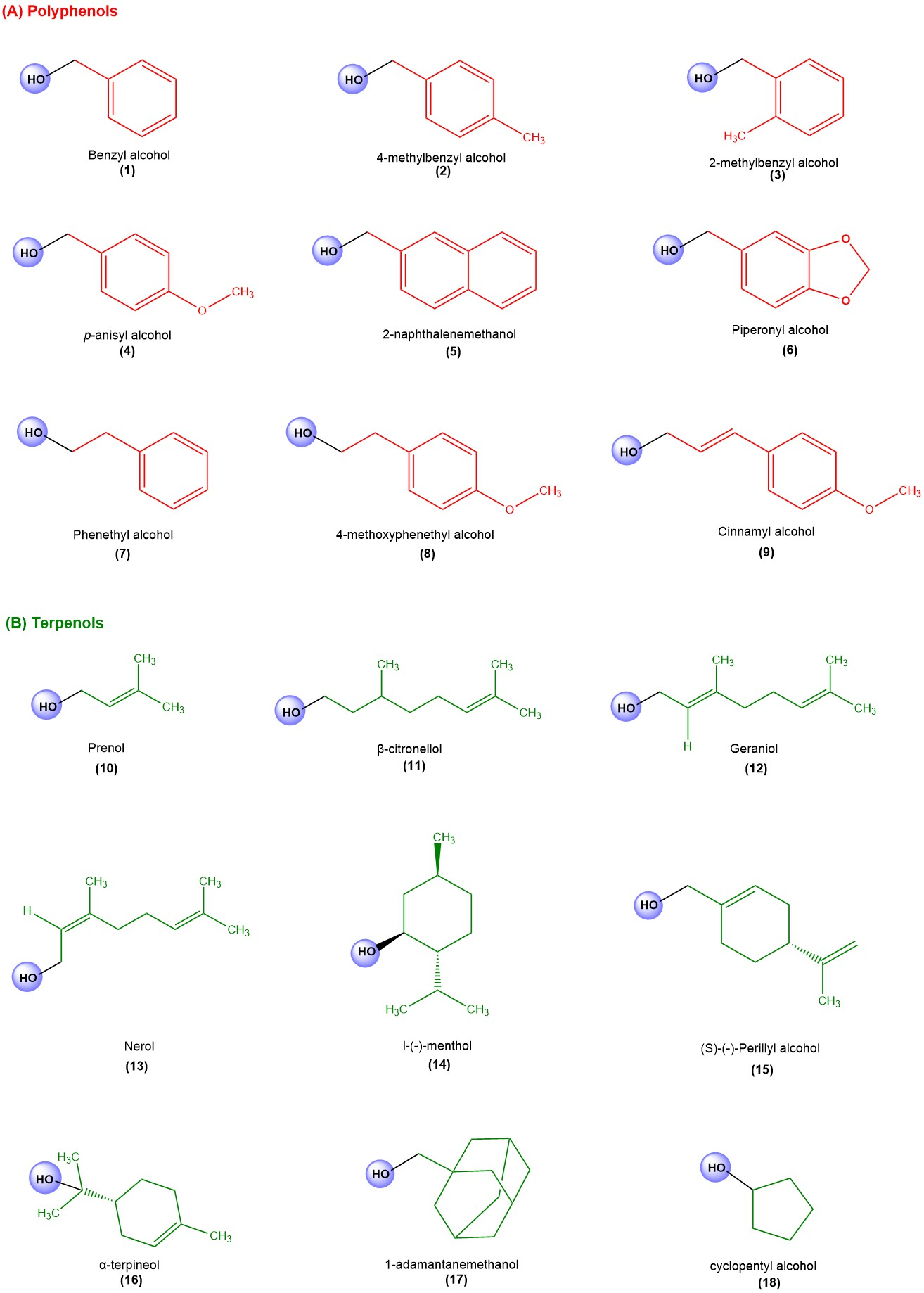
3.1. Small Library of Natural Products
3.2. Antiparasitic Activity
3.3. ADMET In Silico Properties
4. Discussion
4.1. Antiparasitic Activity
4.1.1. Trypanocidal Activity
4.1.2. Leishmanicidal Activity
4.2. ADMET In Silico Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- World Health Organization. Chagas Disease, American Trypanosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 27 June 2025).
- Swett, M.C.; Rayes, D.L.; Campos, S.V.; Kumar, R.N. Chagas Disease: Epidemiology, Diagnosis, and Treatment. Curr. Cardiol. Rep. 2024, 26, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 27 June 2025).
- World Health Organization. Control of the Leishmaniases: Report of a WHO Expert Committee = Lutte contre les leishmanioses: Rapport d’un Comité OMS d’experts. Wkly. Epidemiol. Rec. 1991, 66, 88. [Google Scholar]
- Mitra, A.K.; Mawson, A.R. Neglected Tropical Diseases: Epidemiology and Global Burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef]
- Ji, H.F.; Li, X.J.; Zhang, H.Y. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases—Part II. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; John Wiley & Sons: Zurich, Switzerland, 2000; pp. 1250–1318. [Google Scholar]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Chanaj-Kaczmarek, J.; Derda, M.; Budzianowska, A.; Thiem, B.; Ekiert, H.; Szopa, A. The Evaluation of Phenolic Acids and Flavonoids Content and Antiprotozoal Activity of Eryngium Species Biomass Produced by Biotechnological Methods. Molecules 2022, 27, 363. [Google Scholar] [CrossRef] [PubMed]
- Jayawardene, K.L.T.D.; Palombo, E.A.; Boag, P.R. Natural Products Are a Promising Source for Anthelmintic Drug Discovery. Biomolecules 2021, 11, 1457. [Google Scholar] [CrossRef]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasit. Vectors 2019, 12, 306. [Google Scholar] [CrossRef]
- Strothmann, A.L.; Berne, M.E.A.; Capella, G.A.; de Moura, M.Q.; da Silva Terto, W.D.; da Costa, C.M.; Pinheiro, N.B. Antiparasitic treatment using herbs and spices: A review of the literature of the phytotherapy. Braz. J. Vet. Med. 2022, 44, e00472. [Google Scholar] [CrossRef]
- Vargas-Munévar, L.; Borja-Fajardo, J.; Sandoval-Aldana, A.; García, W.Q.; Moreno, E.M.; Henriquez, J.C.; Stashenko, E.; García, L.T.; García-Beltrán, O. Microencapsulation of Theobroma cacao L polyphenols: A high-value approach with in vitro anti-Trypanosoma cruzi, immunomodulatory and antioxidant activities. Biomed. Pharmacother. 2024, 173, 116307. [Google Scholar] [CrossRef]
- Clemente, C.M.; Robledo, S.M.; Ravetti, S. Menthol carbonates as potent antiparasitic agents: Synthesis and in vitro studies along with computer-aided approaches. BMC Complement. Med. Ther. 2022, 22, 156. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.E.; Tekiel, V.; Campo, V.A. In vitro evaluation of Resveratrol as a potential pre-exposure prophylactic drug against Trypanosoma cruzi infection. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 54–64. [Google Scholar] [CrossRef]
- Jooste, J.; Legoabe, L.J.; Ilbeigi, K.; Caljon, G.; Beteck, R.M. Hydrazinated geraniol derivatives as potential broad-spectrum antiprotozoal agents. Arch. Pharm. 2024, 357, e2400430. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodríguez, A.; Campo-Colín, A.S.M.D.; Domínguez-Díaz, L.R.; Posadas-Jiménez, A.L.; Matadamas-Martínez, F.; Yépez-Mulia, L. Molecular Identification and Drug Susceptibility of Leishmania spp. Clinical Isolates Collected from Two Regions of Oaxaca, Mexico. Microorganisms 2025, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Díaz, L.R.; Eugenia Ochoa, M.; Soto-Castro, D.; Fárfan, N.; Morales-Chamorro, M.; Yépez-Mulia, L.; Pérez-Campos, E.; Santillán, R.; Moreno-Rodríguez, A. In vitro, ex vivo and in vivo short-term screening of DHEA nitrate derivatives activity over Trypanosoma cruzi Ninoa and TH strains from Oaxaca State, México. Bioorg. Med. Chem. 2021, 48, 116417. [Google Scholar] [CrossRef]
- Moreno-Rodríguez, A.; Cerecetto, H.; González, M.; Vieites, M.; Gil, D.M.; Otero, L. In vitro antiparasitic activity of new thiosemicarbazones in strains of Trypanosoma cruzi. Eur. J. Med. Chem. 2014, 87, 23–29. [Google Scholar] [CrossRef]
- Delgado-Maldonado, T.; Navarrete-Carriola, D.V.; Vázquez-Jiménez, L.K.; Paz-González, A.D.; Wan, B.; Franzblau, S.; Mohammed, O.M.; Rodríguez-Páez, L.; Aguirre-Alvarado, C.; Alcántara-Farfán, V.; et al. Nifuroxazide and 4-Hydroxybenzhydrazone Derivatives as New Antiparasitic (Trypanosoma cruzi and Leishmania mexicana) and Anti-Mycobacterium tuberculosis Agents. Pharmaceutics 2025, 17. [Google Scholar] [CrossRef]
- Abdul Rahman, S.M.; Bhatti, J.S.; Thareja, S.; Monga, V. Current development of 1,2,3-triazole derived potential antimalarial scaffolds: Structure-activity relationship (SAR) and bioactive compounds. Eur. J. Med. Chem. 2023, 259, 115699. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Chuanfeng, L.; Han, J.; Ruifang, J.; Shenghua, G.; Xinyong, L.; Menéndez-Arias, L.; Peng, Z. Chapter 2—Chalcones: Diverse biological activities and structure–activity relationships. In Privileged Scaffolds in Drug Discovery; Academic Press: New York, NY, USA, 2023; pp. 21–39. [Google Scholar]
| Secondary Metabolites | T. cruzi | Cytotoxicity CC50 J774.2 | |||
|---|---|---|---|---|---|
| IC50 (µM) | SI | ||||
| NINOA | A1 | NINOA | A1 | ||
| (1) | >200 | 62.28 ± 0.2 | 0.1 | 0.4 | 26.46 ± 2.1 |
| (2) | 51.85 ± 0.01 | 50.43 ± 1.8 | 3.9 | 4.0 | >200 |
| (3) | >200 | >200 | 0.3 | 0.3 | 64.20 ± 0.2 |
| (4) | 118.75 ± 2.9 | 79.49 ± 0.3 | 1.7 | 2.5 | >200 |
| (5) | 124.92 ± 3.2 | >200 | 1.6 | 1.0 | >200 |
| (6) | >200 | >200 | 0.3 | 0.3 | 62.18 ± 3.2 |
| (7) | 48.24 ± 0.3 | >200 | 1.9 | 0.4 | 89.63 ± 0.0 |
| (8) | >200 | >200 | 1.0 | 1.0 | >200 |
| (9) | >200 | 22.12 ± 0.2 | 0.2 | 2.1 | 47.35 ± 0.1 |
| (10) | 27.33 ± 1.0 | >200 | 7.3 | 1.0 | >200 |
| (11) | 53.57 ± 3.3 | 21.54 ± 0.2 | 3.7 | 9.3 | >200 |
| (12) | 41.04 ± 1.2 | 124.51 ± 5.5 | 4.9 | 1.6 | >200 |
| (13) | 58.30 ± 0.3 | 65.66 ± 3.1 | 3.4 | 3.0 | >200 |
| (14) | 24.52 ± 0.7 | >200 | 8.2 | 1.0 | >200 |
| (15) | >200 | >200 | 1.0 | 1.0 | >200 |
| (16) | >200 | 14.02 ± 0.2 | 0.3 | 4.5 | 63.41 ± 0.9 |
| (17) | >200 | >200 | 0.1 | 0.1 | 12.69 ± 0.5 |
| (18) | >200 | 10.83 ± 0.1 | 0.1 | 2.3 | 24.42 ± 3.2 |
| Nfx | 19.30 ± 0.1 | 7.09 ± 0.1 | 8.5 | 23.1 | 164.20 ± 0.2 |
| Bzn | 39.08 ± 0.1 | 30.3 ± 0.03 | 3.4 | 4.4 | 133.90 ± 0.06 |
| Secondary Metabolites | L. mexicana | Cytotoxicity CC50 J774.2 | |||
|---|---|---|---|---|---|
| IC50 (µM) | SI | ||||
| M379 | FCQEPS | M379 | FCQEPS | ||
| (1) | >200 | 104.01 ± 0.3 | 0.1 | 0.2 | 26.46 ± 2.1 |
| (2) | >200 | >200 | 1.0 | 1.0 | >200 |
| (3) | 14.23 ± 0.2 | 92.97 ± 0.2 | 4.5 | 0.7 | 64.20 ± 0.2 |
| (4) | 34.89 ± 3.0 | 19.09 ± 0.4 | 5.7 | 10.4 | >200 |
| (5) | 40.10 ± 4.7 | >200 | 5.0 | 1.0 | >200 |
| (6) | 23.61 ± 0.2 | 49.11 ± 0.1 | 2.6 | 1.2 | 62.18 ± 3.2 |
| (7) | 21.70 ±1.7 | 42.24 ± 0.1 | 4.1 | 2.1 | 89.63 ± 0.0 |
| (8) | 52.08 ± 0.5 | >200 | 3.8 | 1.0 | >200 |
| (9) | 12.07 ± 0.2 | 29.06 ± 0.1 | 3.9 | 1.6 | 47.35 ± 0.1 |
| (10) | >200 | >200 | 1.0 | 1.0 | >200 |
| (11) | 70.98 ± 1.0 | >200 | 2.8 | 1.0 | >200 |
| (12) | 57.00 ± 5.1 | >200 | 3.5 | 1.0 | >200 |
| (13) | 106.40 ± 6.0 | >200 | 1.9 | 1.0 | >200 |
| (14) | 81.46 ± 4.7 | >200 | 2.5 | 1.0 | >200 |
| (15) | 34.42 ± 2.8 | >200 | 5.8 | 1.0 | >200 |
| (16) | 14.04 ± 0.2 | 75.57 ± 0.03 | 4.5 | 0.8 | 63.41 ± 0.9 |
| (17) | 35.09 ± 0.2 | 30.20 ± 0.2 | 0.3 | 0.4 | 12.69 ± 0.5 |
| (18) | 5.48 ± 0.1 | 125.37 ± 0.2 | 4.4 | 0.1 | 24.42 ± 3.2 |
| Glu | >200 | 133.96 ± 4.3 | 1.3 | 2.0 | >273.2 |
| Compounds | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Physicochemical | MW (g/mol) | 108.1 | 122.2 | 122.2 | 136.2 | 158.2 | 152.1 |
| Rotatable bonds | 1 | 1 | 1 | 2 | 1 | 1 | |
| Hydrogen bond acceptors | 1 | 1 | 1 | 2 | 1 | 3 | |
| Hydrogen bond donors | 1 | 1 | 1 | 1 | 1 | 1 | |
| TPSA (Å2) | 20.2 | 20.2 | 20.2 | 29.5 | 20.2 | 38.7 | |
| Log P | 1.7 | 1.6 | 1.9 | 2.0 | 2.1 | 1.9 | |
| Log S | Very soluble | Soluble | Soluble | Very soluble | Soluble | Very soluble | |
| Pharmacokinetic | GI Absorption | High | High | High | High | High | High |
| Permeability BBB | Yes | Yes | Yes | Yes | Yes | Yes | |
| P-gp subtrate | No | No | No | No | No | No | |
| CYP1A2 inhibitor | Yes | Yes | Yes | Yes | Yes | No | |
| CYP2C19 inhibitor | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | No | |
| CYP2D6inhibitor | No | No | No | No | No | No | |
| Toxicity | Hepatoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Carcinogenicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
| Mutagenicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
| cytotoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
| Compounds | |||||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | ||
| Physicochemical | MW (g/mol) | 126.16 | 152.19 | 134.18 | 86.13 | 156.26 | 154.25 |
| Rotatable bonds | 2 | 3 | 2 | 1 | 5 | 4 | |
| Hydrogen bond acceptors | 1 | 2 | 1 | 1 | 1 | 1 | |
| Hydrogen bond donors | 1 | 1 | 1 | 1 | 1 | 1 | |
| TPSA (Å2) | 20.23 | 29.46 | 20.23 | 20.23 | 20.23 | 20.23 | |
| Log P | 1.70 | 2.10 | 1.98 | 1.60 | 2.72 | 2.52 | |
| Log S | Very soluble | Soluble | Soluble | Very soluble | Soluble | Soluble | |
| Pharmacokinetic | GI Absorption | High | High | High | High | High | High |
| Permeability BBB | Yes | Yes | Yes | Yes | Yes | Yes | |
| P-gp subtrate | No | No | No | No | No | No | |
| CYP1A2 inhibitor | Yes | Yes | Yes | No | No | No | |
| CYP2C19 inhibitor | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | No | |
| CYP2D6inhibitor | No | No | No | No | No | No | |
| Toxicity | Hepatoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Carcinogenicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
| Mutagenicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
| cytotoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
| Compounds | |||||||
|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | 16 | 17 | 18 | ||
| Physicochemical | MW (g/mol) | 154.25 | 156.27 | 152.23 | 154.25 | 166.26 | 86.13 |
| Rotatable bonds | 4 | 1 | 2 | 1 | 1 | 0 | |
| Hydrogen bond acceptors | 1 | 1 | 1 | 1 | 1 | 1 | |
| Hydrogen bond donors | 1 | 1 | 1 | 1 | 1 | 1 | |
| TPSA (Å2) | 20.23 | 20.23 | 20.23 | 20.23 | 20.23 | 20.23 | |
| Log P | 2.75 | 2.55 | 2.50 | 2.51 | 2.33 | 1.59 | |
| Log S | Soluble | Soluble | Soluble | Soluble | Soluble | Very soluble | |
| Pharmacokinetic | GI Absorption | High | High | High | High | High | High |
| Permeability BBB | Yes | Yes | Yes | Yes | Yes | Yes | |
| P-gp subtrate | No | No | No | No | No | No | |
| CYP1A2 inhibitor | No | No | No | No | No | No | |
| CYP2C19 inhibitor | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | No | |
| CYP2D6inhibitor | No | No | No | No | No | No | |
| Toxicity | Hepatoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive |
| Carcinogenicity | Inactive | Inactive | Inactive | Inactive | Active | Inactive | |
| Mutagenicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
| cytotoxicity | Inactive | Inactive | Inactive | Inactive | Inactive | Inactive | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete-Carriola, D.V.; Rivera, G.; Ortiz-Pérez, E.; Paz-González, A.D.; Martínez-Vázquez, A.V.; Aquino-González, L.V.; Argueta-Figueroa, L.; Doyle, M.P.; Moreno-Rodríguez, A. Antiparasitic Effect of Polyphenols and Terpenes from Natural Products Against Trypanosoma cruzi and Leishmania mexicana. Metabolites 2025, 15, 560. https://doi.org/10.3390/metabo15080560
Navarrete-Carriola DV, Rivera G, Ortiz-Pérez E, Paz-González AD, Martínez-Vázquez AV, Aquino-González LV, Argueta-Figueroa L, Doyle MP, Moreno-Rodríguez A. Antiparasitic Effect of Polyphenols and Terpenes from Natural Products Against Trypanosoma cruzi and Leishmania mexicana. Metabolites. 2025; 15(8):560. https://doi.org/10.3390/metabo15080560
Chicago/Turabian StyleNavarrete-Carriola, Diana V., Gildardo Rivera, Eyra Ortiz-Pérez, Alma D. Paz-González, Ana Verónica Martínez-Vázquez, Laura Victoria Aquino-González, Liliana Argueta-Figueroa, Michael P. Doyle, and Adriana Moreno-Rodríguez. 2025. "Antiparasitic Effect of Polyphenols and Terpenes from Natural Products Against Trypanosoma cruzi and Leishmania mexicana" Metabolites 15, no. 8: 560. https://doi.org/10.3390/metabo15080560
APA StyleNavarrete-Carriola, D. V., Rivera, G., Ortiz-Pérez, E., Paz-González, A. D., Martínez-Vázquez, A. V., Aquino-González, L. V., Argueta-Figueroa, L., Doyle, M. P., & Moreno-Rodríguez, A. (2025). Antiparasitic Effect of Polyphenols and Terpenes from Natural Products Against Trypanosoma cruzi and Leishmania mexicana. Metabolites, 15(8), 560. https://doi.org/10.3390/metabo15080560








