Anti-Hypochlorite, Antioxidant, and Catalytic Activity of Three Polyphenol-Rich Super-Foods Investigated with the Use of Coumarin-Based Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Methods
2.3.1. Extracts Preparations for Total Phenolic Content, Antioxidant and Anti-Hypochlorite Analysis
2.3.2. Total Phenolic Content
2.3.3. Antioxidant Assay
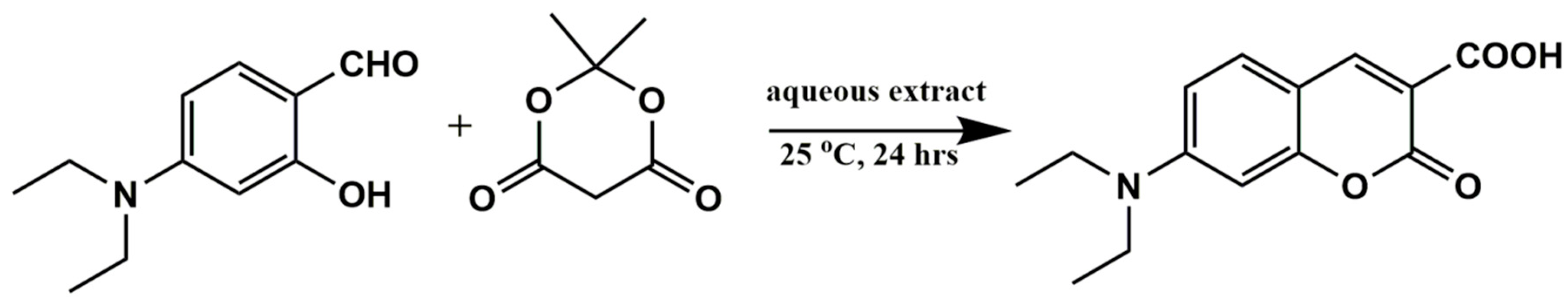
2.3.4. Green Synthesis of 7-DCCA Fluorescent Probe
2.3.5. Anti-Hypochlorite Assay
2.3.6. Preparation of Standard Solutions and Plant Extracts for LC-MS/MS Analysis
2.3.7. LC-MS/MS Conditions for Polyphenols Determination
3. Results and Discussion
3.1. Total Phenolic Content in Aqueous Extracts of the Açaí, Goji, and Schisandra Berries
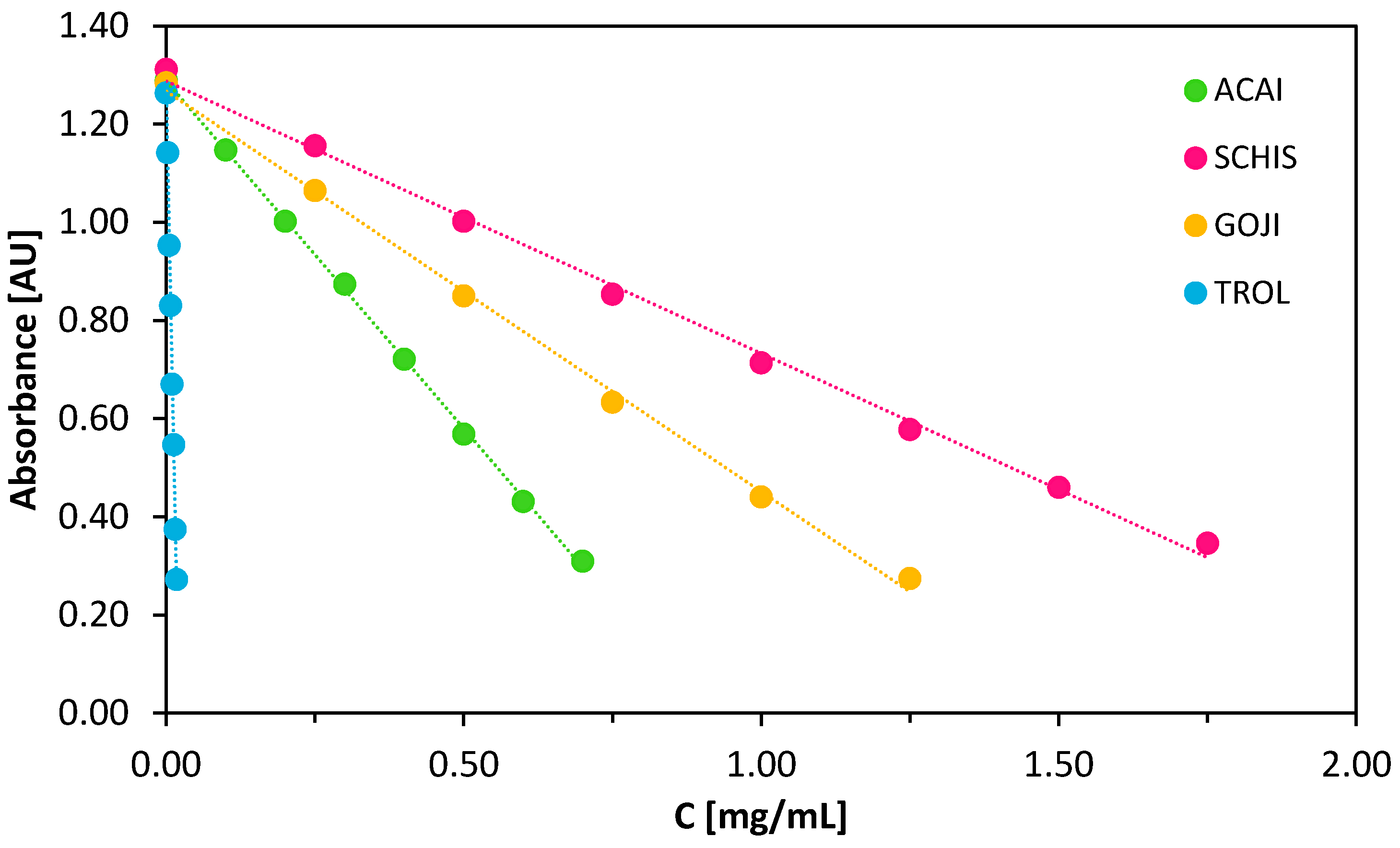
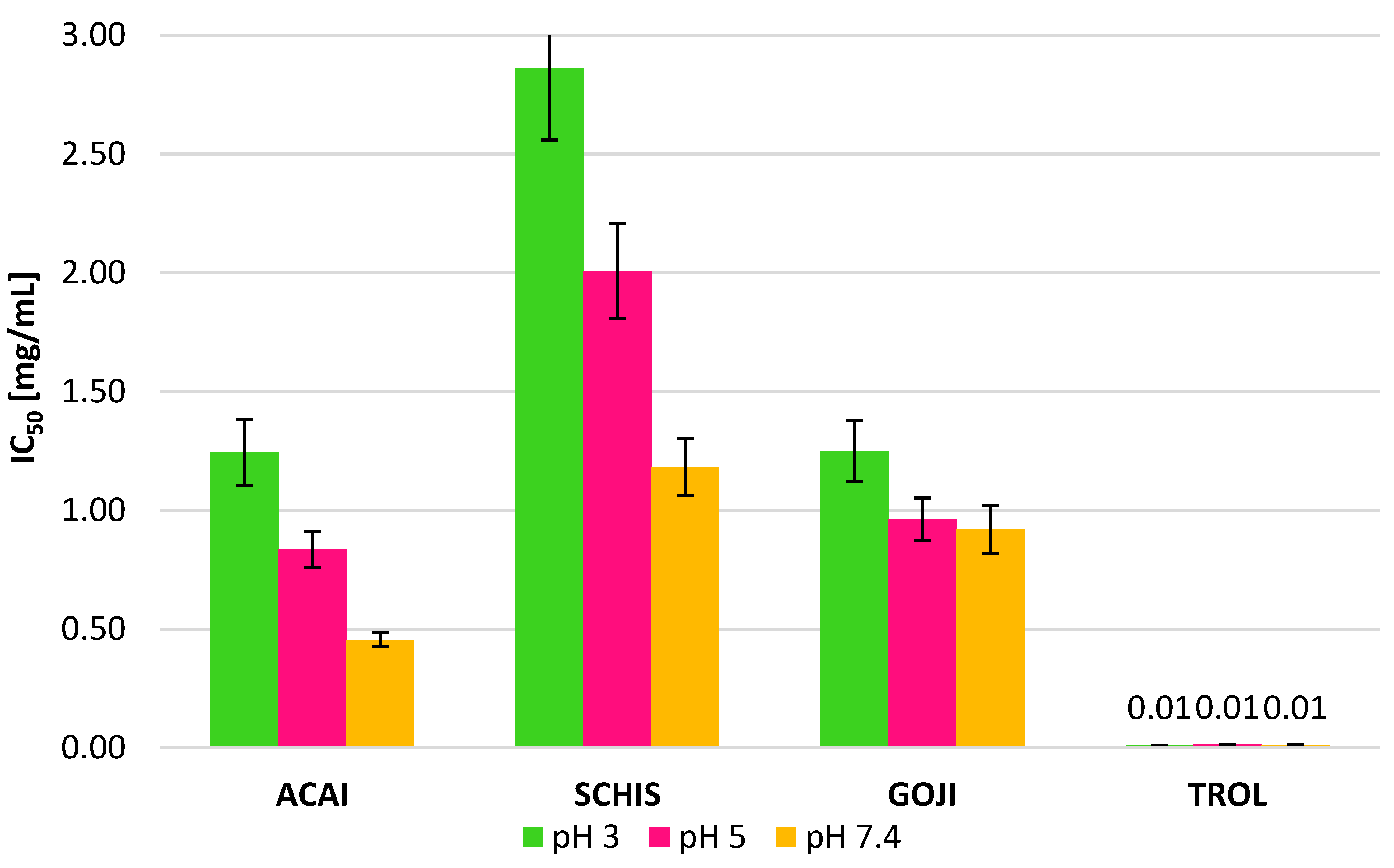
3.2. Antioxidant Assay
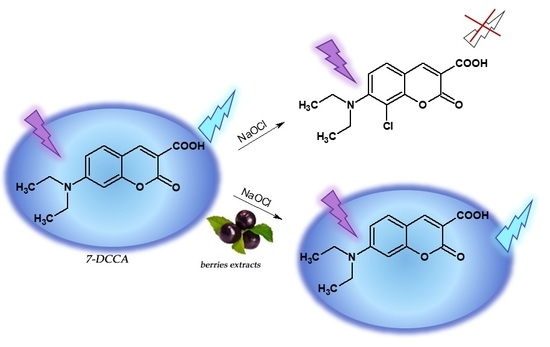
3.3. Synthesis of Hypochlorite-Sensitive Fluorescent Probe 7-DCCA
3.4. Anti-Hypochlorite Assay
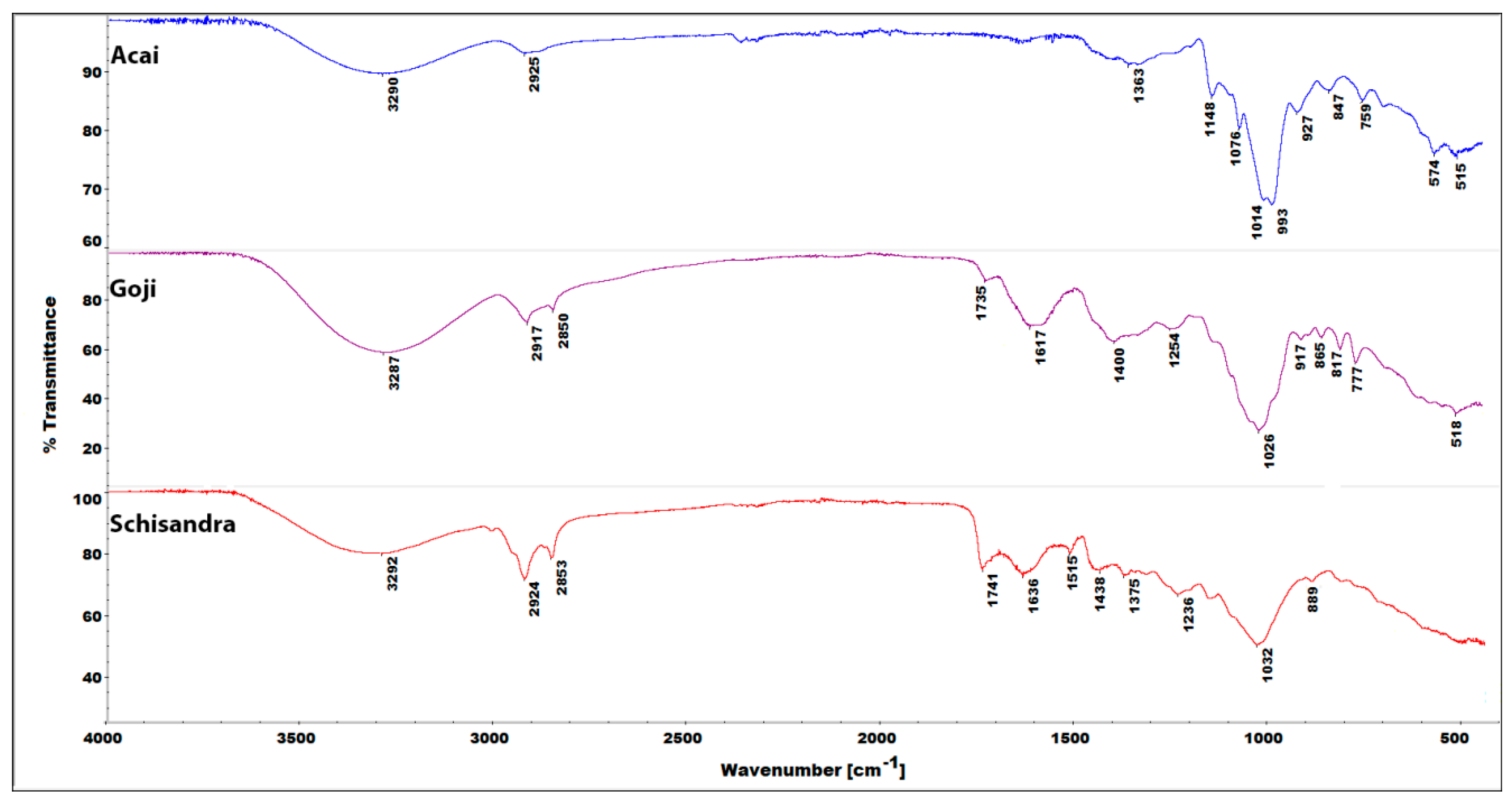
3.5. IR Spectroscopy of Plant Powder Samples
3.6. Polyphenols Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramos, C.L.; Pou, S.; Britigan, B.E.; Cohen, M.S.; Rosen, G.M. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J. Biol. Chem. 1992, 267, 8307–8312. [Google Scholar] [PubMed]
- Pullar, J.M.; Visser, M.C.M.; Winterbourn, C.C. Living with a Killer: The Effects of Hypochlorous Acid on Mammalian Cells. IUBMB Life 2000, 50, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.W.; Whiteman, M.; Cheung, N.S. Chlorinative stress: An under appreciated mediator of neurodegeneration? Cell Signal. 2007, 19, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Haenen, G.R.M.M.; Bast, A. Glutathione revisited: A better scavenger than previously thought. Front. Pharmacol. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Daels-Rakotoarison, D.A.; Gressier, B.; Trotin, F.; Brunet, C.; Luyckx, M.; Dine, T.; Bailleul, F.; Cazin, M.; Cazin, J.C. Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother. Res. 2002, 16, 157–161. [Google Scholar] [CrossRef]
- Wybraniec, S.; Starzak, K.; Szneler, E.; Pietrzkowski, Z. Separation of chlorinated diastereomers of decarboxy-betacyanins in myeloperoxidase catalyzed chlorinated Beta vulgaris L. extract. J. Chromatogr. B 2016, 1036–1037, 20–32. [Google Scholar] [CrossRef]
- Wybraniec, S.; Starzak, K.; Pietrzkowski, Z. Chlorination of Betacyanins in Several Hypochlorous Acid Systems. J. Agric. Food Chem. 2016, 64, 2865–2874. [Google Scholar] [CrossRef]
- Pietrzkowski, Z.; Argumedo, R.; Shu, C.; Nemzer, B.; Wybraniec, S.; Reyes-Izquierdo, T. Betalain-rich red beet concentrate improves reduced knee discomfort and joint function: A double blind, placebo-controlled pilot clinical study. Nutr. Diet. Suppl. 2014, 4, 9–13. [Google Scholar] [CrossRef]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jimenez, R.; Michałowski, T.; Wybraniec, S. Influence of betalain-rich extract on reduction of discomfort associated with osteoarthritis. New Med. 2010, 14, 12–17. [Google Scholar]
- Silva, C.F.; Rosalen, P.L.; Soares, J.C.; Massarioli, A.P.; Campestrini, L.H.; Semarini, R.A.; Ikegaki, M.; Alencar, S.M. Polyphenols in Brazilian organic honey and their scavenging capacity against reactive oxygen and nitrogen species. J. Apic. Res. 2020, 59, 136–145. [Google Scholar] [CrossRef]
- Schaffer, S.; Eckert, G.P.; Müller, W.E.; Llorach, R.; Rivera, D.; Grande, S.; Galli, C.; Visioli, F. Hypochlorous Acid Scavenging Properties of Local Mediterranean Plant Foods. Lipids 2004, 39, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, L. Antioxidative Properties of Cardoon (Cynara cardunculus L.) Infusion Against Superoxide Radical, Hydroxyl Radical, and Hypochlorous Acid. J. Agric. Food Chem. 2002, 50, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- Starzak, K.; Matwijczuk, A.; Creaven, B.; Matwijczuk, A.; Wybraniec, S.; Karcz, D. Fluorescence Quenching-Based Mechanism for Determination of Hypochlorite by Coumarin-Derived Sensors. Int. Mol. J. Sci. 2019, 20, 281. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.; Zhi, W.; Ye, D.; Zhang, W.; Ni, L. Rapid detection of hypochlorite by a coumarin-based hydrazide in aqueous solution and its application in live-cell imaging. Sens. Actuators B 2018, 255, 1112–1118. [Google Scholar] [CrossRef]
- Song, X.; Dong, B.; Kong, X.; Wang, C.; Zhang, N.; Lin, W. Construction of a ratiometric fluorescent probe with an extremely large emission shift for imaging hypochlorite in living cells. Spectrochim. Acta Part A 2018, 188, 394–399. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.; Zhao, Y.; Fan, D.; Chen, L.; Qiu, Y.; Qian, L.; Zhang, K.; Yang, C. Crystal structure and photoluminescence of 7-(N,N’-diethylamino)-coumarin-3-carboxylic acid. Spectrochim. Acta A 2008, 69, 1136–1139. [Google Scholar] [CrossRef]
- Chatterjee, A.; Seth, D. Photophysical Properties of 7-(diethylamino)Coumarin-3-carboxylic Acid in the Nanocage of Cyclodextrins and in Different Solvents and Solvent Mixtures. Photochem. Photobiol. 2013, 89, 280–293. [Google Scholar] [CrossRef]
- Chatterjee, A.; Maity, B.; Seth, D. The photophysics of 7-(N,N′-diethylamino)coumarin-3-carboxylic acid in water/AOT/isooctane reverse micelles: An excitation wavelength dependent study. Phys. Chem. Chem. Phys. 2013, 15, 1894–1906. [Google Scholar] [CrossRef]
- Starzak, K.; Creaven, B.; Matwijczuk, A.; Matwijczuk, A.; Karcz, D. Anti-Hypochlorite and Catalytic Activity of Commercially Available Moringa oleifera Diet Supplement. Molecules 2019, 24, 3330. [Google Scholar] [CrossRef]
- Karcz, D.; Boroń, B.; Matwijczuk, A.; Furso, J.; Staroń, J.; Ratuszna, A.; Fiedor, L. Lessons from chlorophylls: Modifications of porphyrinoids towards optimized solar energy conversion. Molecules 2014, 19, 15938–15954. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Pedro, A.C.; Maurer, J.B.B.; Zawadzki-Baggio, S.F.; Avila, S.; Maciel, G.M.; Haminiuk, C.W.I. Bioactive compounds of organic goji berry (Lycium barbarum L.) prevents oxidative deterioration of soybean oil. Ind. Crops Prod. 2018, 11, 90–97. [Google Scholar] [CrossRef]
- Shash, T.; Bule, M.; Niaz, K. Goji Berry (Lycium barbarum)—A Superfood. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: London, UK, 2019; pp. 257–264. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Int. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Schauss., A.G. Açaí (Euterpe oleracea Mart.): A Macro and Nutrient Rich Palm Fruit from the Amazon Rain Forest with Demonstrated Bioactivities In Vitro and In Vivo. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Academic Press: London, UK, 2010; pp. 479–490. [Google Scholar] [CrossRef]
- Schaus, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababik, J.P. Phytochemical and Nutrient Composition of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef] [PubMed]
- Schauss, A.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant Capacity and Other Bioactivities of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Zhang, W.; Lan, G.; Zhang, L. Comparative studies on the chemical composition and antioxidant activities of Schisandra chinensis and Schisandra sphenanthera fruits. J. Med. Plants Res. 2011, 5, 102–1216. [Google Scholar]
- Zhang, Q.; Chen, W.; Zhao, J.; Xi, W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016, 200, 230–236. [Google Scholar] [CrossRef]
- Lichtenthäler, R.; Rodrigues, R.B.; Maia, J.G.S.; Papagiannopoulos, M.; Fabricius, H.; Marx, F. Total oxidant scavenging capacities of Euterpe oleracea Mart. (Açaí) fruits. Int. J. Food Sci. Nutr. 2005, 56, 53–64. [Google Scholar] [CrossRef]
- Henning, S.M.; Zhang, Y.; Rontoyanni, V.G.; Huang, J.; Lee, R.P.; Trang, A.; Nuernberger, G.; Heber, D. Variability in the Antioxidant Activity of Dietary Supplements from Pomegranate, Milk Thistle, Green Tea, Grape Seed, Goji, and Acai: Effects of in Vitro Digestion. J. Agric. Food Chem. 2014, 62, 4313–4321. [Google Scholar] [CrossRef]
- Protti, M.; Gualandi, I.; Mandrioli, R.; Zappoli, S.; Tonelli, D.; Mercolini, L. Analytical profiling of selected antioxidants and total antioxidantcapacity of goji (Lycium spp.) berries. J. Pharm. Biomed. Anal. 2017, 143, 252–260. [Google Scholar] [CrossRef]
- Mocan, A.; Crișan, G.; Vlase, L.; Crișan, O.; Vodnar, D.C.; Raita, O.; Gheldiu, A.M.; Toiu, A.; Oprean, R.; Tilea, I. Comparative Studies on Polyphenolic Composition, Antioxidant and Antimicrobial Activities of Schisandra chinensis Leaves and Fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, H.; Komiya, S. The Turnover of Body Water as an Indicator of Health. J. Physiol. Anthropol. Appl. Hum. Sci. 2000, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Gomes, A.L.; da Silva, M.G.; Alves, A.B.; Angol, W.H.D.; Ferrari, R.A.; Carvalho, P.R.N.; Pacheco, M.T.B. Antioxidant Capacity and Chemical Characterization of Açaí (Euterpe oleracea Mart.) Fruit Fractions. Food Sci. Tech. 2016, 4, 95–102. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crisan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotens and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Fiorito, S.; Taddeo, V.A.; Genovese, S.; Epifano, F. A green chemical synthesis of coumarin-3-carboxylic and cinnamic acids using crop-derived products and waste waters as solvents. Tetrahedron Lett. 2016, 57, 4795–4798. [Google Scholar] [CrossRef]
- Bagul, S.D.; Rajput, J.D.; Bendre, R.S. Synthesis of 3-carboxycoumarins at room temperature in water extract of banana peels. Environ. Chem. Lett. 2017, 15, 725–731. [Google Scholar] [CrossRef]
- Kettle, A.J.; Albrett, A.M.; Chapman, A.L.; Dickerhof, N.; Forbes, L.V.; Khalilova, I.; Turner, R. Measuring chlorine bleach in biology and medicine. Biochim. Biophys. Acta 2014, 1840, 781–793. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukocyte Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, G.; Wang, W.; Niu, J.; Zhang, N. Fluorescence sensing and intracellular imaging for hydroxyl radical using coumarin-modified cyclodextrin derivatives. Supramol. Chem. 2012, 24, 799–802. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Song, J. Ratiometric fluorescent detection ofintracellularhydroxyl radicals based on a hybrid coumarin–cyanine platform. Chem. Commun. 2010, 46, 7930–7932. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska-Matysek, M.; Matwijczuk, A.; Florek, M.; Barłowska, J.; Wolanciuk, A.; Matwijczuk, A.; Chruściel, E.; Walkowiak, R.; Karcz, D.; Gładyszewska, B. Application of FTIR spectroscopy for analysis of the quality of honey. BIO Web Conf. 2018, 10, 02008. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 72–126. [Google Scholar]
- Brunschwig, C.; Leba, L.J.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.C. Chemical Composition and Antioxidant Activity of Euterpe oleracea Roots and Leaflets. Int. J. Mol. Sci. 2017, 18, 61. [Google Scholar] [CrossRef]
- Forino, M.; Tartaglione, L.; Dell’Aversano, C.; Ciminiello, P. NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 2016, 194, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Ghisoni, S.; Baccolo, G.; Blasi, F.; Montesano, D.; Trevisan, M.; Lucini, L. UHPLC-ESI-QTOF-MS profile of polyphenols in Goji berries (Lycium barbarum L.) and its dynamics during in vitro gastrointestinal digestion and fermentation. J. Funct. Foods 2018, 40, 564–572. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Mayr, U.; Treutter, D.; Santos-Buelga, C.; Bauer, H.; Feucht, W. Developmental changes in the phenol concentrations of ‘Golden delicious’ apple fruits and leaves. Phytochemistry 1995, 38, 1151–1155. [Google Scholar] [CrossRef]
- Shao, X.; Bai, N.; He, K.; Ho, C.T.; Yang, C.S.; Sang, S. Apple Polyphenols, Phloretin and Phloridzin: New Trapping Agents of Reactive Dicarbonyl Species. Chem. Res. Toxicol. 2008, 21, 2042–2050. [Google Scholar] [CrossRef]
- Koksal, Z.; Kalin, R.; Kalin, P.; Karaman, M.; Gulcin, I.; Ozdemir, H. Lactoperoxidase inhibition of some natural phenolic compounds: Kinetics and molecular docking studies. J. Food Biochem. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Mistry, N. Guidelines for Formulating Anti-Pollution Products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef]
- McDaniel, D.H.; Waugh, J.M.; Jiang, L.I.; Stephens, T.J.; Yaroshinsky, A.; Mazur, C.; Wortzman, M.; Nelson, D.B. Evaluation of the Antioxidant Capacity and Protective Effects of a Comprehensive Topical Antioxidant Containing Water-soluble, Enzymatic, and Lipid-soluble Antioxidants. J. Clin. Aesthet. Dermatol. 2019, 12, 46–53. [Google Scholar]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Liu, S.R.; Wu, S.P. Hypochlorous Acid Turn-on Fluorescent Probe Based on Oxidation of Diphenyl Selenide. Org. Lett. 2013, 15, 878–881. [Google Scholar] [CrossRef]
- Liu, S.R.; Vedamalai, M.; Wu, S.P. Hypochlorous acid turn-on boron dipyrromethene probe based on oxidation of methyl phenyl sulphide. Anal. Chim. Acta 2013, 800, 71–76. [Google Scholar] [CrossRef]

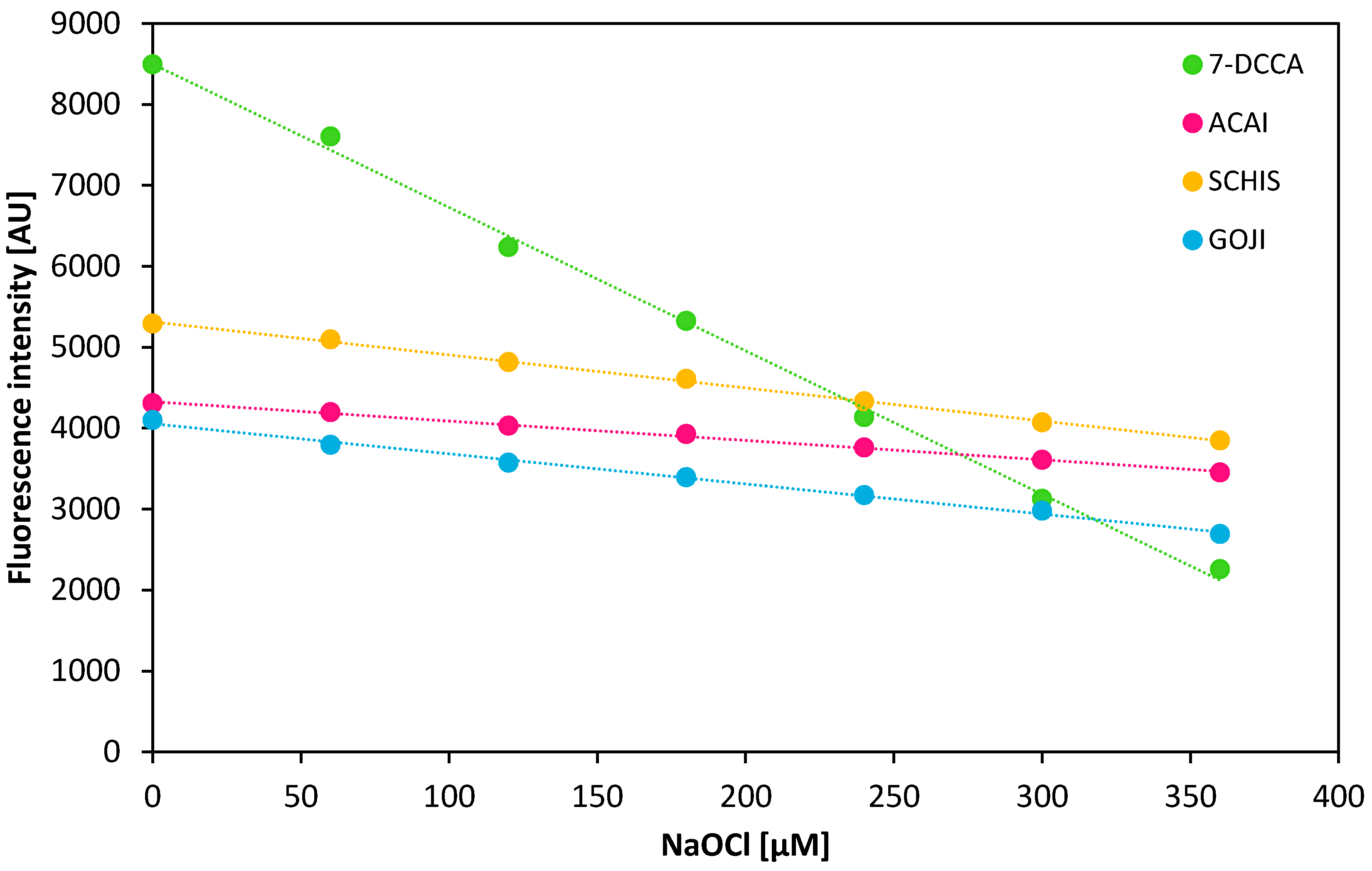
| Decrease of Fluorescence Intensity [%] | |||
|---|---|---|---|
| Sample | pH 3 | pH 5 | pH 7.4 |
| 7-DCCA | 92.1 | 72.0 | 73.4 |
| açaí | 4.0 | 22.8 | 19.9 |
| goji | 12.2 | 17.0 | 34.3 |
| schisandra | 21.7 | 20.3 | 27.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starzak, K.; Świergosz, T.; Matwijczuk, A.; Creaven, B.; Podleśny, J.; Karcz, D. Anti-Hypochlorite, Antioxidant, and Catalytic Activity of Three Polyphenol-Rich Super-Foods Investigated with the Use of Coumarin-Based Sensors. Biomolecules 2020, 10, 723. https://doi.org/10.3390/biom10050723
Starzak K, Świergosz T, Matwijczuk A, Creaven B, Podleśny J, Karcz D. Anti-Hypochlorite, Antioxidant, and Catalytic Activity of Three Polyphenol-Rich Super-Foods Investigated with the Use of Coumarin-Based Sensors. Biomolecules. 2020; 10(5):723. https://doi.org/10.3390/biom10050723
Chicago/Turabian StyleStarzak, Karolina, Tomasz Świergosz, Arkadiusz Matwijczuk, Bernadette Creaven, Janusz Podleśny, and Dariusz Karcz. 2020. "Anti-Hypochlorite, Antioxidant, and Catalytic Activity of Three Polyphenol-Rich Super-Foods Investigated with the Use of Coumarin-Based Sensors" Biomolecules 10, no. 5: 723. https://doi.org/10.3390/biom10050723
APA StyleStarzak, K., Świergosz, T., Matwijczuk, A., Creaven, B., Podleśny, J., & Karcz, D. (2020). Anti-Hypochlorite, Antioxidant, and Catalytic Activity of Three Polyphenol-Rich Super-Foods Investigated with the Use of Coumarin-Based Sensors. Biomolecules, 10(5), 723. https://doi.org/10.3390/biom10050723