Impact of Plant Origin on Eurasian Propolis on Phenolic Profile and Classical Antioxidant Activity
Abstract
:1. Introduction
- investigate chemical profiles of Eurasian propolis samples, including weakly researched, rare samples from Kyrgyzstan and Kazakhstan through ultra-high-performance liquid chromatography–diode array detector–mass spectrometry (UPLC-DAD-MS);
- evaluate the classical antioxidant properties, which are based on electron donation mechanism, by using DPPH radical scavenging assay, oxygen radical absorbance capacity (ORAC), and ferric reducing antioxidant power (FRAP) assays;
- assess the relations between the polyphenolic profile of propolis, plant origin, and classical antioxidant activity.
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. UPLC-DAD-MS
2.4. Antioxidant and Reducing Activity Determination
2.5. Statistical Analysis
3. Results and Discussion
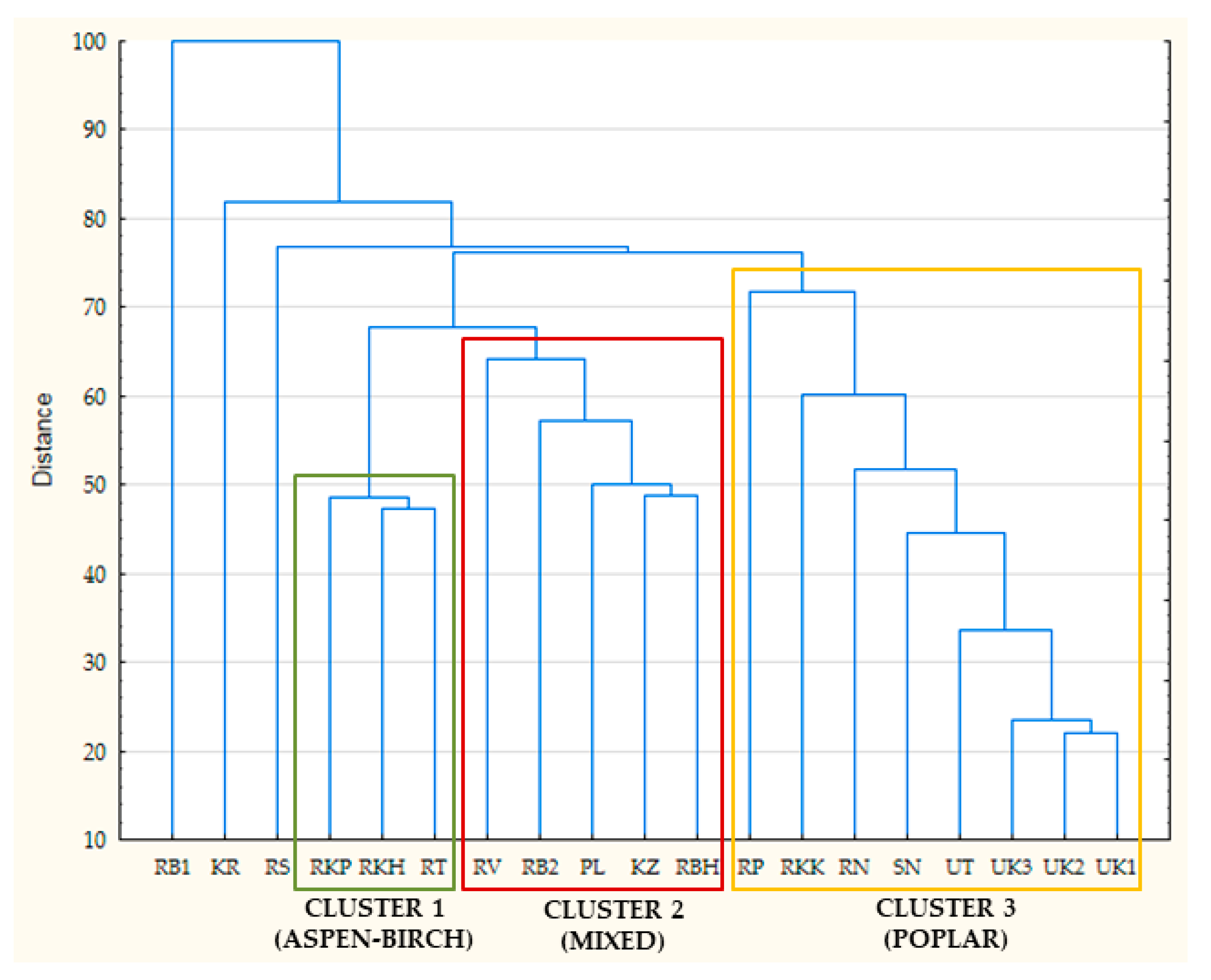
3.1. Profile of Polyphenols and Classification of Eurasian Propolis
3.2. Antioxidant Properties and Phenolic Content of Eurasian Propolis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bogdanov, S. Propolis: Composition, Health, Medicine: A Review. Bee Prod. Sci. 2014, 1, 1–40. [Google Scholar]
- Bankova, V.; de Castro, S.; Marcucci, M. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective behaviour of honeybees in acquiring European propolis plant precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Fedotova, V.V.; Konovalov, D.A. Propolis research in Russia. Indian J. Pharm. Educ. Res. 2019, 53, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Zhilyakova, E.T.; Novikov, O.O.; Novikova, M.Y.; Pisarev, D.I.; Lupina, T.V. Chemical study of the composition and quantitative analysis of hydroxycinnamic acids in experimental samples of propolis. Int. J. Green Pharm. 2017, 11, 470–475. [Google Scholar]
- Ambi, A.; Bryan, J.; Borbon, K.; Centeno, D.; Liu, T.; Chen, T.P.; Cattabiani, T.; Traba, C. Are Russian propolis ethanol extracts the future for the prevention of medical and biomedical implant contaminations? Phytomedicine 2017, 30, 50–58. [Google Scholar] [CrossRef]
- Ristivojević, P.; Stević, T.; Starović, M.; Pavlović, S.; Özcan, M.M.; Berić, T.; Dimkić, I. Phenolic composition and biological activities of geographically different type of propolis and black cottonwood resins against oral streptococci, vaginal microbiota and phytopathogenic Fusarium species. J. Appl. Microbiol. 2020, 129, 296–310. [Google Scholar] [CrossRef]
- Ambi, A.; Vera, C.; Parikh, N.; Perez, N.; Lopez Rojas, A.; Kumar, S.; Stradford, C.; Borbon, K.; Bryan, J.; Traba, C. Plasma-initiated graft polymerization as an immobilization platform for metal free Russian propolis ethanol extracts designed specifically for biomaterials. Biofouling 2018, 34, 557–568. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef]
- Okinczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Zbikowska, B.; Krzyzanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean propolis from the adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, ftiratr, UHPLC-DAD-QQTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Vardar-Ünlü, G.; Silici, S.; Ünlü, M. Composition and in vitro antimicrobial activity of Populus buds and poplar-type propolis. World J. Microbiol. Biotechnol. 2008, 24, 1011–1017. [Google Scholar] [CrossRef]
- Bankova, V. Recent trends and important developments in propolis research. Evid.-Based Complementary Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2019, 58, 7366–7376. [Google Scholar] [CrossRef] [Green Version]
- Bankova, V.; Christov, R.; Kujumgiev, A.; Marcucci, M.C.; Podov, S. Chemical composition and antibacterial activity of Brazilian propolis. Z. Nat. C 1995, 50, 167–172. [Google Scholar] [CrossRef]
- Bertrams, J.; Müller, M.B.; Kunz, N.; Kammerer, D.R.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food Qual. 2013, 86, 143–153. [Google Scholar]
- Isidorov, V.A.; Brzozowska, M.; Czyzewska, U.; Glinka, L. Gas chromatographic investigation of phenylpropenoid glycerides from aspen (Populus tremula L.) buds. J. Chromatogr. A 2008, 1198–1199, 196–201. [Google Scholar] [CrossRef]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bȩben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type propolis: Chemical composition, botanical origin and biological activity. Nat. Prod. Commun. 2015, 11, 1869–1876. [Google Scholar] [CrossRef] [Green Version]
- Trudić, B.; Anđelković, B.; Orlović, S.; Tešević, V.; Pilipović, A.; Cvetković, M.; Stanković, J. HPLC/MS-TOF analysis of surface resins from three poplar clones grown in Serbia. South-East Eur. For. 2016, 2, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Yang, H.; Zhang, X.; Yu, L. Identification and quantification of phytochemical composition and anti-inflammatory and radical scavenging properties of methanolic extracts of Chinese propolis. J. Agric. Food Chem. 2012, 60, 12403–12410. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, 608–617. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Yang, H.; Zhang, X.; Sheng, Y.; Huang, H.; Yu, L. Isolation and characterization of five glycerol esters from Wuhan propolis and their potential anti-inflammatory properties. J. Agric. Food Chem. 2012, 60, 10041–10047. [Google Scholar] [CrossRef]
- Volpi, N.; Bergonzini, G. Analysis of flavonoids from propolis by on-line HPLC-electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 354–361. [Google Scholar] [CrossRef]
- Greenaway, W.; May, J.; Scaysbrook, T.; Whatley, F.R. Compositions of bud and leaf exudates of some Populus species compared. Z. Nat. C 1992, 47, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Maciejewicz, W. Isolation of flavonoid aglycones from propolis by a column chromatography method and their identification by GC-MC and TLC methods. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1171–1179. [Google Scholar] [CrossRef]
- Asakawa, Y.; Schillo, D.; Lehmann, U.; Heidrun, W. A novel caffeic acid derivative and other constituents of populus bud excretion and propolis (bee-glue). Z. Nat. C 1987, 42, 1030–1034. [Google Scholar]
- Falcão, S.I.; Calhelha, R.C.; Touzani, S.; Lyoussi, B.; Ferreira, I.C.F.R.; Vilas-Boas, M. In vitro interactions of moroccan propolis phytochemical’s on human tumor cell lines and anti-inflammatory properties. Biomolecules 2019, 9, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martos, I.; Cossentini, M.; Ferreres, F.; Tomás-Barberán, F.A. Flavonoid composition of Tunisian honeys and propolis. J. Agric. Food Chem. 1997, 45, 2824–2829. [Google Scholar] [CrossRef]
- Trusheva, B.; Ivanova, D.; Popova, M.; Bankova, V. Insights into the essential oil compositions of Brazilian red and Taiwanese green propolis. Nat. Prod. Commun. 2017, 12, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Medana, C.; Carbone, F.; Aigotti, R.; Appendino, G.; Baiocchi, C. Selective analysis of phenolic compounds in propolis by HPLC-MS/MS. Phytochem. Anal. 2008, 19, 32–39. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Y.; Du, H.; Shi, H.; Peng, Y.; Li, X. Antioxidant compounds from propolis collected in Anhui, China. Molecules 2011, 16, 3444–3455. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Khismatullin, R.; Gavrilova, N.; Legotkina, G.; Lyapunov, J.; Bankova, V. The triple botanical origin of Russian propolis from the Perm Region, its phenolic content and antimicrobial activity. Nat. Prod. Commun. 2013, 8, 617–620. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, Y.; Takemoto, T.; Wollenweber, E.; Aratani, T. Lasiocarpin A, B and C, three novel phenolic triglycerides from Populus lasiocarpa. Phytochemistry 1977, 16, 1791–1795. [Google Scholar] [CrossRef]
- Isidorov, V.; Szczepaniak, L.; Wróblewska, A.; Piroznikow, E.; Vetchinnikova, L. Gas chromatographic-mass spectrometric examination of chemicalcompositionoftwo Eurasian birch (Betula L.) bud exudates and its taxonomical implication. Biochem. Syst. Ecol. 2014, 52, 41–48. [Google Scholar] [CrossRef]
- Vedernikov, D.N.; Roshchin, V.I. Extractive compounds of betulaceae family birch buds (Betula pendula Roth.): IV. Composition of sesquiterpene diols, triols, and flavonoids. Russ. J. Bioorganic Chem. 2012, 38, 753–761. [Google Scholar] [CrossRef]
- Przybylski, T.; Białobok, S.; Bugała, W.; Hejnowicz, A.; Jakuszewski, T.; Środoń, A.; Żelawski, A.; Jankiewicz, L.S.; Szuszka, D.; Obmiński, Z.; et al. Nasze Drzewa Leśne: Topole Populus L., 1st ed.; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1973; pp. 1–40. [Google Scholar]
- Seyoum, A.; Asres, K.; El-Fiky, F.K. Structure-radical scavenging activity relationships of flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Al-Amiery, A.A.; Bagnati, R. Theoretical, antioxidant and cytotoxic activities of caffeic acid phenethyl ester and chrysin. Int. J. Food Sci. Nutr. 2014, 65, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [Green Version]

| No. | Component | RT | UV λmax (nm) | [M − H]− | Mass Fragments | Ref |
| 1 | * Caffeoylglycerol b,c | 1.88 | 324, 298sh, 242 | 253 | 179, 161, 135 | [20] |
| 2 | p-Hydroxy benzoic acid a,b,c | 2.13 | 256 | 137 | - | [21] |
| 3 | Caffeic acid a,b,c | 2.17 | 324, 298sh, 242 | 179 | 135 | [10,22,23,24,25,26,27] |
| 4 | * p-Coumaroylglycerol b,c | 2.63 | 310, 300sh, 229 | 237 | 163, 145, 119 | [11,19,20] |
| 5 | p-Coumaric acid a,b,c | 3.25 | 310, 300sh, 229 | 163 | 119 | [10,22,23,24,25,26,27] |
| 7 | Vanilline a,b,c | 3.42 | 310, 280, 231 | 151 | - | [11,22,27] |
| 8 | Ferulic acid a,b,c | 3.70 | 324, 298sh, 236 | 193 | 149, 134 | [11,19,20] |
| 9 | Isoferulic acid a,b,c | 4.11 | 323, 295sh, 221 | 193 | 149, 134 | [11,19,20] |
| 10 | Benzoic acid a,b,c | 5.97 | 281sh, 274sh, 236 | 121 | 103, 77 | [11,22,27] |
| 11 | * Ferulic acid derivate | 6.82 | 326, 298sh, 236 | 389 | 193, 175, 148, 134 | - |
| 12 | Acetyl-p-coumraoylglycerol b,c | 7.40 | 311 | 279 | 219, 163, 145, 119 | [11,19,20] |
| 13 | * Apigenin-7-O-glucoside b,c | 8.07 | 315sh, 265 | 431 | 268, 239, 195 | [24] |
| 14 | 3,4-Dimethylcaffeic acid b,c | 8.52 | 322, 294sh, 236 | 207 | 163, 133 | [11,23,24] |
| 15 | Luteolin a,b,c | 11.30 | 349, 290sh, 270sh, 254, 227sh | 285 | 213, 151 | [11,23,24] |
| 16 | Quercetin a,b,c | 11.43 | 368, 293sh, 270sh, 256 | 301 | 271, 151, 121, 107 | [21,24] |
| 17 | Pinobanksin-5-methyl ether b,c | 12.32 | 322sh, 288, 228 | 285 | 267, 252, 239, 224, 208, 195, 180, 165, 152,136 | [23,24,26] |
| 18 | Cinnamic acid a,b,c | 12.94 | 277 | 147 | 103 | [11,23,24,27] |
| 19 | Quercetin-3-methyl ether b,c | 13.68 | 355, 293sh, 268sh, 255 | 315 | 300, 271, 243 | [11,23] |
| 20 | * 1-Caffeoyl-3-p-coumaroyl glycerol c | 15.23 | 315, 298sh, 235 | 399 | 253,235, 219, 179, 163, 135, 119 | [10,11,24] |
| 21 | Naringenin a,b,c | 15.83 | 330sh, 290, 230 | 271 | 269, 227, 177, 165, 151, 119, 107, 93 | [24,28] |
| 22 | Pinobanksin a,b,c | 16.30 | 332sh, 292, 229 | 271 | 253, 225, 209, 185, 151, 107 | [10,11,23,24] |
| 23 | Apigenin a,b,c | 16.40 | 338, 290sh, 268, 226 | 269 | 227, 181, 151, 149, 117, 107 | [11,23,24] |
| 24 | * Caffeoyl-feruloylglycerol b,c | 16.12 | 326, 298sh, 240 | 429 | 267, 253, 249, 235, 193, 179, 149, 135, 134 | [11,19,20] |
| 25 | Chrysin-5-methyl ether b,c | 16.80 | 314sh, 264, 247sh | 267 | 252, 242, 180 | [11,25] |
| 26 | Kaempferol a,b,c | 17.48 | 366, 266, 248sh | 285 | - | [10,11,24,27] |
| 27 | Isorhamnetin a,b,c | 19.11 | 371, 298sh, 268sh, 255, 233sh | 315 | 300, 151 | [10,24,26,27] |
| 28 | * Quercetin-methyl ether b,c | 19.57 | 371, 298sh, 268sh, 255, 233sh | 315 | 300,165, 151, 193, 121, 109 | [3,5] |
| 29 | Luteolin-5-methyl ether b,c | 20.15 | 350, 298sh, 266, 232sh | 299 | 284, 255, 227, 211 | [11,23,25] |
| 30 | * 1,3-Di-p-coumaroylglycerol b,c | 20.25 | 310, 300sh, 233 | 383 | 237, 219, 163, 145, 119 | [10,11] |
| 31 | Quercetine-5,7-dimethyl ether b,c | 20.71 | 356, 296sh, 269sh, 255 | 329 | 314, 299, 285, 271, 257, 243, 227 | [3,5,7] |
| 32 | * p-Coumaroyl-feruloylglycerol b,c | 21.37 | 316, 298sh, 233 | 413 | 249, 237, 219, 179, 163, 149, 134, 119 | [11,24,29] |
| 33 | * di-1,3-Feruloylglycerol b,c | 21.95 | 323, 298sh | 443 | 249, 193 | [10,11,24] |
| 34 | 2-Acetyo-1,3-di-caffeoylglycerol b,c | 22.57 | 328, 298sh, 244 | 457 | 397, 295, 235, 179, 163, 161, 135 | [11,24,29] |
| 35 | β-Styrylacrilic acid (cinnamylideneacetic acid) b,c | 23.82 | 311, 240sh | 173 | - | [23] |
| 36 | Galangin-5-methyl ether b,c | 25.42 | 352, 300sh, 261, 240sh | 283 | 268, 239, 211 | [23,26] |
| 37 | Pinobanksin-3-O-acetyl-5-methyl-ether b,c | 25.60 | 289 | 327 | 285, 267, 252, 224 | [23,26] |
| 38 | * Caffeic acid butenic or isobutenic ester b,c | 24.73 | 326, 298sh, 248 | 233 | 179, 161, 135 | [11] |
| No. | Component | RT | UV λmax (nm) | [M − H]− | Mass Fragments | Ref |
| 39 | Rhamnetin a,b,c | 25.92 | 354, 298sh, 268sh, 255 | 315 | 300,165, 151, 193, 121, 109 | [11,24] |
| 40 | Quercetin-3,3-dimethyl ether b,c | 27.22 | 356, 292sh, 268sh, 256 | 329 | 314, 299, 271, 243, 227 | [11,23] |
| 41 | 2-Acetyl-1-caffeoyl-3-p-coumaroylglycerol b,c | 29.23 | 316, 299sh 235 | 441 | 381, 295, 235, 179, 163, 135, 119 | [10,11,19,24] |
| 42 | * Quercetin-dimethyl ether b,c | 29.46 | 356, 292sh, 268sh, 256 | 329 | 300,165, 151, 193, 121, 109 | [11,23,24,25,26,27] |
| 43 | * Caffeic acid butyl or isobutyl ester b,c | 30.20 | 326, 298sh, 242 | 235 | 179, 161, 135 | [11] |
| 44 | 2-Acetyl-3-caffeoyl-1-feruloylglycerol b,c | 30.28 | 328, 300sh, 244 | 471 | 411, 295, 235, 193, 179, 149, 135 | [11,19,26] |
| 45 | Quercetin-3,7-dimethyl ether b,c | 30.63 | 356, 292sh, 268sh, 256 | 329 | 300,165, 151, 193, 121, 109 | [21,24] |
| 46 | * Caffeic acid prenyl or isoprenyl ester 1 b,c | 31.92 | 326, 298sh, 246 | 247 | 179, 161, 135 | [23,24,25,26,27] |
| 47 | Chrysin a,b,c | 32.11 | 314sh, 268, 246sh | 253 | 209, 181, 167, 165, 151, 107, 145, 143, 119 | [10,11,23,24,25,26,27] |
| 48 | Sakuranetin b,c | 35.16 | 328sh, 291, 270sh, 247sh | 285 | 165, 119 | [24,27] |
| 49 | Pinocembrin a,b,c | 33.68 | 330sh, 290, 235 | 255 | 213, 187,151, 145, 136 | [11,23,26,27] |
| 50 | Acacetin a,b,c | 34.08 | 335, 299sh, 268, 228 | 283 | 268, 239, 211, 183, 151, 107 | [30] |
| 51 | * Caffeic acid prenyl or isoprenyl ester 2 b,c | 34.10 | 326, 298sh, 246 | 247 | 179, 161, 135 | [23,24,25,27] |
| 52 | Pinocembrin chalcone b,c | 34.14 | 345 | 255 | 213, 151, 101 | [10,31] |
| 53 | * Caffeic acid prenyl or isoprenyl derivate 1 b,c | 34.21 | 326, 298sh, 246 | 247 | 179, 161, 135 | [11,23,24,25,26] |
| 54 | Caffeic acid benzyl ester b,c | 34.62 | 328, 298sh, 244 | 269 | 178, 161, 134 | [11,23,24,25,26] |
| 55 | * Caffeic acid prenyl or isoprenyl derivate 2 b,c | 34.58 | 326, 298sh, 246 | 247 | 179, 161, 135 | [11,23,24,25,26] |
| 56 | * Flavonoid b,c | 33.62 | 332sh, 291, 232 | 285 | 255, 145, 139, 124 | - |
| 57 | Genkwanin a,b,c | 35.64 | 337, 267, 242sh | 283 | 268, 239, 211, 183, 171, 151, 117, 107 | [32] |
| 58 | Galangin a,b,c | 36.07 | 360, 314sh, 290sh, 266, 240sh | 269 | 227, 197, 183, 151 | [10,11,21,23,24,25] |
| 59 | Kaempferide (Kaempferol 4’-methyl ether) b,c | 37.31 | 366, 322sh, 292sh, 266, 250sh | 299 | 284, 255, 227, 164, 151 | [21,27] |
| 60 | 2-Acetyl-1,3-di-p-coumaroylglycerol b,c | 37.55 | 360sh, 312, 232 | 425 | 365, 321, 215, 163, 119 | [10,19,24] |
| 61 | 3-O-Acetyl-pinobanksin b,c | 37.82 | 332sh, 294, 238 | 313 | 271, 253, 209, 181, 165, 143, 151, 107 | [10,11,23,24] |
| 62 | * Quercetin-dimethyl ether b,c | 38.33 | 370, 268sh, 255 | 329 | 314, 299, 284, 271 | [11,21,23,26,27] |
| 63 | * 2-Acetyl-3-p-coumaroyl-1-feruloylglycerol b,c | 38.96 | 318, 299sh 235 | 455 | 395, 351, 193, 163, 149, 119 | [10,11,29] |
| 64 | * Metoxychrysin b,c | 39.27 | 340sh, 310sh, 266, 245sh | 283 | 268, 239, 211, 195 | [11,23,26] |
| 65 | 3-Acetyl-1,2-di-p-coumaroylglycerol b,c | 40.09 | 312, 300sh, 238 | 425 | 365, 163 | [11,20] |
| 66 | Caffeic acid phenethyl ester (CAPE) b,c | 40.15 | 321, 300sh, 264 232 | 283 | 179, 161, 119 | [11,23,26] |
| 67 | 2-Acetyl-1,3-di-feruloyl glycerol b,c | 40.37 | 328, 298sh, 243 | 485 | 425, 249, 230, 193, 175, 149, 134 | [10,11,29] |
| 68 | Ermanin (Kaempferol 3,4’-dimethyl ether) b,c | 41.05 | 348, 267, 246sh | 313 | 298, 283, 269, 255, 227, 211, 183, 155, 117 | [33] |
| 69 | * Caffeic acid pentyl or isopentyl ester 1 b,c | 41.90 | 326, 298sh, 247 | 249 | 179, 161, 134 | [34] |
| 70 | Ayanin (3,7,4’-Trimethylquercetin) b,c | 42.53 | 343, 271, 248sh | 343 | 328, 313, 298, 285, 270, 255, 242, 214, 186, 163, 145, 129, 113 | [35] |
| 71 | * p-Coumaric acid prenyl ester 1 b,c | 45.42 | 311, 299sh, 245sh | 233 | 163, 145, 119 | [11,23,25,26] |
| 72 | * p-Coumaric acid prenyl ester 2 b,c | 45.42 | 311, 299sh, 245sh | 233 | 163, 145, 119 | [11,23,25,26] |
| 73 | p-Coumaric acid benzyl ester b,c | 45.80 | 312, 298sh, 244sh | 253 | 162, 145, 118 | [10,11,23,26] |
| 74 | * Ferulic or isoferulic acid benzyl ester b,c | 47.13 | 326, 298sh | 283 | - | [10,11,36] |
| 75 | Caffeic acid cinnamic ester b,c | 48.04 | 326, 300sh, 243 | 295 | 178, 163, 134, 92 | [11,23,24] |
| 76 | 3-O-propyl-pinobanksin b,c | 48.58 | 329sh, 294, 234 | 327 | 271, 253, 225, 209, 181, 165, 143 | [11,23,24] |
| 77 | p-Coumaric acid phenethyl ester b,c | 49.21 | 312, 300sh, 242sh | 267 | 163, 145, 119 | [11,25] |
| No. | Component | RT | UV λmax (nm) | [M − H]− | Mass Fragments | Rf |
| 78 | Pinostrobin chalcone b,c | 49.67 | 345, 309sh, 267 | 269 | 254, 226, 198, 171, 165, 136, 122 | [11,24] |
| 79 | Tectochrysin (Chrysin-7-methyl ether) b,c | 51.23 | 310sh, 268 | 267 iw | - | [37] |
| 80 | Pinostrobin (Pinocembrin-5-methyl ether) a,b,c | 51.40 | 328sh, 290, 248sh | 269 iw | - | [11,12,28] |
| 81 | 3-O-butyl or isobutyl pinobanksin b,c | 51.65 | 320sh, 293, 240sh | 341 | 271, 253, 209, 181, 165, 151, 107 | [23,26] |
| 82 | Galangin-7-methyl ether b,c | 52.21 | 353, 268 | 283 iw | - | [38] |
| 83 | 3-O-pentyl or isopentyl pinobanksin 1 b,c | 53.07 | 332sh, 293, 242 | 355 | 253, 209, 181, 165, 143, 107, 101 | [23,24,26] |
| 84 | 3-O-pentyl or isopentyl pinobanksin 2 b,c | 53.24 | 320sh, 295, 250 | 353 | 271, 253, 209, 181, 165, 151, 107 | [23,24,26] |
| 85 | 3-O-hexyl-pinobanksin b,c | 54.09 | 282 | 369 | 271, 253, 209, 151, 143 | [23,24,26] |
| 86 | * Metoxycinnamic acid cinnamyl ester b,c | 54.26 | 280 | 293 | - | [23] |
| No. | Component | UK1 | UK2 | UK3 | UT | RBH | RB1 | RB2 | RKH | RKK | RKP | RN | RP | RS | RT | RV | KR | KZ | SL | PL |
| 1 | * Caffeoyl-glycerol | tr | tr | tr | tr | tr | tr | - | tr | tr | - | tr | tr | tr | tr | - | tr | tr | tr | tr |
| 2 | p-Hydroxy benzoic acid | tr | tr | tr | tr | tr | - | tr | tr | tr | tr | tr | - | tr | tr | tr | - | tr | tr | tr |
| 3 | Caffeic acid | + | + | + | + | + | + | + | + | + | tr | + | + | + | tr | tr | + | + | + | tr |
| 4 | p-Coumaroylglycerol | - | - | - | - | tr | tr | tr | tr | - | - | tr | - | tr | tr | tr | - | tr | tr | tr |
| 5 | p-Coumaric acid | + | + | + | + | ++ | ++ | ++ | ++ | + | + | + | tr | ++ | +++ | ++ | - | + | + | ++ |
| 7 | Vanilline | tr | - | - | - | tr | tr | tr | tr | - | + | tr | - | tr | + | + | - | tr | tr | + |
| 8 | Ferulic acid | + | + | + | + | tr | + | + | ++ | + | + | + | tr | ++ | ++ | + | - | + | + | + |
| 9 | Isoferulic acid | + | + | + | + | + | + | + | tr | + | + | + | + | tr | tr | tr | - | tr | tr | tr |
| 10 | Benzoic acid | + | tr | tr | tr | + | + | + | + | tr | + | tr | tr | ++ | + | + | - | + | tr | ++ |
| 11 | * Ferulic acid derivate | tr | tr | tr | tr | tr | tr | tr | tr | - | - | tr | - | tr | tr | tr | - | tr | tr | tr |
| 12 | Acetyl-p-coumaroylglycerol | tr | - | - | - | tr | tr | tr | tr | tr | - | - | - | tr | - | tr | - | tr | tr | tr |
| 13 | 3,4-Dimethylcaffeic acid (DMCA) | tr | tr | tr | tr | + | + | + | - | + | - | tr | + | - | - | tr | - | tr | tr | tr |
| 14 | * Apigenin-O-glucoside | tr | tr | tr | tr | - | - | - | - | tr | - | tr | - | - | - | - | - | tr | tr | - |
| 15 | Luteolin | tr | tr | tr | tr | tr | tr | - | tr | - | - | tr | tr | tr | - | tr | - | tr | tr | - |
| 16 | Quercetin | tr | + | + | + | tr | tr | tr | tr | tr | tr | tr | tr | tr | - | tr | + | tr | tr | tr |
| 17 | Pinobanksin-5-methyl ether | + | + | + | + | tr | + | - | tr | tr | - | tr | - | tr | - | - | - | tr | + | tr |
| 18 | Cinnamic acid | tr | - | - | - | tr | - | tr | tr | tr | Tr | - | - | tr | - | tr | - | - | tr | tr |
| 19 | Quercetin-3-methyl ether | tr | tr | tr | tr | tr | tr | tr | tr | - | tr | tr | tr | tr | - | + | tr | + | tr | |
| 20 | * 1-Caffeoyl-3-p-coumaroyl glycerol | - | - | - | - | tr | tr | tr | tr | - | - | tr | - | tr | tr | tr | - | tr | - | tr |
| 21 | Naringenine | tr | tr | tr | tr | tr | tr | tr | tr | tr | - | tr | tr | tr | tr | tr | - | tr | - | tr |
| 22 | Pinobanksin | + | + | + | + | tr | + | tr | tr | + | tr | + | + | tr | tr | tr | + | + | + | tr |
| 23 | Apigenin | + | tr | tr | tr | + | tr | + | + | + | + | tr | tr | tr | + | + | + | + | + | + |
| 24 | Caffeoyl-feruloylglycerol | - | - | - | - | - | - | tr | tr | - | - | - | - | tr | tr | tr | - | tr | - | - |
| 25 | Chrysin-5-methyle ether | tr | tr | tr | tr | - | - | - | - | tr | - | tr | - | - | - | - | - | tr | tr | tr |
| No. | Component | UK1 | UK2 | UK3 | UT | RBH | RB1 | RB2 | RKH | RKK | RKP | RN | RP | RS | RT | RV | KR | KZ | SL | PL |
| 26 | Kempferol | + | + | + | + | + | tr | tr | + | tr | + | + | tr | tr | + | + | + | + | + | + |
| 27 | Isorhamnetin | tr | tr | tr | tr | + | tr | tr | tr | tr | + | tr | tr | tr | + | + | - | + | tr | tr |
| 28 | Quercetin-methyl ether | tr | - | - | - | tr | - | tr | - | tr | - | tr | tr | tr | tr | tr | - | tr | tr | |
| 29 | Luteolin-5-methyl ether | + | tr | tr | tr | tr | - | tr | + | + | tr | + | tr | tr | + | + | + | + | + | tr |
| 30 | ** 1,3-Di-p-coumaroylglycerol | tr | tr | tr | tr | Tr | + | + | + | tr | tr | tr | - | + | + | tr | - | tr | tr | tr |
| 31 | Quercetine-5,7-dimethyl ether | tr | tr | tr | tr | tr | - | tr | tr | tr | tr | tr | tr | tr | + | + | tr | + | tr | tr |
| 32 | p-Coumaroyl-feruloylglycerol | - | tr | tr | tr | tr | tr | tr | tr | - | tr | tr | tr | tr | tr | tr | - | tr | - | - |
| 33 | Di-feruloyoglicerol | tr | - | - | - | tr | tr | tr | tr | - | tr | tr | - | tr | - | tr | - | tr | tr | - |
| 34 | 2-Acetyo-1,3-di-caffeoylglycerol | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | - | tr | - | tr | - | tr | tr | tr |
| 35 | β-styrylacrilic acid | tr | tr | tr | tr | - | - | - | - | - | tr | - | - | - | - | - | - | tr | tr | |
| 36 | Galangin-5-methyl ether | tr | tr | tr | tr | tr | tr | - | - | tr | - | tr | - | - | - | tr | - | tr | tr | |
| 37 | Pinobanksin-3-O-acetyl-5-methyl-ether | tr | tr | tr | tr | tr | - | - | - | tr | - | tr | - | - | - | tr | - | tr | tr | - |
| 38 | * Caffeic acid butenic or isobutenic ester b,c | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | tr | - | - |
| 39 | Rhamnetin | tr | + | + | + | tr | tr | tr | tr | tr | - | tr | - | tr | tr | tr | - | tr | + | tr |
| 40 | Quercetin-dimethyl ether | - | tr | tr | tr | tr | tr | tr | tr | - | tr | tr | tr | tr | tr | tr | - | tr | tr | tr |
| 41 | 2-Acetyl-1-caffeoyl-3-p-coumaroylglycerol | tr | tr | tr | tr | tr | tr | tr | + | tr | - | tr | - | + | tr | tr | - | tr | tr | tr |
| 42 | Quercetin-dimethyl ether | tr | tr | tr | tr | tr | - | tr | tr | tr | - | tr | tr | tr | tr | - | - | tr | + | tr |
| 43 | Caffeic acid butyl or isobutyl ester | - | - | - | - | - | - | tr | - | tr | - | tr | + | - | - | - | - | tr | - | - |
| 44 | 2-Acetyl-3-caffeoyl-1-feruloylglycerol | tr | - | - | - | tr | tr | tr | + | tr | - | tr | - | + | tr | tr | - | tr | tr | tr |
| 45 | Quercetin-trimethyl ether | - | tr | tr | tr | - | - | - | - | tr | - | - | - | - | - | - | - | tr | - | |
| 46 | Caffeic acid prenyl or isoprenyl ester 1 | tr | + | + | tr | + | +++ | tr | - | tr | - | + | + | + | tr | - | + | tr | tr | tr |
| 47 | Chrysin | +++ | +++ | +++ | +++ | + | tr | + | tr | +++ | tr | ++ | ++ | + | tr | tr | ++ | + | +++ | + |
| 48 | Sakuranetin | + | + | + | + | ++ | + | + | ++ | tr | - | + | +++ | + | +++ | ++ | + | ++ | + | ++ |
| 49 | Pinocembrin | +++ | +++ | +++ | +++ | + | + | + | tr | + | ++ | ++ | + | + | tr | tr | +++ | ++ | +++ | ++ |
| 50 | Acacetin | - | - | - | - | ++ | tr | + | ++ | - | + | + | tr | - | +++ | ++ | - | ++ | Tr | + |
| 51 | Caffeic acid prenyl or isoprenyl derivate 1 | tr | - | - | - | tr | - | tr | - | ++ | - | - | tr | + | tr | tr | - | tr | + | - |
| 52 | Pinocembrin chalcone | - | - | - | - | - | - | - | - | + | - | - | + | - | - | - | ++ | - | - | - |
| 53 | Caffeic acid prenyl or isoprenyl ester 2 | tr | + | + | + | tr | - | tr | - | tr | - | + | + | tr | tr | - | ++ | tr | tr | - |
| 54 | Caffeic acid benzyl ester | + | + | + | ++ | tr | ++ | tr | tr | + | + | + | + | tr | tr | - | - | tr | tr | tr |
| 55 | Caffeic acid prenyl or isoprenyl derivate 2 | tr | - | - | - | + | + | + | - | - | - | + | ++ | tr | tr | - | - | tr | + | tr |
| 56 | Flavonoid | tr | - | - | - | - | - | tr | + | + | - | - | tr | tr | tr | - | - | - | - | - |
| 57 | Genkwanin | - | tr | tr | tr | tr | + | + | + | tr | + | tr | tr | tr | + | + | - | + | tr | + |
| 58 | Galangin | +++ | +++ | +++ | +++ | ++ | + | + | tr | ++ | + | ++ | ++ | tr | - | + | +++ | + | ++ | + |
| 59 | Kaempheride | tr | tr | tr | tr | ++ | tr | tr | tr | tr | + | + | tr | tr | tr | ++ | - | ++ | tr | ++ |
| 60 | 2-Acetyl-1,3-di-p-Coumaroylglycerol | tr | tr | tr | tr | ++ | ++ | ++ | +++ | + | +++ | + | tr | ++ | +++ | + | - | ++ | + | + |
| 61 | 3-O-acetyl-pinobanksin | +++ | ++ | ++ | ++ | + | + | + | - | ++ | - | ++ | ++ | tr | + | - | +++ | + | +++ | + |
| 62 | Quercetin-dimethyl | - | - | - | - | + | - | tr | + | tr | + | tr | - | tr | tr | tr | - | + | - | + |
| 63 | * 2-Acetyl-3-p-coumaroyl-1-feruloylglycerol | tr | tr | tr | tr | + | + | + | + | tr | + | tr | - | + | + | + | - | + | - | + |
| 64 | Metoxychrysin | + | + | + | + | tr | + | tr | - | tr | - | + | tr | tr | tr | - | + | tr | tr | - |
| No. | Component | UK1 | UK2 | UK3 | UT | RBH | RB1 | RB2 | RKH | RKK | RKP | RN | RP | RS | RT | RV | KR | KZ | SL | PL |
| 65 | * 3-Acetyl-1,2-di-p-coumaroylglycerol | tr | - | - | tr | tr | tr | tr | - | tr | tr | - | tr | - | - | - | tr | - | tr | |
| 66 | Caffeic acid phenethyl ester (CAPE) | + | + | + | + | + | tr | + | - | ++ | - | + | + | tr | tr | - | - | + | + | - |
| 67 | 2-Acetyl-1,3-di-feruloylglycerol | tr | - | - | - | tr | tr | tr | + | - | + | tr | - | + | tr | + | - | tr | - | tr |
| 68 | Dimethyl luteolin | tr | tr | tr | tr | ++ | + | + | + | + | ++ | + | tr | tr | ++ | ++ | - | ++ | tr | + |
| 69 | Caffeic acid pentyl or isopentyl ester 1 | - | - | - | - | tr | - | tr | - | tr | - | + | + | tr | tr | - | - | - | - | - |
| 70 | Flavonoid dimethyl ether | tr | - | - | - | + | - | + | tr | tr | + | - | - | - | + | tr | - | + | - | - |
| 71 | p-Coumaric acid prenyl ester 1 | tr | tr | tr | tr | tr | - | - | - | - | - | - | - | tr | - | - | - | - | tr | |
| 72 | p-Coumaric acid prenyl ester 2 | tr | tr | tr | tr | - | - | - | - | tr | - | tr | - | tr | - | - | - | - | tr | + |
| 73 | p-Coumaric acid benzyl ester | + | + | + | + | ++ | + | ++ | ++ | + | + | + | - | ++ | +++ | + | - | + | + | ++ |
| 74 | Ferulic or isoferulic acid benzyl ester | + | + | + | + | + | + | + | ++ | + | + | + | - | + | + | + | - | + | + | + |
| 75 | Caffeic acid cinnamic ester | ++ | tr | tr | tr | tr | tr | tr | - | - | + | tr | - | tr | - | tr | - | tr | + | tr |
| 76 | 3-O-propyl-pinobanksin | + | + | + | tr | - | - | tr | - | + | - | tr | - | tr | - | - | + | tr | + | - |
| 77 | p-Coumaric acid phenethyl ester | - | - | - | - | - | - | tr | tr | tr | - | - | - | - | - | tr | - | - | tr | + |
| 78 | Pinostrobin chalcone | - | tr | tr | tr | tr | - | + | - | + | - | tr | ++ | tr | - | - | - | tr | tr | + |
| 79 | Tectochrysin (Chrysin-7-methyl ether) | + | + | + | + | tr | tr | tr | - | - | - | tr | + | - | - | - | tr | - | + | - |
| 80 | Pinostrobin (Pinocembrin-5-methyl ether) | tr | tr | tr | + | + | +++ | + | - | + | + | + | ++ | tr | + | - | tr | + | + | + |
| 81 | 3-O-butyl or isobutyl pinobanksin | + | + | + | tr | + | tr | - | - | + | - | + | - | - | - | - | - | + | + | tr |
| 82 | Galangin-7-methyl ether | + | tr | tr | + | + | + | + | + | tr | + | + | + | - | + | + | - | tr | tr | tr |
| 83 | 3-O-pentyl or isopentyl pinobanksin 1 | tr | + | + | tr | + | tr | - | - | tr | - | tr | - | - | - | - | + | tr | tr | - |
| 84 | 3-O-pentyl or isopentyl pinobanksin 2 | tr | tr | tr | tr | - | - | - | - | tr | - | tr | - | - | tr | - | - | + | tr | - |
| 85 | 3-O-hexyl- pinobanksin | tr | tr | tr | tr | - | - | - | - | - | - | tr | - | - | - | - | - | tr | tr | - |
| 86 | Metoxycinnamic acid cinnamyl ester | tr | tr | tr | tr | + | + | tr | - | tr | - | tr | tr | ++ | + | - | - | tr | tr | tr |
| Country | Plant Origin | TPC [mg GAE/g] | TFC [mg QE/g] | [TPC-TFC] [mg/g] | DPPH [mg GAE/g] | FRAP [mmol Fe2+/g] | ORAC [mmol Trx/g] |
|---|---|---|---|---|---|---|---|
| UK1 | P | 128.43 ± 2.15 | 55.24 ± 0.82 | 73.19 | 36.10 ± 1.40 | 0.25 ± 0.01 | 3.85 ± 0.13 |
| UK2 | P | 135.89 ± 1.17 | 55.79 ± 1.76 | 80.10 | 32.83 ± 0.64 | 0.29 ± 0.02 | 3.99 ± 0.23 |
| UK3 | P | 120.30 ± 1.22 | 53.90 ± 2.36 | 66.40 | 30.98 ± 0.83 | 0.34 ± 0.01 | 4.17 ± 0.16 |
| UT | P | 145.24 ± 2.24 | 82.71 ± 2.46 | 62.53 | 42.69 ± 0.91 | 0.40 ± 0.04 | 4.23 ± 0.24 |
| RB1 | A-B-P | 121.26 ± 0.29 | 25.84 ± 0.57 | 95.42 | 64.47 ± 0.70 | 1.03 ± 0.02 | 3.46 ± 0.16 |
| RB2 | A-B-P | 120.02 ± 2.00 | 26.77±0.71 | 93.25 | 53.55 ± 0.31 | 0.91 ± 0.03 | 3.56 ± 0.19 |
| RBH | A-B-P | 48.89 ± 0.37 | 31.63±2.81 | 93.25 | 39.39 ± 0.19 | 1.28 ± 0.07 | 3.68 ± 0.27 |
| RKP | A-B | 50.39 ± 0.43 | 30.48 ± 0.82 | 19,91 | 31.76 ± 2.53 | 0.13 ± 0.01 | 3.55 ± 0.22 |
| RKH | A-B | 52.81 ± 0.41 | 33.93 ± 1.06 | 17,26 | 34.65 ± 2.74 | 0.15 ± 0.01 | 3.63 ± 0.17 |
| RKK | P | 129.21 ± 0.91 | 53.13 ± 1.26 | 76.08 | 29.02 ± 1.60 | 0.17 ± 0.01 | 3.56 ± 0.14 |
| RN | P | 99.98 ± 0.52 | 41.65 ± 1.58 | 58.34 | 25.27 ± 0.73 | 0.12 ± 0.01 | 3.67 ± 0.11 |
| RP | P | 81.65 ± 2.09 | 27.34 ± 0.94 | 54.31 | 32.22 ± 0.39 | 0.11 ± 0.01 | 3.96 ± 0.34 |
| RS | A-P | 109.55 ± 0.49 | 10.45 ± 0.46 | 99.10 | 41.19 ± 1.78 | 0.08 ± 0.01 | 4.12 ± 0.26 |
| RT | A-B | 40.02 ± 0.79 | 32.49 ± 1.15 | 7.53 | 12.59 ± 0.52 | 0.06 ± 0.01 | 3.78 ± 0.49 |
| RV | A-B-P | 47.13 ± 0.50 | 31.97 ± 0.79 | 15.16 | 33.70 ± 0.49 | 1.17 ± 0.06 | 4.17 ± 0.12 |
| KR | P | 30.28 ± 0.46 | 17.75 ± 0.72 | 12.52 | 8.83 ± 0.49 | 0.10 ± 0.01 | 1.95 ± 0.36 |
| KZ | A-B-P | 45.04 ± 0.45 | 44.89 ± 1.45 | 0.14 | 24.67 ± 1.00 | 0.85 ± 0.03 | 2.89 ± 0.46 |
| PL | A-B-P | 46.91 ± 1.10 | 40.94 ± 1.35 | 2.05 | 28.34 ± 0.42 | 1.15 ± 0.07 | 3.27 ± 0.38 |
| SN | P | 42.47 ± 0.81 | 40.42 ± 1.07 | 5.97 | 28.28 ± 0.18 | 0.92 ± 0.01 | 3.73 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okińczyc, P.; Widelski, J.; Szperlik, J.; Żuk, M.; Mroczek, T.; Skalicka-Woźniak, K.; Sakipova, Z.; Widelska, G.; Kuś, P.M. Impact of Plant Origin on Eurasian Propolis on Phenolic Profile and Classical Antioxidant Activity. Biomolecules 2021, 11, 68. https://doi.org/10.3390/biom11010068
Okińczyc P, Widelski J, Szperlik J, Żuk M, Mroczek T, Skalicka-Woźniak K, Sakipova Z, Widelska G, Kuś PM. Impact of Plant Origin on Eurasian Propolis on Phenolic Profile and Classical Antioxidant Activity. Biomolecules. 2021; 11(1):68. https://doi.org/10.3390/biom11010068
Chicago/Turabian StyleOkińczyc, Piotr, Jarosław Widelski, Jakub Szperlik, Magdalena Żuk, Tomasz Mroczek, Krystyna Skalicka-Woźniak, Zuriyadda Sakipova, Gabriela Widelska, and Piotr Marek Kuś. 2021. "Impact of Plant Origin on Eurasian Propolis on Phenolic Profile and Classical Antioxidant Activity" Biomolecules 11, no. 1: 68. https://doi.org/10.3390/biom11010068






