Assessing Protein Biomarkers to Detect Lethal Acute Traumatic Brain Injuries in Cerebrospinal Fluid
Abstract
1. Introduction
2. Material/Methods
2.1. Sample Collection, Assessment and Selection
2.2. Statistical Analyses
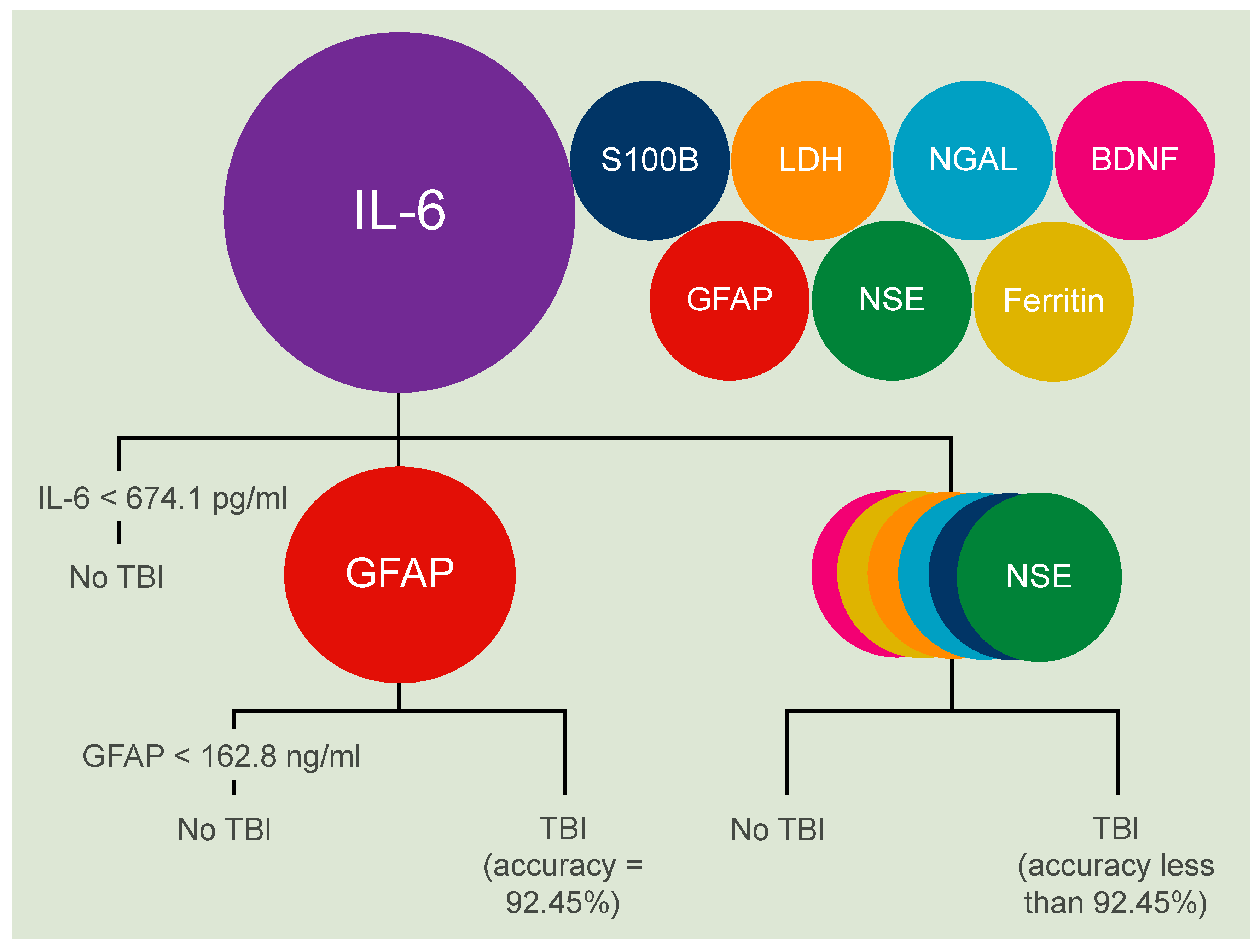
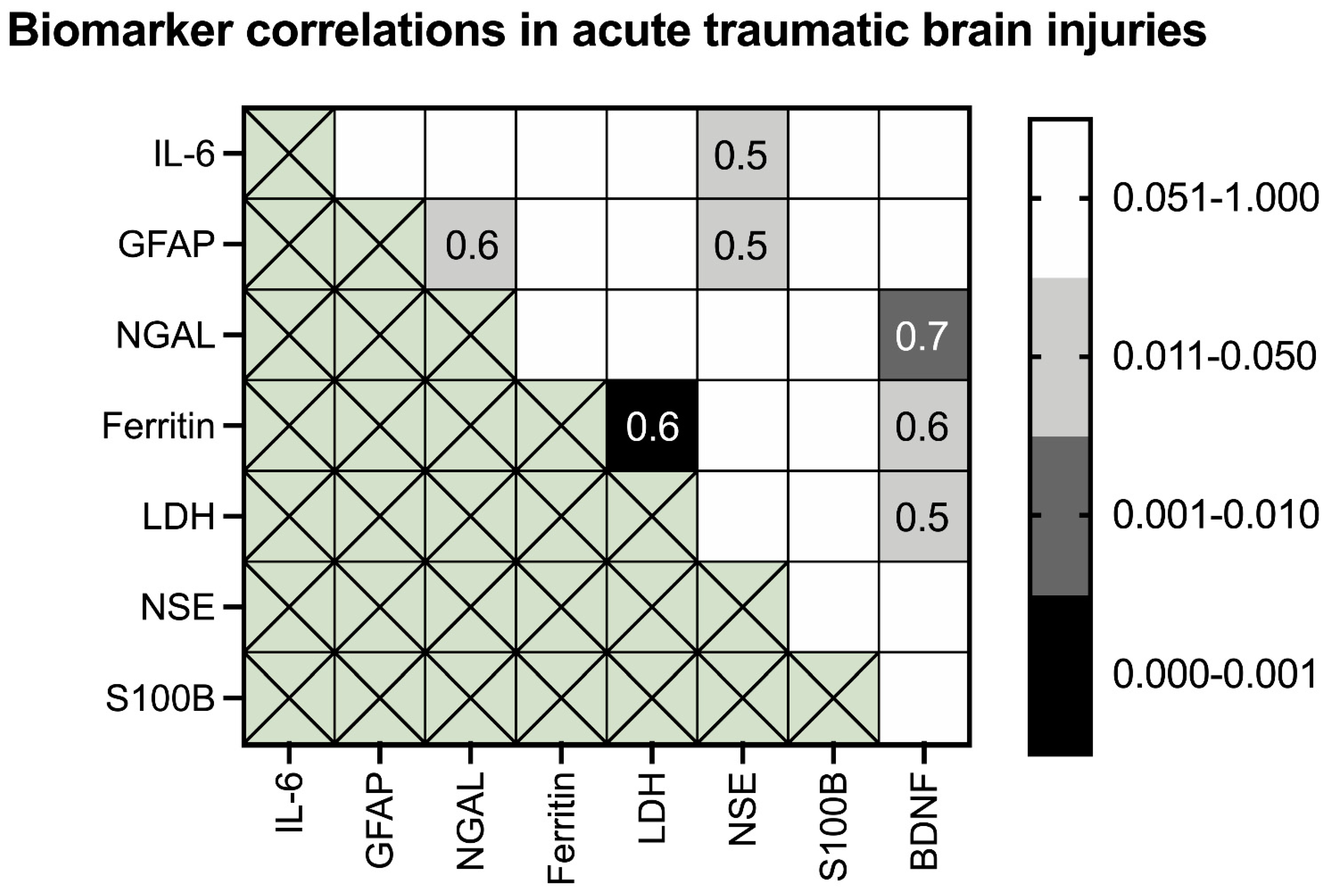
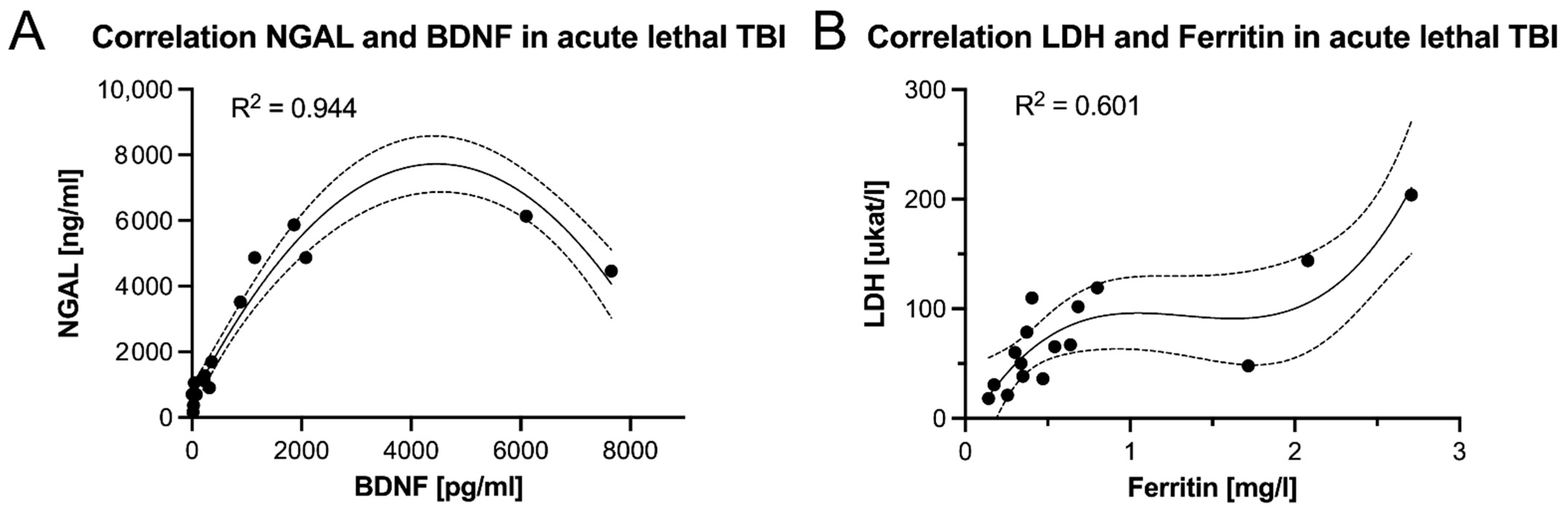
3. Results
BDNF Significantly Correlates with Three Acute Phase Proteins in Acute Lethal TBIs
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- GBD 2016 Traumatic Brain Injury; Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Zetterberg, H.; Blennow, K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat. Rev. Neurol. 2016, 12, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Li, D.R.; Michiue, T.; Zhu, B.L.; Ishikawa, T.; Quan, L.; Zhao, D.; Yoshida, C.; Chen, J.H.; Wang, Q.; Komatsu, A.; et al. Evaluation of postmortem S100B levels in the cerebrospinal fluid with regard to the cause of death in medicolegal autopsy. Legal Med. (Tokyo) 2009, 11 (Suppl. 1), S273–S275. [Google Scholar] [CrossRef]
- Li, D.R.; Zhu, B.L.; Ishikawa, T.; Zhao, D.; Michiue, T.; Maeda, H. Postmortem serum protein S100B levels with regard to the cause of death involving brain damage in medicolegal autopsy cases. Legal Med. (Tokyo) 2006, 8, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ondruschka, B.; Pohlers, D.; Sommer, G.; Schober, K.; Teupser, D.; Franke, H.; Dressler, J. S100B and NSE as useful postmortem biochemical markers of traumatic brain injury in autopsy cases. J. Neurotrauma 2013, 30, 1862–1871. [Google Scholar] [CrossRef]
- Ondruschka, B.; Schuch, S.; Pohlers, D.; Franke, H.; Dressler, J. Acute phase response after fatal traumatic brain injury. Int. J. Legal Med. 2018, 132, 531–539. [Google Scholar] [CrossRef]
- Ondruschka, B.S.M.; Kirsten, H.; Franke, H.; Dressler, J. Measurement of Cerebral Biomarkers Proving Traumatic Brain Injuries in Post-Mortem Body Fluids. J. Neurotrauma 2018, 35, 2044–2055. [Google Scholar] [CrossRef]
- Sieber, M.; Dressler, J.; Franke, H.; Pohlers, D.; Ondruschka, B. Post-mortem biochemistry of NSE and S100B: A supplemental tool for detecting a lethal traumatic brain injury? J. Forensic Legal Med. 2018, 55, 65–73. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Regner, A.; Miotto, K.D.; Moura, S.; Ikuta, N.; Vargas, A.E.; Chies, J.A.; Simon, D. Increased levels of interleukin-6, -8 and -10 are associated with fatal outcome following severe traumatic brain injury. Brain Inj. 2014, 28, 1311–1316. [Google Scholar] [CrossRef]
- Lustenberger, T.; Kern, M.; Relja, B.; Wutzler, S.; Stormann, P.; Marzi, I. The effect of brain injury on the inflammatory response following severe trauma. Immunobiology 2016, 221, 427–431. [Google Scholar] [CrossRef]
- Olczak, M.; Niderla-Bielinska, J.; Kwiatkowska, M.; Samojlowicz, D.; Tarka, S.; Wierzba-Bobrowicz, T. Tau protein (MAPT) as a possible biochemical marker of traumatic brain injury in postmortem examination. Forensic Sci. Int. 2017, 280, 1–7. [Google Scholar] [CrossRef]
- Olczak, M.; Poniatowski, L.A.; Niderla-Bielinska, J.; Kwiatkowska, M.; Chutoranski, D.; Tarka, S.; Wierzba-Bobrowicz, T. Concentration of microtubule associated protein tau (MAPT) in urine and saliva as a potential biomarker of traumatic brain injury in relationship with blood-brain barrier disruption in postmortem examination. Forensic Sci. Int. 2019, 301, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J.J.; Biberthaler, P.; Welch, R.D.; Lewis, L.M.; Barzo, P.; Bogner-Flatz, V.; Gunnar Brolinson, P.; Buki, A.; Chen, J.Y.; Christenson, R.H.; et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018, 17, 782–789. [Google Scholar] [CrossRef]
- Diaz-Arrastia, R.; Wang, K.K.; Papa, L.; Sorani, M.D.; Yue, J.K.; Puccio, A.M.; McMahon, P.J.; Inoue, T.; Yuh, E.L.; Lingsma, H.F.; et al. Acute biomarkers of traumatic brain injury: Relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 2014, 31, 19–25. [Google Scholar] [CrossRef]
- Maeda, H.; Zhu, B.L.; Ishikawa, T.; Quan, L.; Michiue, T. Significance of postmortem biochemistry in determining the cause of death. Legal Med. (Tokyo) 2009, 11 (Suppl. 1), S46–S49. [Google Scholar] [CrossRef]
- Zwirner, J.; Anders, S.; Bohnert, S.; Burkhardt, R.; Da Broi, U.; Hammer, N.; Pohlers, D.; Tse, R.; Ondruschka, B. Screening for Fatal Traumatic Brain Injuries in Cerebrospinal Fluid Using Blood-Validated CK and CK–MB Immunoassays. Biomolecules 2021, 11, 1061. [Google Scholar] [CrossRef]
- Zwirner, J.; Lier, J.; Franke, H.; Hammer, N.; Matschke, J.; Trautz, F.; Tse, R.; Ondruschka, B. GFAP positivity in neurons following traumatic brain injuries. Int. J. Legal Med. 2021, 135, 2323–2333. [Google Scholar] [CrossRef]
- Olczak, M.; Poniatowski, L.A.; Siwinska, A.; Kwiatkowska, M.; Chutoranski, D.; Wierzba-Bobrowicz, T. Elevated serum and urine levels of progranulin (PGRN) as a predictor of microglia activation in the early phase of traumatic brain injury: A further link with the development of neurodegenerative diseases. Folia Neuropathol. 2021, 59, 81–90. [Google Scholar] [CrossRef]
- Vazquez, M.D.; Sanchez-Rodriguez, F.; Osuna, E.; Diaz, J.; Cox, D.E.; Perez-Carceles, M.D.; Martinez, P.; Luna, A.; Pounder, D.J. Creatine kinase BB and neuron-specific enolase in cerebrospinal fluid in the diagnosis of brain insult. Am. J. Forensic Med. Pathol. 1995, 16, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Osuna, E.; Perez-Carceles, M.D.; Luna, A.; Pounder, D.J. Efficacy of cerebro-spinal fluid biochemistry in the diagnosis of brain insult. Forensic Sci. Int. 1992, 52, 193–198. [Google Scholar] [CrossRef]
- Bohnert, S.; Ondruschka, B.; Bohnert, M.; Schuhmann, M.K.; Monoranu, C.M. Post-mortem cerebrospinal fluid diagnostics: Cytology and immunocytochemistry method suitable for routine use to interpret pathological processes in the central nervous system. Int. J. Legal Med. 2019, 133, 1141–1146. [Google Scholar] [CrossRef]
- Trautz, F.; Franke, H.; Bohnert, S.; Hammer, N.; Muller, W.; Stassart, R.; Tse, R.; Zwirner, J.; Dressler, J.; Ondruschka, B. Survival-time dependent increase in neuronal IL-6 and astroglial GFAP expression in fatally injured human brain tissue. Sci. Rep. 2019, 9, 11771. [Google Scholar] [CrossRef]
- Maier, B.; Laurer, H.L.; Rose, S.; Buurman, W.A.; Marzi, I. Physiological levels of pro- and anti-inflammatory mediators in cerebrospinal fluid and plasma: A normative study. J. Neurotrauma 2005, 22, 822–835. [Google Scholar] [CrossRef]
- Hans, V.H.; Kossmann, T.; Lenzlinger, P.M.; Probstmeier, R.; Imhof, H.G.; Trentz, O.; Morganti-Kossmann, M.C. Experimental axonal injury triggers interleukin-6 mRNA, protein synthesis and release into cerebrospinal fluid. J. Cereb. Blood Flow Metab. 1999, 19, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Gutierrez, E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994, 179, 53–56. [Google Scholar] [CrossRef]
- Benveniste, E.N.; Sparacio, S.M.; Norris, J.G.; Grenett, H.E.; Fuller, G.M. Induction and regulation of interleukin-6 gene expression in rat astrocytes. J. Neuroimmunol. 1990, 30, 201–212. [Google Scholar] [CrossRef]
- Woodroofe, M.N.; Sarna, G.S.; Wadhwa, M.; Hayes, G.M.; Loughlin, A.J.; Tinker, A.; Cuzner, M.L. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: Evidence of a role for microglia in cytokine production. J. Neuroimmunol. 1991, 33, 227–236. [Google Scholar] [CrossRef]
- Li, D.R.; Ishikawa, T.; Zhao, D.; Michiue, T.; Quan, L.; Zhu, B.L.; Maeda, H. Histopathological changes of the hippocampus neurons in brain injury. Histol. Histopathol. 2009, 24, 1113–1120. [Google Scholar] [CrossRef]
- Davidoff, M.S.; Middendorff, R.; Kofuncu, E.; Muller, D.; Jezek, D.; Holstein, A.F. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem. 2002, 104, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Zetterberg, H.; Hampel, H.; Blennow, K. Biomarker-based dissection of neurodegenerative diseases. Prog. Neurobiol. 2011, 95, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Silvestri, S.; Brophy, G.M.; Giordano, P.; Falk, J.L.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Demery, J.A.; Dixit, N.K.; et al. GFAP out-performs S100beta in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J. Neurotrauma 2014, 31, 1815–1822. [Google Scholar] [CrossRef]
- Papa, L.; Brophy, G.M.; Welch, R.D.; Lewis, L.M.; Braga, C.F.; Tan, C.N.; Ameli, N.J.; Lopez, M.A.; Haeussler, C.A.; Mendez Giordano, D.I.; et al. Time Course and Diagnostic Accuracy of Glial and Neuronal Blood Biomarkers GFAP and UCH-L1 in a Large Cohort of Trauma Patients With and Without Mild Traumatic Brain Injury. JAMA Neurol. 2016, 73, 551–560. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Blyth, B.J.; He, H.; Mookerjee, S.; Jones, C.; Kiechle, K.; Moynihan, R.; Wojcik, S.M.; Grant, W.D.; Secreti, L.M.; et al. Classification accuracy of serum Apo A-I and S100B for the diagnosis of mild traumatic brain injury and prediction of abnormal initial head computed tomography scan. J. Neurotrauma 2013, 30, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- McGee, S. Simplifying likelihood ratios. J. Gen. Intern. Med. 2002, 17, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Z.Z.; Huang, Y.L.; Song, H.J.; Wang, Y.J. Value of free/total prostate-specific antigen (f/t PSA) ratios for prostate cancer detection in patients with total serum prostate-specific antigen between 4 and 10 ng/mL: A meta-analysis. Medicine (Baltimore) 2018, 97, e0249. [Google Scholar] [CrossRef] [PubMed]
- Elsafi, S.H.; Alqahtani, N.I.; Zakary, N.Y.; Al Zahrani, E.M. The sensitivity, specificity, predictive values, and likelihood ratios of fecal occult blood test for the detection of colorectal cancer in hospital settings. Clin. Exp. Gastroenterol. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Grady, D.; Barclay, J.; Sickles, E.A.; Ernster, V. Likelihood ratios for modern screening mammography. Risk of breast cancer based on age and mammographic interpretation. JAMA 1996, 276, 39–43. [Google Scholar] [CrossRef]
- Bolliger, S.A.; Thali, M.J.; Ross, S.; Buck, U.; Naether, S.; Vock, P. Virtual autopsy using imaging: Bridging radiologic and forensic sciences. A review of the Virtopsy and similar projects. Eur. Radiol. 2008, 18, 273–282. [Google Scholar] [CrossRef]
- Anim, J.T. Autopsy Practice in Ghana-Reflections of a Pathologist. Ghana Med. J. 2015, 49, 112–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ondruschka, B.; Woydt, L.; Bernhard, M.; Franke, H.; Kirsten, H.; Loffler, S.; Pohlers, D.; Hammer, N.; Dressler, J. Post-mortem in situ stability of serum markers of cerebral damage and acute phase response. Int. J. Legal Med. 2019, 133, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Woydt, L.; Bernhard, M.; Kirsten, H.; Burkhardt, R.; Hammer, N.; Gries, A.; Dressler, J.; Ondruschka, B. Intra-individual alterations of serum markers routinely used in forensic pathology depending on increasing post-mortem interval. Sci. Rep. 2018, 8, 12811. [Google Scholar] [CrossRef]
- Weustink, A.C.; Hunink, M.G.; van Dijke, C.F.; Renken, N.S.; Krestin, G.P.; Oosterhuis, J.W. Minimally invasive autopsy: An alternative to conventional autopsy? Radiology 2009, 250, 897–904. [Google Scholar] [CrossRef]

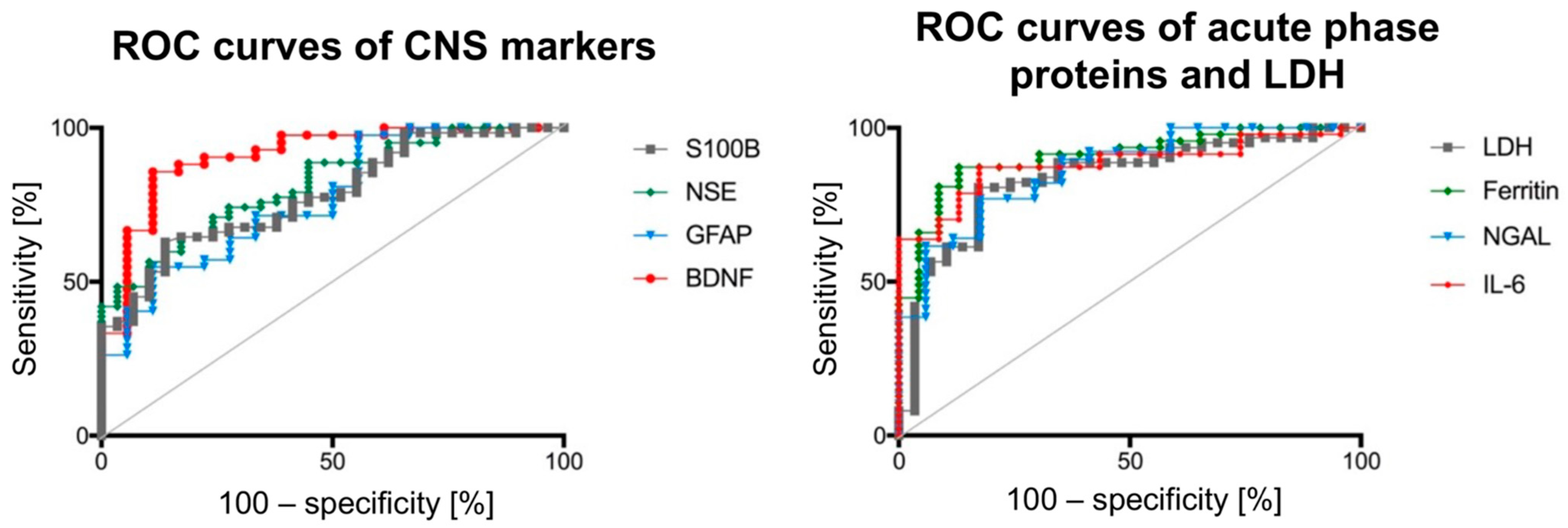
| Biomarker | AUC | 95% Confidence Interval | Threshold Value | Sensitivity: Specificity Ratio [%] | Positive Likelihood Ratio | Accuracy [%] |
|---|---|---|---|---|---|---|
| TBI biomarker of the central nervous system | ||||||
| S100B | 0.78 | 0.69–0.88 | 2267 ng/mL | 37.1:96.6 | 10.76 | 79 |
| NSE | 0.82 | 0.73–0.90 | 598.5 ng/mL | 48.4:96.5 | 14.03 | 83 |
| GFAP | 0.77 | 0.64–0.89 | 134.4 ng/mL | 40.5:94.4 | 7.29 | 78 |
| BDNF | 0.91 | 0.83–0.99 | 11.1 pg/mL | 64.3:95.7 | 14.68 | 86 |
| Acute phase proteins as TBI biomarkers | ||||||
| IL-6 | 0.88 | 0.80–0.96 | 99.1 pg/mL | 63.8:95.7 | 14.68 | 86 |
| Ferritin | 0.91 | 0.83–0.98 | 1.73 mg/L | 66.0:95.7 | 15.17 | 87 |
| LDH | 0.84 | 0.75–0.93 | 16.71 µkat/L | 41.9:96.5 | 12.16 | 81 |
| NGAL | 0.86 | 0.76–0.96 | 334.4 ng/mL | 61.5:94.1 | 10.46 | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwirner, J.; Bohnert, S.; Franke, H.; Garland, J.; Hammer, N.; Möbius, D.; Tse, R.; Ondruschka, B. Assessing Protein Biomarkers to Detect Lethal Acute Traumatic Brain Injuries in Cerebrospinal Fluid. Biomolecules 2021, 11, 1577. https://doi.org/10.3390/biom11111577
Zwirner J, Bohnert S, Franke H, Garland J, Hammer N, Möbius D, Tse R, Ondruschka B. Assessing Protein Biomarkers to Detect Lethal Acute Traumatic Brain Injuries in Cerebrospinal Fluid. Biomolecules. 2021; 11(11):1577. https://doi.org/10.3390/biom11111577
Chicago/Turabian StyleZwirner, Johann, Simone Bohnert, Heike Franke, Jack Garland, Niels Hammer, Dustin Möbius, Rexson Tse, and Benjamin Ondruschka. 2021. "Assessing Protein Biomarkers to Detect Lethal Acute Traumatic Brain Injuries in Cerebrospinal Fluid" Biomolecules 11, no. 11: 1577. https://doi.org/10.3390/biom11111577
APA StyleZwirner, J., Bohnert, S., Franke, H., Garland, J., Hammer, N., Möbius, D., Tse, R., & Ondruschka, B. (2021). Assessing Protein Biomarkers to Detect Lethal Acute Traumatic Brain Injuries in Cerebrospinal Fluid. Biomolecules, 11(11), 1577. https://doi.org/10.3390/biom11111577






