2.1. General Chemistry Methods
Chromatographic purification of products was accomplished using forced-flow chromatography on Merck
® (Merck, Darmstadt, Germany) Kieselgel 60 F
254 230–400 mesh. Thin-layer chromatography (TLC) was performed on aluminum-backed silica plates (0.2 mm, 60 F
254). Visualization of the developed chromatogram was performed by fluorescence quenching using phosphomolybdic acid, ninhydrin, or potassium permagnate stains. Melting points were determined on a Buchi
® 530 (Buchi, Flawil, Switzerland) spectrometer and were uncorrected.
1H and
13C NMR spectra were recorded on a Varian
® Mercury (Varian, Palo Alto, CA, USA) (200 MHz and 50 MHz, respectively), a Bruker Avance Neo (400 MHz and 100 MHz, respectively) (Bruker, Faellanden, Switzerland), or a Bruker Avance (500 MHz and 125 MHz, respectively) (Bruker, Santa Barbara, CA, USA), and are internally referenced to residual solvent signals. Data for
1H NMR are reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, br s = broad signal), coupling constant, integration, and peak assignment. Data for
13C NMR are reported in terms of chemical shift (δ ppm). IR spectra were recorded with an OSTEC, IROS-05, FTIR spectrophotometer equipped with ATR diamond crystal (Simex Co., Ltd., Nizhny Novgorod, Russia). Mass spectra (ESI) were recorded on a Finnigan
® Surveyor MSQ LC-MS spectrometer (Thermo, Darmstadt, Germany). High-resolution mass spectrometry (HRMS) spectra were recorded on a Bruker
® Maxis Impact QTOF (Bruker Daltonics, Bremen, Germany) spectrometer. A microwave synthesizer, Discover (CEM, Charlotte, NC, USA), was used for the microwave synthesis.
1H NMR and
13C NMR spectra of the final products are shown in the
Supplementary Materials.
Compounds
18,
19,
20a,
21a, and
22a were synthesized as previously described [
21], and their analytical data were in accordance with literature.
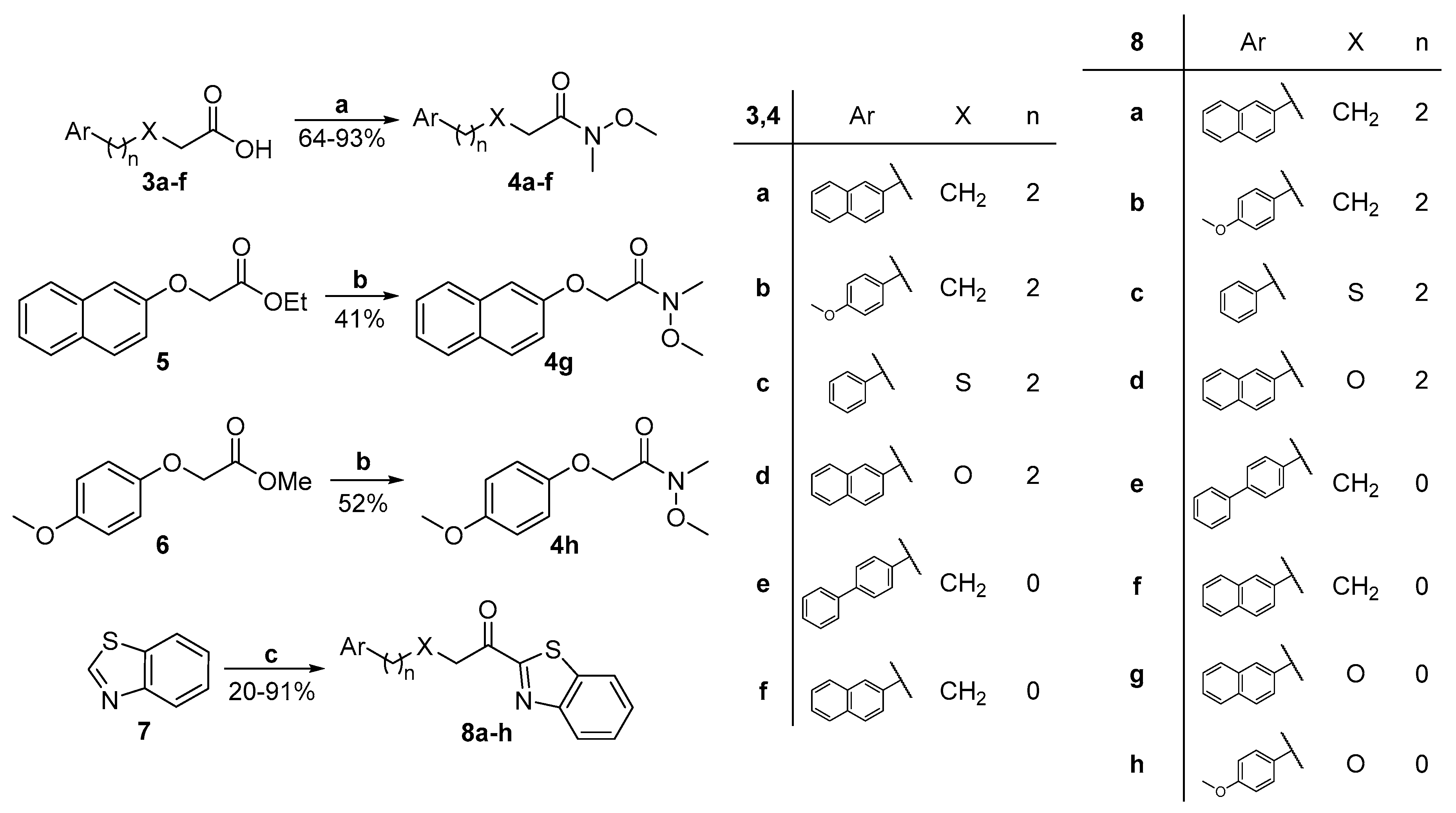
General procedure for the synthesis of Weinreb amides 4a–f from carboxylic acids.
To a stirred solution of the carboxylic acid 3a–f (1 mmol) in dry CH2Cl2 (7 mL), 4-dimethylaminopyridine (DMAP) (1 mmol), N,O-dimethyl hydroxylamine hydrochloride (1 mmol), N-methylmorpholine (1 mmol), and N-(3-dimethylaminopropyl)-N’-ethyl carbodiimide hydrochloride (WSCI·HCl) (1 mmol) were added consecutively at room temperature. The reaction mixture was left stirring for 18 h. It was then washed with an aqueous solution of 10% citric acid (3 × 10 mL), brine (10 mL), an aqueous solution of 5% NaHCO3 (3 × 10 mL), and brine (10 mL). The organic layer was dried over Na2SO4 and concentrated under reduced pressure. The amide was purified by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) to afford the desired product.
N-Methoxy-
N-methyl-5-(naphthalen-2-yl) pentanamide (
4a) [
19]. Yield 75%; Colorless oil;
1H NMR (200 MHz, CDCl
3):
δ = 7.85–7.70 (m, 3H, 3 × ArH), 7.61 (s, 1H, ArH), 7.50–7.27 (m, 3H, 3 × ArH), 3.64 (s, 3H, OCH
3), 3.16 (s, 3H, NCH
3), 2.80 (t,
J = 7.0 Hz, 2H, CH
2), 2.45 (t,
J = 7.0 Hz, 2H, CH
2), 1.87–1.60 (m, 4H, 2 × CH
2);
13C NMR (50 MHz, CDCl
3):
δ = 175.8, 139.9, 133.6, 131.9, 127.8, 127.6, 127.4, 127.3, 126.3, 125.8, 125.0, 61.2, 35.9, 31.7, 31.1, 24.4; MS (ESI)
m/z (%): 272 [(M+H)
+, 100].
N-Methoxy-5-(4-methoxyphenyl)-N-methylpentanamide (4b). Yield 64%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.10 (d, J = 8.0 Hz, 2H, 2 × ArH), 6.82 (d, J = 8.0 Hz, 2H, 2×ArH), 3.78 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 3.17 (s, 3H, NCH3), 2.59 (t, J = 6.0 Hz, 2H, CH2), 2.44 (t, J = 6.0 Hz, 2H, CH2), 1.73–1.61 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 176.4, 157.5, 134.3, 129.2, 113.6, 61.1, 55.1, 34.7, 31.6, 31.4, 24.2; MS (ESI) m/z (%): 252.2 [(M+H)+, 100].
N-Methoxy-N-methyl-2-(phenethylthio)acetamide (4c). Yield 83%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.31−7.10 (m, 5H, 5 × ArH), 3.63 (s, 3H, OCH3), 3.31 (s, 2H, SCH2), 3.16−3.04 (m, 4H, SCH2,CH2), 2.87 (s, 3H, NCH3); 13C NMR (50 MHz, CDCl3): δ = 159.2, 156.7, 128.1, 128.0, 125.9, 61.1, 35.3, 33.3, 31.2; HRMS (ESI) [M+Na]+ m/z: 262.0872; (calculated for [C12H17NNaO2S]+ 262.0872).
N-Methoxy-N-methyl-2-(2-(naphthalen-2-yl)ethoxy)acetamide (4d). Yield 72%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.83−7.35 (m, 7H, 7 × ArH), 4.24 (s, 2H, OCH2), 3.85 (t, J = 7.1 Hz, 2H, OCH2), 3.52 (s, 3H, OCH3), 3.17−3.03 (m, 5H, NCH3, CH2); 13C NMR (50 MHz, CDCl3): δ = 170.7, 135.9, 133.2, 131.8, 127.6, 127.3, 127.22, 127.17, 126.9, 125.6, 125.0, 72.2, 68.1, 61.0, 36.0, 31.9; HRMS (ESI) [M+Na]+ m/z: 296.1254; (calculated for [C16H19NNaO3]+ 296.1257).
3-([1,1′-Biphenyl]-4-yl)-N-methoxy-N-methylpropanamide (4e). Yield 88%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.60−7.23 (m, 9H, 9 × ArH), 3.63 (s, 3H, OCH3), 3.19 (s, 3H, NCH3), 3.00 (t, J = 8.1 Hz, 2H, CH2), 2.81 (t, J = 8.2 Hz, 2H, CH2); 13C NMR (50 MHz, CDCl3): δ = 140.9, 140.4, 139.0, 128.8, 128.7, 127.1, 127.0, 126.9, 61.2, 33.7, 32.2, 30.2; HRMS (ESI) [M+Na]+ m/z: 292.1308; (calculated for [C17H19NNaO2]+ 292.1308).
N-Methoxy-N-methyl-3-(naphthalen-2-yl)propanamide (4f). Yield 93%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.82−7.32 (m, 7H, 7 × ArH), 3.59 (s, 3H, OCH3), 3.23−3.07 (m, 5H, NCH3, CH2), 2.91−2.76 (m, 2H, CH2); 13C NMR (50 MHz, CDCl3): δ = 173.4, 138.7, 133.4, 131.9, 127.9, 127.5, 127.3, 127.1, 126.4, 125.8, 125.1, 61.1, 33.5, 32.0, 30.7; HRMS (ESI) [M+Na]+ m/z: 266.1151; (calculated for [C15H17NNaO2]+ 266.1151).
General procedure for the synthesis of Weinreb amides 4g,h from esters.
To a stirred solution of ester 5 or 6 (1 mmol) in dry tetrahydrofuran (2 mL) at −20 °C, N,O-dimethyl hydroxylamine hydrochloride (1.5 mmol) was added. Isopropyl magnesium chloride was then added dropwise over 15 min and the reaction mixture was left stirring for 35 min at −20 °C. The reaction mixture was quenched with a saturated solution of NH4Cl (5 mL) and the reaction mixture was extracted with diethyl ether (2 × 10 mL). The combined extracts were dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) afforded the desired product.
N-Methoxy-N-methyl-2-(naphthalen-2-yloxy)acetamide (4g). Yield 41%; White solid; mp: 74–75 °C; 1H NMR (200 MHz, CDCl3): δ = 7.88–6.99 (m, 7H, 7 × ArH), 4.91 (s, 2H, CH2), 3.77 (s, 3H, OCH3), 3.25 (s, 3H, NCH3); 13C NMR (50 MHz, CDCl3): δ = 201.7, 156.2, 144.2, 134.4, 129.7, 127.7, 126.9, 126.5, 124.0, 118.9, 107.3, 65.7, 61.8, 32.5. HRMS (ESI) [M+Na]+ m/z: 268.0940; (calculated for [C14H15NNaO3]+ 268.0944).
N-Methoxy-2-(4-methoxyphenoxy)-N-methylacetamide (4h). Yield 52%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 6.88–6.66 (m, 4H, 4 × ArH), 4.67 (s, 2H, CH2), 3.66 (s, 3H, OCH3), 3.64 (s, 3H, OCH3), 3.13 (s, 3H, NCH3); 13C NMR (50 MHz, CDCl3): δ = 169.3, 154.1, 152.3, 115.8, 114.4, 66.2, 61.4, 55.4, 32.1. HRMS (ESI) [M+Na]+ m/z: 248.0891; (calculated for [C11H15NNaO4]+ 248.0893.
General procedure for the synthesis of α-ketobenzothiazoles 8a–h.
To a stirred solution of benzothiazole (3 mmol) in dry Et2O (20 mL) at −78 °C, under a dry argon atmosphere, a solution of n-BuLi (1.6 M in hexane, 3 mmol) was added dropwise over a period of 10 min. The resulting orange solution was stirred for 45 min. Then, a solution of the Weinreb amide (1 mmol) in dry Et2O (2 mL) was slowly added giving the mixture a dark brown color. After stirring for 30 min at −78 °C, the mixture was allowed to warm up to room temperature over a period of 2 h. Then, saturated aqueous ammonium chloride solution was added, and the reaction mixture was extracted with diethyl ether (2 × 10 mL). The combined extracts were washed with brine (10 mL) and then dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) afforded the desired product.
1-(Benzo[
d]thiazol-2-yl)-5-(naphthalen-2-yl)pentan-1-one (
8a) [
19]. Yield 72%; Yellow solid;
1H NMR (400 MHz, CDCl
3):
δ = 8.18 (d,
J = 8.0 Hz, 1H, ArH), 7.97 (d,
J = 8.0 Hz, 1H, ArH), 7.83−7.72 (m, 3H, 3 × ArH), 7.63 (s, 1H, ArH), 7.61−7.49 (m, 2H, 2 × ArH), 7.49−7.38 (m, 2H, 2 × ArH), 7.35 (dd,
J1 = 8.4,
J2 = 1.7 Hz, 1H, ArH), 3.34 (t,
J = 6.9 Hz, 2H, CH
2CO), 2.86 (t,
J = 7.1 Hz, 2H, CH
2Ar), 1.98–1.79 (m, 4H, 2 × CH
2);
13C NMR (100 MHz, CDCl
3):
δ = 195.5, 166.7, 153.7, 139.8, 137.4, 133.8, 132.1, 128.0, 127.8, 127.7, 127.6, 127.5, 127.1, 126.6, 126.0, 125.5, 125.2, 122.6, 38.5, 36.0, 30.9, 23.8; IR:
= 3052, 1687, 1601, 1551, 1490 cm
−1; HRMS (ESI) [M+Na]
+ m/z: 368.1084; (calculated for [C
22H
19NNaOS]
+ 368.1080).
1-(Benzo[d]thiazol-2-yl)-5-(4-methoxyphenyl)pentan-1-one (8b). Yield 52%; Orange solid; mp: 65–66 °C; 1H NMR (200 MHz, CDCl3): δ = 8.18 (d, J = 7.6 Hz, 1H, ArH), 7.96 (d, J = 7.2 Hz, 1H, ArH), 7.62−7.45 (m, 2H, 2 × ArH), 7.12 (d, J = 8.6 Hz, 2H, 2 × ArH), 6.83 (d, J = 8.6 Hz, 2H, 2 × ArH), 3.77 (s, 3H, OCH3), 3.30 (t, J = 7.1 Hz, 2H, CH2), 2.64 (t, J = 7.3 Hz, 2H, CH2), 1.95−1.64 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 195.4, 166.5, 157.7, 153.6, 137.3, 134.2, 129.3, 127.6, 127.0, 125.4, 122.5, 113.7, 55.2, 38.4, 34.8, 31.2, 23.6; HRMS (ESI) [M+Na]+ m/z: 348.1032; (calculated for [C19H19NNaO2S]+ 348.1029).
1-(Benzo[d]thiazol-2-yl)-2-(phenethylthio)ethan-1-one (8c). Yield 86%; Orange solid; mp: 63–65 °C; 1H NMR (500 MHz, CDCl3): δ = 8.24 (d, J = 8.0 Hz, 1H, ArH), 8.03 (d, J = 7.8 Hz, 1H, ArH), 7.63 (t, J = 7.3 Hz, 1H, ArH), 7.58 (t, J = 7.0 Hz, 1H, ArH), 7.39−7.33 (m, 2H, 2 × ArH), 7.32−7.26 (m, 3H, 3 × ArH), 4.14 (s, 2H, SCH2), 3.03−2.96 (m, 4H, SCH2, CH2); 13C NMR (125 MHz, CDCl3): δ = 188.9, 165.4, 153.5, 140.1, 137.7, 128.62, 128.56, 127.9, 127.1, 126.5, 125.6, 122.5, 36.2, 35.7, 33.9; IR: = 3060, 1678, 1603, 1556 cm−1; HRMS (ESI) [M+Na]+ m/z: 336.0484; (calculated for [C17H15NNaOS2]+ 336.0487).
1-(Benzo[d]thiazol-2-yl)-2-(2-(naphthalen-2-yl)ethoxy)ethan-1-one (8d). Yield 20%; Orange solid; mp: 57–59 °C; 1H NMR (200 MHz, CDCl3): δ = 8.22−8.08 (m, 1H, ArH), 8.06−7.92 (m, 1H, ArH), 7.91−7.69 (m, 4H, 4 × ArH), 7.60−7.38 (m, 5H, 5 × ArH), 5.15 (s, 2H, OCH2), 3.99 (t, J = 7.2 Hz, 2H, OCH2), 3.21 (t, J = 7.2 Hz, 2H, CH2Ar); 13C NMR (50 MHz, CDCl3): δ = 191.2, 164.0, 153.5, 136.9, 136.0, 133.7, 132.3, 128.1, 128.0, 127.74, 127.66, 127.4, 127.3, 126.1, 125.5, 122.6, 73.7, 73.0, 36.5; IR: = 3058, 1708, 1634, 1589 cm−1; HRMS (ESI) [M+Na]+ m/z: 370.0883; (calculated for [C21H17NNaO2S]+ 370.0872).
3-([1,1′-Biphenyl]-4-yl)-1-(benzo[d]thiazol-2-yl)propan-1-one (8e). Yield 91%; Yellowish solid; mp: 44–46 °C; 1H NMR (400 MHz, CDCl3): δ = 8.20 (d, J = 8.1 Hz, 1H, ArH), 7.97 (d, J = 7.9 Hz, 1H, ArH), 7.65–7.50 (m, 6H, 6 × ArH), 7.46 (t, J = 7.6 Hz, 2H, 2 × ArH), 7.42-7.33 (m, 3H, 3 × ArH), 3.69 (t, J = 7.6 Hz, 2H, CH2), 3.22 (t, J = 7.6 Hz, 2H, CH2); 13C NMR (100 MHz, CDCl3): δ = 194.4, 166.2, 153.6, 141.0, 139.8, 139.2, 137.3, 129.0, 128.8, 127.7, 127.3, 127.2, 127.1, 127.0, 125.5, 122.5, 40.2, 29.4; HRMS (ESI) [M+Na]+ m/z: 366.0934; (calculated for [C22H17NNaOS]+ 366.0923).
1-(Benzo[d]thiazol-2-yl)-3-(naphthalen-2-yl)propan-1-one (8f). Yield 91%; Yellowish solid of low melting point; 1H NMR (400 MHz, CDCl3): δ = 8.20 (d, J = 8.2 Hz, 1H, ArH), 7.96 (d, J = 8.0 Hz, 1H, ArH), 7.87−7.78 (m, 3H, 3 × ArH), 7.75 (s, 1H, ArH), 7.60−7.41 (m, 5H, 5 × ArH), 3.73 (t, J = 7.6 Hz, 2H, CH2), 3.32 (t, J = 7.6 Hz, 2H, CH2); 13C NMR (100 MHz, CDCl3): δ = 194.4, 166.2, 153.6, 138.2, 137.3, 133.7, 132.2, 128.2, 127.71, 127.68, 127.6, 127.3, 127.0, 126.7, 126.1, 125.5, 125.4, 122.5, 40.2, 29.9; HRMS (ESI) [M+Na]+ m/z: 340.0779; (calculated for [C20H15NNaOS]+ 340.0767).
1-(Benzo[d]thiazol-2-yl)-2-(naphthalen-2-yloxy)ethan-1-one (8g). Yield 22%; Pale yellow solid; mp: 144–145 °C; 1H NMR (400 MHz, CDCl3): δ = 8.28 (d, J = 8.1 Hz, 1H, ArH), 8.07 (d, J = 7.9 Hz, 1H, ArH), 7.86−7.79 (m, 2H, 2 × ArH), 7.76 (d, J = 8.2 Hz, 1H, ArH), 7.70−7.59 (m, 2H, 2 × ArH), 7.50−7.44 (m, 1H, ArH), 7.42−7.34 (m, 2H, 2 × ArH), 7.28 (d, J = 8.9 Hz, 1H, ArH), 5.81 (s, 2H, CH2); 13C NMR (100 MHz, CDCl3): δ = 188.7, 163.6, 155.9, 153.5, 137.1, 134.3, 129.8, 129.5, 128.1, 127.7, 127.4, 126.9, 126.5, 125.6, 124.1, 122.6, 118.7, 107.6, 70.3; IR: = 3058, 1715, 1634, 1598, 1484 cm-1; HRMS (ESI) [M+Na]+ m/z: 342.0568; (calculated for [C19H13NNaO2S]+ 342.0559).
1-(Benzo[d]thiazol-2-yl)-2-(4-methoxyphenoxy)ethan-1-one (8h). Yield 27%; Pale yellow solid of low melting point; 1H NMR (400 MHz, CDCl3): δ = 8.20 (d, J = 8.0 Hz, 1H, ArH), 8.02 (d, J = 7.9 Hz, 1H, ArH), 7.67−7.53 (m, 2H, 2 × ArH), 6.99 (d, J = 8.4 Hz, 2H, 2 × ArH), 6.85 (d, J = 8.4 Hz, 2H, 2 × ArH), 5.61 (s, 2H, CH2), 3.77 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 189.5, 163.7, 154.8, 153.6, 152.3, 137.1, 128.2, 127.4, 125.6, 122.7, 116.4, 114.9, 71.5, 55.9; HRMS (ESI) [M+Na]+ m/z: 322.0514; (calculated for [C16H13NNaO3S]+ 322.0508).
tert-Butyl 2-(2-(naphthalen-2-yl)ethoxy)acetate (10). Alcohol 9 (1 mmol), tert-butyl bromoacetate (1.2 mmol), and Bu4NHSO4 (0.2 mmol) were diluted in toluene (1 mL) and in an aqueous solution of 50% NaOH (1 mL). The reaction mixture was stirred for 18 h. After completion of the reaction, the organic layer was collected and washed with brine (2 mL), and then dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography eluting with a mixture of EtOAc:petroleum ether (40–60 °C) 2:8 afforded the desired product. Yield 94%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.90–7.31 (m, 7H, 7 × ArH), 4.01 (s, 2H, OCH2), 3.85 (t, J = 7.2 Hz, 2H, OCH2), 3.14 (t, J = 7.2 Hz, 2H, CH2Ar), 1.50 (s, 9H, 3 × CH3); 13C NMR (50 MHz, CDCl3): δ = 169.6, 136.0, 133.5, 132.1, 127.8, 127.5, 127.4, 127.1, 125.8, 125.2, 81.5, 72.3, 68.8, 36.3, 28.0. HRMS (ESI) [M+Na]+ m/z: 309.1458; (calculated for [C18H22NaO3]+ 309.1461).
2-(2-(Naphthalen-2-yl)ethoxy)acetic acid (3d). To a stirred solution of tert-butyl ester 10 (1 mmol) in dry CH2Cl2 (2 mL), under an inert argon atmosphere, trifluoroacetic acid (2 mL) was added and the reaction mixture was left stirring for 2 h. Toluene (2 mL) was then added and the solvents were evaporated under reduced pressure. The latter was repeated until complete removal of trifluoroacetic acid. The residue was diluted in H2O (5 mL) and diethyl ether (5 mL) and transferred to a separating funnel. An aqueous solution of 5% NaHCO3 (5 mL) was added, and the aqueous layer was acidified with an aqueous solution of 5% citric acid (5 mL) and then extracted with diethyl ether (3 × 5 mL). Concentration of the combined organic layers under reduced pressure afforded the desired product. Yield 81%; White solid; mp: 63–67 °C; 1H NMR (200 MHz, CD3OD): δ = 7.67−7.19 (m, 7H, 7 × ArH), 5.43 (br s, 1H, COOH), 4.02−3.88 (m, 2H, OCH2), 3.71−3.56 (m, 2H, OCH2), 2.97−2.85 (m, 2H, CH2Ar); 13C NMR (50 MHz, CD3OD): δ = 174.1, 137.3, 134.9, 133.5, 128.8, 128.4, 128.1, 126.8, 126.2, 73.1, 68.6, 36.9. HRMS (ESI) [M-H]- m/z: 229.0866; (calculated for [C14H13O3]- 229.0870).
1-(Benzo[d]thiazol-2-yl)-5-(naphthalen-2-yl)pentan-1-ol (12). To a stirred solution of benzothiazole (1.2 mmol) in dry diethyl ether (6.5 mL), at −78 °C, under an inert argon atmosphere, a solution of n-BuLi 1M (1.2 mmol) was added dropwise and the reaction mixture was stirred for 1h at −78 °C. A solution of aldehyde 11 (1 mmol) in dry diethyl ether (1.5 mL) was then added, and the reaction mixture was further stirred for 1 h at −78 °C and for 16 h at room temperature. The reaction was quenched with a saturated NH4Cl aqueous solution (5 mL), and the aqueous layer was collected and extracted with diethyl ether (2 x 10 mL). The combined organic layers were washed with brine (10 mL), dried over Na2SO4, and concentrated under reduced pressure. Purification by flash chromatography eluting with a mixture of EtOAc:petroleum ether (40–60 °C) 3:7 afforded the desired product. Yield 55%; Orange solid of low melting point; 1H NMR (400 MHz, CDCl3): δ = 7.98 (d, J = 8.1 Hz, 1H, ArH), 7.91−7.70 (m, 4H, 4 × ArH), 7.59 (s, 1H, ArH), 7.50−7.34 (m, 4H, ArH), 7.30 (d, J = 8.4 Hz, 1H, ArH), 5.10 (dd, J1 = 7.5, J2 = 4.7 Hz, 1H, CHOH), 3.83 (br s, 1H, OH), 2.77 (t, J = 7.6 Hz, 2H, CH2Ar), 2.14−1.93 (m, 2H, CH2), 1.84−1.70 (m, 2H, CH2), 1.70−1.50 (m, 2H, CH2); 13C NMR (100 MHz, CDCl3): δ = 176.8, 152.8, 140.0, 134.8, 133.7, 132.0, 127.9, 127.7, 127.5, 127.4, 126.4, 126.2, 125.9, 125.12, 125.09, 122.9, 121.9, 72.2, 38.0, 35.9, 31.1, 25.0; HRMS (ESI) [M+H]+ m/z: 348.1425; (calculated for [C22H22NOS]+ 348.1417).
General procedure for the synthesis of cyanohydrins 14a,b and 15.
To a stirred solution of aldehydes 13a,b, 11 (1 mmol) in CH2Cl2 (1.3 mL), a solution of NaHSO3 (1.5 mmol, 156 mg) in water (0.3 mL) was added at room temperature. After stirring for 30 min, the organic solvent was concentrated under reduced pressure, water (0.3 mL) was added, and the reaction mixture was cooled to 0 °C. Then, a solution of KCN (1.5 mmol, 98 mg) in water (0.3 mL) was added dropwise over 1 h, and the reaction mixture was left stirring for 16 h. After the completion of the reaction, CH2Cl2 (5 mL) was added to extract the product and the organic layer was washed with brine (10 mL), dried over Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) afforded the desired product.
2-Hydroxy-6-(4-methoxyphenyl)hexanenitrile (14a). Yield 78%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.16 (d, J = 8.0 Hz, 2H, 2 × ArH), 6.90 (d, J = 8.0 Hz, 2H, 2 × ArH), 4.45 (q, J = 6.0 Hz, 1H, CHOH), 4.11 (br s, 1H, OH), 3.82 (s, 3H, OCH3), 2.62 (t, J = 6.0 Hz, 2H, CH2Ar), 1.93−1.83 (m, 2H, CH2), 1.72−1.55 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 157.2, 133.9, 128.9, 119.9, 113.4, 60.6, 54.9, 34.5, 34.3, 30.6, 23.8; MS (ESI) m/z (%): 237.2 [(M+NH4)+, 100].
2-Hydroxy-3-(4-methoxyphenethoxy)propanenitrile (14b). Yield 40%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.14 (d, J = 8.6 Hz, 2H, 2 × ArH), 6.86 (d, J = 8.8 Hz, 2H, 2 × ArH), 4.56−4.45 (m, 1H, CHOH), 3.79 (s, 3H, OCH3), 3.77−3.65 (m, 4H, 2 × CH2), 2.86 (t, J = 6.9 Hz, 2H, CH2); HRMS (ESI) [M+Na]+ m/z: 244.0944; (calculated for [C12H15NNaO3]+ 244.0944).
2-Hydroxy-6-(naphthalen-2-yl)hexanenitrile (15). Yield 45%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.93−7.28 (m, 7H, 7 × ArH), 4.39 (q, J = 6.2 Hz, 1H, CHOH), 3.41 (br s, 1H, OH), 2.81 (t, J = 7.4 Hz, 2H, CH2Ar), 1.82−1.68 (m, 4H, 2 × CH2), 1.59−1.55 (m, 2H, CH2); 13C NMR (50 MHz, CDCl3): δ = 139.4, 133.4, 131.8, 127.8, 127.5, 127.3, 127.1, 126.3, 125.9, 125.1, 120.0, 60.9, 35.6, 34.8, 30.4, 24.1; HRMS (ESI) [M+Na]+ m/z: 262.1202; (calculated for [C16H17NNaO]+ 262.1202).
General procedure for the synthesis of α-hydroxy benzoxazoles (16a,c,d) and α-hydroxy benzimidazoles (16b,e) from cyanohydrins.
To a stirred mixture of chloroform (0.5 M) and absolute ethanol (0.5 M) cooled at 0 °C, under an inert dry argon atmosphere, acetyl chloride (0.46 mL) was added dropwise over 15 min. Then, a solution of cyanohydrins 14a,b and 15 (1 mmol) in CHCl3 (0.5 M) was added and the reaction mixture was stirred at 0 °C for 1 h. The solvent was evaporated under reduced pressure and at a temperature not higher than 25 °C. The reaction mixture was then dissolved in absolute ethanol (1.2 M), 2-aminophenol (for the benzoxazole compounds 16a,c,d) or 2-phenylenediamine (for the benzimidazole compounds 16b,e) (1.1 mmol) was added and the final reaction mixture was refluxed under an inert argon atmosphere for 16 h. After completion of the reaction, the solvent was evaporated under reduced pressure. Purification by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) afforded the desired product.
1-(Benzo[d]oxazol-2-yl)-5-(4-methoxyphenyl)pentan-1-ol (16a). Yield 57%; Pale yellow oil; 1H NMR (200 MHz, CDCl3): δ = 7.73−7.67 (m, 1H, ArH), 7.57−7.48 (m, 1H, ArH), 7.39−7.30 (m, 2H, 2 × ArH), 7.08 (d, J = 8.0 Hz, 2H, 2 × ArH), 6.81 (d, J = 8.0 Hz, 2H, 2 × ArH), 4.97 (t, J = 6.0 Hz, 1H, CHOH), 3.84 (br s, 1H, OH), 3.78 (s, 3H, OCH3), 2.57 (t, J = 6.0 Hz, 2H, CH2Ar), 2.15–1.94 (m, 2H, CH2), 1.73−1.47 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 167.8, 157.5, 150.6, 140.2, 134.3, 129.1, 125.1, 124.4, 119.8, 113.6, 110.7, 67.9, 55.1, 35.3, 34.7, 31.3, 24.5; MS (ESI) m/z (%): 312.2 [(M+H)+, 100].
1-(1H-Benzo[d]imidazol-2-yl)-5-(4-methoxyphenyl)pentan-1-ol (16b). Yield 37%; White solid; mp: 165–167 °C; 1H NMR (200 MHz, CD3OD): δ = 7.58−7.54 (m, 2H, 2 × ArH), 7.25−7.21 (m, 2H, 2 × ArH), 7.03 (d, J = 8.0 Hz, 2H, 2 × ArH), 6.75 (d, J = 8.0 Hz, 2H, 2 × ArH), 4.93 (t, J = 6.0 Hz, 1H, CHOH), 3.73 (s, 3H, OCH3), 2.54 (t, J = 6.0 Hz, 2H, CH2Ar), 2.05−1.91 (m, 2H, CH2), 1.66−1.40 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CD3OD): δ = 159.3, 139.4, 135.8, 135.7, 130.4, 123.4, 115.8, 114.7, 69.5, 55.7, 37.8, 35.8, 32.7, 25.7; MS (ESI) m/z (%): 311.2 [(M+H)+, 100].
1-(Benzo[d]oxazol-2-yl)-2-(4-methoxyphenethoxy)ethan-1-ol (16c). Yield 65%; Pale yellow solid of low melting point; 1H NMR (200 MHz, CDCl3): δ = 7.80−7.69 (m, 1H, ArH), 7.56−7.47 (m, 1H, ArH), 7.38−7.30 (m, 2H, 2 × ArH), 7.03 (d, J = 8.5 Hz, 2H, 2 × ArH), 6.71 (d, J = 8.6 Hz, 2H, 2 × ArH), 5.12 (t, J = 4.9 Hz, 1H, CHOH), 4.01−3.91 (m, 2H, OCH2CH), 3.81−3.59 (m, 5H, OCH3, OCH2), 2.79 (t, J = 6.9 Hz, 2H, CH2); 13C NMR (50 MHz, CDCl3): δ = 165.0, 158.0, 157.6, 140.5, 130.5, 129.7, 125.2, 124.5, 120.1, 113.7, 110.8, 72.7, 72.5, 67.5, 55.1, 35.0; HRMS (ESI) [M+Na]+ m/z: 336.1201; (calculated for [C18H19NNaO4]+ 336.1206).
1-(Benzo[d]oxazol-2-yl)-5-(naphthalen-2-yl)pentan-1-ol (16d). Yield 53%; Orange oil; 1H NMR (200 MHz, CDCl3): δ = 7.94–7.20 (m, 11H, 11 × ArH, 5.08–4.93 (m, 1H, CHOH), 4.45 (br s, 1H, OH), 2.79 (t, J = 7.4 Hz, 2H, CH2Ar), 2.10 (m, 2H, CH2), 1.89−1.49 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 168.0, 150.5, 140.2, 139.8, 133.5, 131.8, 127.7, 127.5, 127.3, 127.2, 126.2, 125.8, 125.1, 125.0, 124.4, 119.8, 110.7, 67.8, 35.8, 35.2, 30.9, 24.7; HRMS (ESI) [M+Na]+ m/z: 354.1466; (calculated for [C22H21NNaO2]+ 354.1465).
1-(1H-Benzo[d]imidazol-2-yl)-5-(naphthalen-2-yl)pentan-1-ol (16e). Yield 43%; White solid; 1H NMR (200 MHz, CDCl3): δ = 7.82−7.03 (m, 11H, 11 × ArH), 4.85 (t, J = 6.6 Hz, 1H, CHOH), 4.41 (br s, 1H, OH), 2.65 (t, J = 7.3 Hz, 2H, CH2Ar), 2.03−1.76 (m, 2H, CH2), 1.74−1.25 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 157.4, 139.8, 137.6, 133.4, 131.7, 127.5, 127.4, 127.2, 127.1, 126.1, 125.6, 124.8, 122.3, 114.7, 68.1, 36.3, 35.7, 30.9, 24.7; HRMS (ESI) [M+H]+ m/z: 331.1805; (calculated for [C22H23N2O]+ 331.1805).
General procedure for the synthesis of O-acyl-amidoximes (20b,c).
To a stirred solution of amidoxime 19 (1.0 mmol) in dry CH2Cl2 (20 mL), benzoic acid (for benzoate group) or isobutyric anhydride (for isobutyrate group) (1 mmol, 102 mg) and N,N′-dicyclohexylcarbodiimide (DCC) (1.1 mmol, 227 mg) were added. The reaction mixture was stirred for 24 h at room temperature. After completion of the reaction the organic solvent was evaporated under reduced pressure. Purification by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) afforded the desired product.
(Z)-N’-(Benzoyloxy)-2-((tert-butyldimethylsilyl)oxy)-6-(4-methoxyphenyl)hexanimidamide (20b). Yield 67%; 1H NMR (200 MHz, CDCl3): δ = 8.08−7.98 (m, 2H, 2 × ArH), 7.60−7.50 (m, 1H, ArH), 7.48−7.37 (m, 2H, 2 × ArH), 7.07 (d, J = 8.6 Hz, 2H, 2 × ArH), 6.79 (d, J = 8.6 Hz, 2H, 2 × ArH), 5.07 (s, 2H, NH2), 4.41 (t, J = 6.2 Hz, 1H, OCH), 3.75 (s, 3H, OCH3), 2.55 (t, J = 7.2 Hz, 2H, CH2Ar), 1.66−1.42 (m, 4H, 2 × CH2), 1.32−1.08 (m, 2H, CH2), 0.90 (s, 9H, 3 × CCH3), 0.09 (s, 6H, 2 × SiCH3); 13C NMR (50 MHz, CDCl3): δ = 164.2, 161.0, 157.8, 134.7, 133.1, 129.9, 129.6, 129.5, 128.6, 113.9, 70.5, 55.4, 37.8, 35.0, 31.7, 25.9, 24.9, 18.3, -4.9; HRMS (ESI) [M+H]+ m/z: 471.2670; (calculated for [C26H39N2O4Si]+ 471.2674).
(Z)-2-((tert-Butyldimethylsilyl)oxy)-N’-(isobutyryloxy)-6-(4-methoxyphenyl)hexanimidamide (20c). Yield 95%; 1H NMR (200 MHz, CDCl3): δ = 7.05 (d, J = 8.5 Hz, 2H, 2 × ArH), 6.79 (d, J = 8.6 Hz, 2H, 2 × ArH), 4.92 (s, 2H, NH2), 4.30 (t, J = 6.2 Hz, 1H, OCH), 3.76 (s, 3H, OCH3), [2.77−2.57 m, 1H, CH(CH3)2], 2.52 (t, J = 7.3 Hz, 2H, CH2Ar), 1.78−1.33 (m, 6H, 3 × CH2), 1.22 (d, J = 7.0 Hz, 6H, 2 × CHCH3), 0.87 (s, 9H, 3 × CCH3), 0.05 (s, 6H, 2 × SiCH3); 13C NMR (50 MHz, CDCl3): δ = 174.1, 160.1, 157.6, 134.6, 129.3, 113.7, 70.2, 55.2, 37.5, 34.8, 33.2, 31.5, 25.7, 24.7, 19.3, 18.0, -5.1; HRMS (ESI) [M+H]+ m/z: 437.2826; (calculated for [C23H41N2O4Si]+ 437.2830).
General procedure for the synthesis of α-hydroxy-oxadiazoles (21b,c).
To a stirred solution of O-acyl-amidoximes 20b,c (1.0 mmol) in dry toluene (3 mL) in a microwave vessel, tetrabutylammonium fluoride (TBAF) (1 M in THF, 1.0 mmol) was added. The reaction mixture was left stirring under microwave irradiation (initial setting at 90 W) for 1 h at 120 °C. The organic solvent was evaporated under reduced pressure. Purification by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) afforded the desired product.
5-(4-Methoxyphenyl)-1-(5-phenyl-1,2,4-oxadiazol-3-yl)pentan-1-ol (21b). Yield 38%; White solid; mp: 88–90 °C; 1H NMR (200 MHz, CDCl3): δ = 8.26−8.05 (m, 2H, 2 × ArH), 7.67−7.46 (m, 3H, 3 × ArH), 7.08 (d, J = 8.6 Hz, 2H, 2 × ArH), 6.80 (d, J = 8.6 Hz, 2H, 2 × ArH), 4.93 (t, J = 6.6 Hz, 1H, CHOH), 3.76 (s, 3H, OCH3), 2.97 (br s, 1H, OH), 2.57 (t, J = 7.3 Hz, 2H, CH2Ar), 2.12−1.91 (m, 2H, CH2), 1.78−1.40 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 176.0, 172.8, 157.7, 134.6, 133.0, 129.3, 129.2, 128.3, 124.1, 113.8, 66.8, 55.3, 35.6, 34.9, 31.5, 24.8; HRMS (ESI) [M+H]+ m/z: 339.1700; (calculated for [C20H23N2O3]+ 339.1703).
1-(5-Isopropyl-1,2,4-oxadiazol-3-yl)-5-(4-methoxyphenyl)pentan-1-ol (21c). Yield 51%; Pale yellow oil; 1H NMR (200 MHz, CDCl3): δ = 7.05 (d, J = 8.7 Hz, 2H, 2 × ArH), 6.79 (d, J = 8.7 Hz, 2H, 2 × ArH), 4.80 (t, J = 6.6 Hz, 1H, CHOH), 3.75 (s, 3H, OCH3), 3.30−3.08 [m, 1H, CH(CH3)2], 2.83 (br s, 1H, OH), 2.54 (t, J = 7.4 Hz, 2H, CH2Ar), 1.98−1.83 (m, 2H, CH2), 1.75−1.47 (m, 4H, 2 × CH2), 1.37 (d, J = 7.0 Hz, 6H, 2 × CHCH3); 13C NMR (50 MHz, CDCl3): δ = 184.3, 171.9, 157.7, 134.6, 129.3, 113.8, 66.7, 55.3, 35.5, 34.9, 31.4, 27.6, 24.8, 20.2; HRMS (ESI) [M+H]+ m/z: 305.1858; (calculated for [C17H25N2O3]+ 305.1860).
General procedure for the oxidation of secondary alcohols to ketones (17a–e, 22b,c).
To a stirred solution of α-hydroxy-heterocyclic compounds 16a–e and 21b,c (1 mmol) in dry CH2Cl2 (0.2 M), under an inert argon atmosphere, Dess–Martin periodinane was added (1.3 mmol, 551 mg). The reaction mixture was stirred for 1 h and after completion of the reaction the solvent was evaporated under reduced pressure and Et2O (30 mL) was added. The organic phase was washed with saturated aqueous NaHCO3 (20 mL) containing Na2S2O3 (1.5 g, 9.5 mmol), H2O (20 mL), dried over Na2SO4, and the organic solvent was evaporated under reduced pressure. Purification by flash chromatography eluting with the appropriate mixture of EtOAc:petroleum ether (40–60 °C) afforded the desired product.
1-(Benzo[d]oxazol-2-yl)-5-(4-methoxyphenyl)pentan-1-one (17a). Yield 88%; White solid; mp: 59–61 °C; 1H NMR (200 MHz, CDCl3): δ = 7.90 (d, J = 7.4 Hz, 1H, ArH), 7.66 (d, J = 7.6 Hz, 1H, ArH), 7.59–7.38 (m, 2H, 2 × ArH), 7.11 (d, J = 8.5 Hz, 2H, 2 × ArH), 6.82 (d, J = 8.5 Hz, 2H, 2 × ArH), 3.77 (s, 3H, OCH3), 3.24 (t, J = 7.1 Hz, 2H, CH2CO), 2.63 (t, J = 7.3 Hz, 2H, CH2Ar), 1.95–1.60 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 190.2, 157.7, 157.2, 150.7, 140.5, 134.1, 129.3, 128.5, 125.8, 122.3, 113.7, 112.0, 55.3, 39.4, 34.7, 31.1, 23.4; HRMS (ESI) [M-H]- m/z: 308.1291; (calculated for [C19H18NO3]- 308.1292).
1-(1H-Benzo[d]imidazol-2-yl)-5-(4-methoxyphenyl)pentan-1-one (17b). Yield 79%; Colorless solid; mp: 101–103 °C; 1H NMR (200 MHz, CDCl3): δ = 10.64 (br s, 1H, NH), 8.00−7.82 (m, 1H, ArH), 7.63−7.31 (m, 3H, 3 × ArH), 7.10 (d, J = 8.4 Hz, 2H, 2 × ArH), 6.81 (d, J = 8.6 Hz, 2H, 2 × ArH), 3.77 (s, 3H), 3.34 (t, J = 7.2 Hz, 2H, CH2CO), 2.62 (t, J = 7.3 Hz, 2H, CH2Ar), 1.96−1.62 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 194.7, 157.8, 151.1, 147.6, 134.3, 129.4, 126.6, 124.0, 122.0, 114.1, 113.8, 112.3, 55.4, 38.2, 34.9, 31.3, 23.7; HRMS (ESI) [M-H]− m/z: 307.1455; (calculated for [C19H19N2O2]- 308.1292).
1-(Benzo[d]oxazol-2-yl)-2-(4-methoxyphenethoxy)ethan-1-one (17c). Yield 35%; Pale yellow solid of low melting point; 1H NMR (500 MHz, CDCl3): δ = 7.87 (d, J = 8.0 Hz, 1H, ArH), 7.66 (d, J = 8.2 Hz, 1H, ArH), 7.55 (t, J = 7.8 Hz, 1H, ArH), 7.47 (t, J = 7.8 Hz, 1H, ArH), 7.18 (d, J = 8.5 Hz, 2H, 2 × ArH), 6.83 (d, J = 8.3 Hz, 2H, 2 × ArH), 5.01 (s, 2H, OCH2CO), 3.84 (t, J = 7.0 Hz, 2H, OCH2), 3.77 (s, 3H, OCH3), 2.97 (t, J = 7.0 Hz, 2H, CH2Ar); 13C NMR (125 MHz, CDCl3): δ = 186.4, 158.3, 155.5, 150.6, 140.4, 130.4, 130.0, 128.9, 126.1, 122.4, 114.0, 112.1, 73.8, 73.4, 55.4, 35.4; IR: = 3091, 1718, 1612, 1515, 1453 cm-1; HRMS (ESI) [M+H]+ m/z: 312.1230; (calculated for [C18H18NO4]+ 312.1230); HRMS (ESI) [M+Na]+ m/z: 334.1051; (calculated for [C18H17NNaO4]+ 334.1050).
1-(Benzo[d]oxazol-2-yl)-5-(naphthalen-2-yl)pentan-1-one (17d). Yield 96%; White solid; 77−82 °C; 1H NMR (400 MHz, CDCl3): δ = 7.89 (d, J = 8.0 Hz, 1H, ArH), 7.84–7.72 (m, 3H, 3 × ArH), 7.69−7.60 (m, 2H, 2 × ArH), 7.56−7.38 (m, 4H, 4 × ArH), 7.34 (dd, J1 = 8.4, J2 = 1.4 Hz, 1H, ArH), 3.27 (t, J = 7.0 Hz, 2H, CH2CO), 2.86 (t, J = 7.2 Hz, 2H, CH2Ar), 1.98−1.79 (m, 4H, 2 × CH2); 13C NMR (100 MHz, CDCl3): δ = 190.1, 157.3, 150.8, 140.6, 139.6, 133.7, 132.1, 128.6, 128.0, 127.7, 127.5, 127.3, 126.5, 126.0, 125.8, 125.2, 122.3, 112.0, 39.4, 35.8, 30.7, 23.6; IR: = 3049, 1701, 1601, 1534, 1506 cm-1; HRMS (ESI) [M+H]+ m/z: 330.1499; (calculated for [C22H20NO2]+ 330.1489).
1-(1H-Benzo[d]imidazol-2-yl)-5-(naphthalen-2-yl)pentan-1-one (17e). Yield 45%; White solid; mp: 127−132 °C; 1H NMR (400 MHz, CDCl3): δ = 10.24 (s, 1H, NH), 7.91 (d, J = 8.0 Hz, 1H, ArH), 7.82–7.73 (m, 3H, 3 × ArH), 7.62 (s, 1H, ArH), 7.53 (d, J = 7.9 Hz, 1H, ArH), 7.46−7.31 (m, 5H, 5 × ArH), 3.35 (t, J = 6.9 Hz, 2H, CH2CO), 2.85 (t, J = 7.2 Hz, 2H, CH2Ar), 1.96−1.80 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 194.4, 147.6, 143.5, 139.8, 133.7, 133.5, 132.1, 128.0, 127.7, 127.5, 127.4, 126.63, 126.56, 126.0, 125.2, 124.0, 122.1, 112.2, 38.2, 35.9, 30.9, 23.7; IR: = 3286, 3055, 1679, 1598, 1512, 1401 cm−1; HRMS (ESI) [M+H]+ m/z: 329.1655; (calculated for [C22H21N2O]+ 329.1648).
5-(4-Methoxyphenyl)-1-(5-phenyl-1,2,4-oxadiazol-3-yl)pentan-1-one (22b). Yield 77%; White solid; 85−87 °C; 1H NMR (200 MHz, CDCl3): δ = 8.30−8.13 (m, 2H, 2 × ArH), 7.72−7.46 (m, 3H, 3 × ArH), 7.10 (d, J = 8.6 Hz, 2H, 2 × ArH), 6.82 (d, J = 8.7 Hz, 2H, 2 × ArH), 3.78 (s, 3H, OCH3), 3.14 (t, J = 7.1 Hz, 2H, CH2CO), 2.62 (t, J = 7.3 Hz, 2H, CH2Ar), 1.93–1.62 (m, 4H, 2 × CH2); 13C NMR (50 MHz, CDCl3): δ = 191.8, 177.2, 166.2, 157.9, 134.1, 133.6, 129.4, 128.6, 123.5, 113.9, 55.3, 40.7, 34.8, 31.1, 23.2; HRMS (ESI) [M+Na]+ m/z: 359.1363; (calculated for [C20H20N2NaO3]+ 359.1366).
1-(5-Isopropyl-1,2,4-oxadiazol-3-yl)-5-(4-methoxyphenyl)pentan-1-one (22c). Yield 45%; Colorless oil; 1H NMR (200 MHz, CDCl3): δ = 7.09 (d, J = 8.4 Hz, 2H, 2 × ArH), 6.81 (d, J = 8.4 Hz, 2H, 2 × ArH), 3.78 (s, 3H, OCH3), 3.38−3.21 [m, 1H, CH(CH3)2], 3.07 (t, J = 7.0 Hz, 2H, CH2CO), 2.59 (t, J = 7.2 Hz, 2H, CH2Ar), 1.84–1.61 (m, 4H, 2 × CH2), 1.44 (d, J = 7.0 Hz, 6H, 2 × CHCH3); 13C NMR (50 MHz, CDCl3): δ = 191.9, 185.7, 165.6, 157.9, 134.2, 129.4, 113.9, 55.4, 40.5, 34.8, 31.1, 27.7, 23.2, 20.2; HRMS (ESI) [M+Na]+ m/z: 325.1521; (calculated for [C17H22N2NaO3]+ 325.1523).