Understanding Emotions: Origins and Roles of the Amygdala
Abstract
:1. Introduction
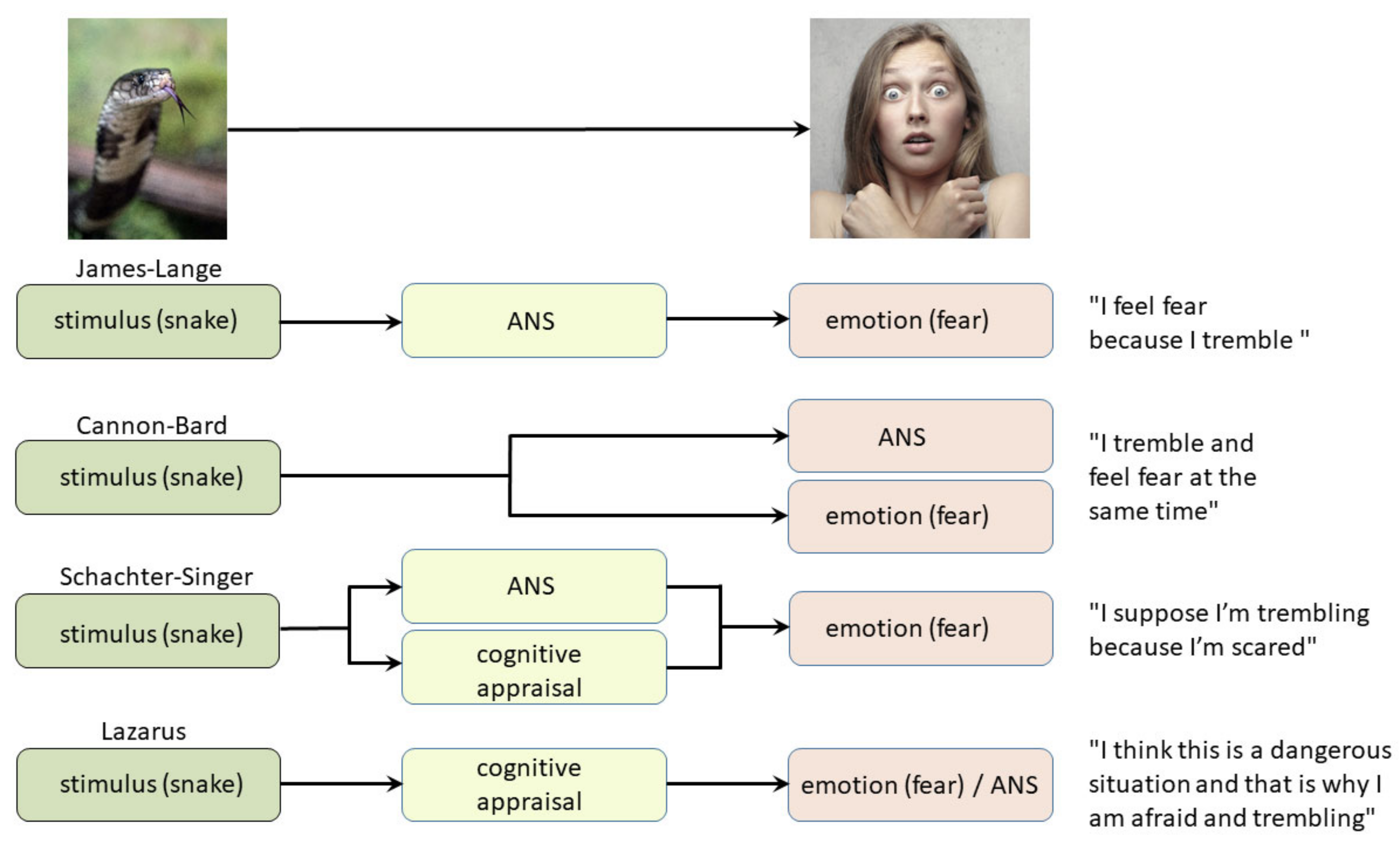
2. Classical Theories of Emotion
2.1. Contemporary Theories of Emotions
2.1.1. Somatic Marker Hypothesis—Interoceptive Theory of Emotions
2.1.2. Theory of Constructed Emotion
2.1.3. Higher-Order Theory of Consciousness and Fear Conditioning
3. The Structure of the Amygdala
3.1. The Lateral Nucleus (LA)
3.2. The Basolateral Nucleus (BLA)
3.3. The Basomedial Nucleus (BM)
3.4. The Amygdalohippocampal Area
3.5. The Paralaminar Nucleus (PL)
3.6. The Intercalated Neurons (IN)
3.7. The Central Nucleus (CE)
3.8. The Medial Nucleus (ME)
3.9. The Cortical Nucleus (Co)
3.10. The Periamygdaloid (Prepiriform) Cortex
4. Connections of the Amygdala
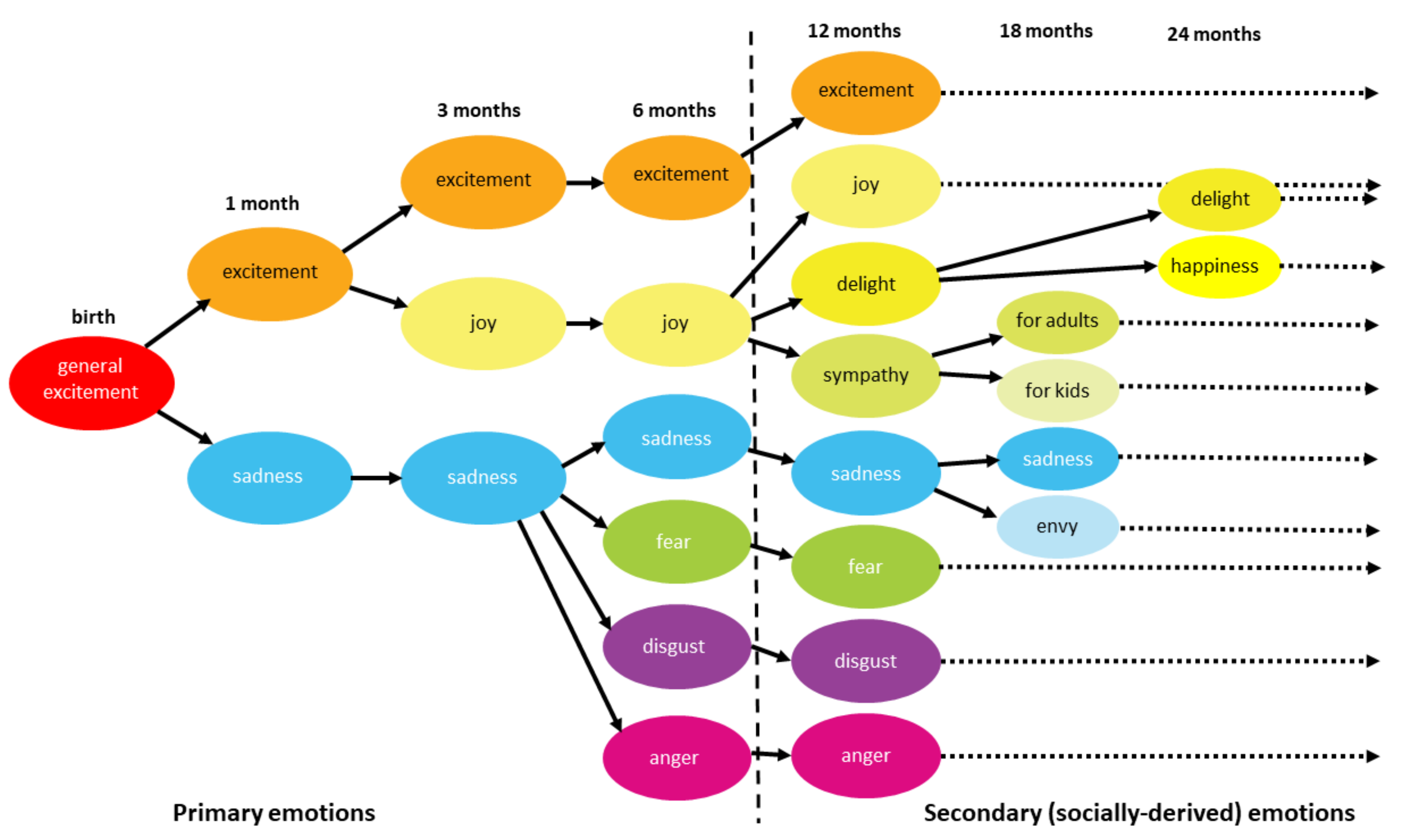
5. Fetal Development of the Amygdala in Human
6. Damage to the Amygdala and Klüver–Bucy Syndrome
7. Emergence of Individual Emotions in the Amygdala
7.1. Aggression
7.2. Fear
8. The Amygdala and Anxiety Disorders
8.1. Generalized Anxiety Disorder
8.2. Social Phobias
8.3. Post-Traumatic Stress Disorder
8.4. Panic Disorder
9. The Role of the Amygdala in Consumption and Negative Effects of Alcohol
10. The Influence of the Amygdala on the Brain Reward System
11. Short Description of Clinical Cases Presenting with Disturbed Emotional Experience and Behavior
12. The Role of the Amygdala in Sensation Seeking, Psychosis, Major Depression and Other Psychiatric Disorders
13. Decision-Making and Interdependence of Emotion and Cognition
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A (AMY)—amygdala |
| 5-HT—5-hydroxytryptamine (serotonin) |
| ACC—anterior cingulate cortex |
| AMPAR—α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors |
| ANS—autonomic nervous system |
| AP-1—transcription factor activating protein 1 |
| ATP/Ado—adenosine triphosphate/adenosine |
| BA—Brodmann’s area |
| BF—basal forebrain |
| BLA—basolateral nucleus of amygdala |
| BNST—bed nucleus of stria terminalis |
| BPD—borderline personality disorder |
| CE—central nucleus of amygdala |
| CN—caudate nucleus |
| CNS—central nervous system |
| Co—cortical nucleus of amygdala |
| CPRN—caudal pontine reticular nucleus |
| CRH/CRF—corticotropin releasing hormone/factor |
| CS—conditioned stimulus |
| DA—dopamine |
| dlPFC—dorsolateral prefrontal cortex |
| dmPFC—dorsomedial prefrontal cortex |
| DRN—dorsal raphe nucleus |
| DSM-5—Diagnostic and Statistica Manual of mental disorders, 5th revision |
| DTN—dorsal tegmental nucleus |
| EC—entorhinal cortex |
| EEG—electroencephalogram |
| FLAIR—fluid attenuated inversion recovery MRI sequence |
| fMRI—functional magnetic resonance imaging |
| GABA—gamma (γ) aminobutyric acid |
| GAD—generalized anxiety disorder |
| H—hippocampus |
| HF—hippocampal formation |
| IN—intercalated neurons of the amygdala |
| ICD-10—International Classification of Diseases, 10th revision |
| LA—lateral nucleus of amygdala |
| LC—locus coeruleus |
| LH—lateral hypothalamus |
| lPFC—lateral prefrontal cortex |
| LTD—long-term depression |
| LTP—long-term potentiation |
| MDD—major depressive disorder |
| MDMA—3,4-methylenedioxymethamphetamine (ecstasy) |
| ME—medial nucleus of amygdala |
| MGN—medial geniculate nucleus of thalamus |
| mPFC—medial prefrontal cortex |
| NAc—nucleus accumbens septi |
| NAc MNS—medium spiny neurons of NAc |
| N. V—trigeminal nerve |
| N. VII—facial nerve |
| NMDAR—N-methyl-D-aspartate receptors |
| OFC—orbitofrontal cortex |
| OXT—oxytocin |
| P—putamen |
| PAG—periaqueductal gray |
| PBN—parabrachial nuclei |
| PCC—posterior cingulate cortex |
| PL—paralaminar nucleus |
| PNS—peripheral nervous system |
| PTSD—post-traumatic stress disorder |
| PVN—periventricular nucleus |
| rmPFC—rostromedial prefrontal cortex |
| RMTg—rostromedial tegmental nucleus |
| BDNF—brain-derived neurotrophic factor |
| DTI—diffusion tensor imaging |
| SNc—substantia nigra, pars compacta |
| TBI—traumatic brain injury |
| UC—unconditioned stimulus |
| vlPFC—ventrolateral prefrontal cortex |
| vmPFC—ventromedial prefrontal cortex |
| VPL—ventroposterolateral nucleus of thalamus |
| VPM—ventroposteromedial nucleus of thalamus |
| VTA—ventral tegmental area |
References
- Vingerhoets, A.; Nykliček, I.; Denollett, J. Emotion Regulation: Conceptual and Clinical Issues; Springer: New York, NY, USA, 2008. [Google Scholar]
- Gračanin, A.; Kardum, I. Primary emotions as modular mechanisms of the human mind. In Brain and Mind: A Lasting Challenge; Žebec, M.S., Sabol, G., Šakić, M., Topić, M.K., Eds.; Institute of Social Sciences “Ivo Pilar”: Zagreb, Croatia, 2006; pp. 89–103. [Google Scholar]
- Fox, E. Emotion Science; J.B. Metzler: Stuttgart, Germany, 2008. [Google Scholar]
- Adolphs, R.; Anderson, D.J. The Neuroscience of Emotion: A New Synthesis; Princeton University Press: Princeton, NJ, USA, 2018. [Google Scholar]
- Ekman, P. An argument for basic emotions. Cogn. Emot. 1992, 6, 169–200. [Google Scholar] [CrossRef]
- Keltner, D.; Ekman, P. Facial expression of emotion. In Handbook of Emotions, 2nd ed.; Lewis, M., Haviland-Jones, J., Eds.; Guilford Publications: New York, NY, USA, 2000; pp. 236–249. [Google Scholar]
- Keltner, D.; Ekman, P.; Gonzaga, G.C.; Beer, J. Facial expression of emotion. In Handbook of Affective Sciences; Davidson, R.J., Scherer, K.R., Goldsmith, H.H., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 415–432. [Google Scholar]
- Matsumoto, D.; Ekman, P. American-Japanese cultural differences in intensity ratings of facial expressions of emotion. Motiv. Emot. 1989, 13, 143–157. [Google Scholar] [CrossRef]
- Wikimedia Commons: Images. Available online: https://commons.wikimedia.org/wiki/Category:Images (accessed on 25 April 2021).
- Šimić, G.; Vukić, V.; Kopić, J.; Krsnik, Ž.; Hof, P.R. Molecules, mechanisms, and disorders of self-domestication: Keys for un-derstanding emotional and social communication from an evolutionary perspective. Biomolecules 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. The Expression of the Emotions in Man and Animals; Murray: London, UK, 1872. [Google Scholar]
- Cosmides, L.; Tooby, J. Evolutionary psychology and the emotions. In Handbook of Emotions; Lewis, M., Haviland-Jones, J.M., Eds.; The Guilford Press: New York, NY, USA, 2000. [Google Scholar]
- Dolan, R.J. Emotion, Cognition, and Behavior. Science 2002, 298, 1191–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shawaf, L.; Conroy-Beam, D.; Asao, K.; Buss, D.M. Human Emotions: An Evolutionary Psychological Perspective. Emot. Rev. 2015, 8, 173–186. [Google Scholar] [CrossRef]
- Sergi, G. Principi di Psicologie: Dolore e Piacere. Storia Naturale dei Sentimenti; Dumolard, F., Ed.; Librai Della Real Casa: Milan, Italy, 1894. [Google Scholar]
- Lange, C. The Emotions; Dunlap, E., Ed.; Williams & Wilkins: Baltimore, MA, USA, 1885. [Google Scholar]
- James, W. What is an emotion? Mind 1884, 34, 188–205. [Google Scholar] [CrossRef]
- Wickens, A. Introduction to Biopsychology; Pearson: Harlow, UK, 2009. [Google Scholar]
- Damasio, A.R. The Feeling of What Happens. Body and Emotion in Making of Consciousness; Heinemann: London, UK, 1999. [Google Scholar]
- Damasio, A.R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 1413–1420. [Google Scholar] [CrossRef]
- Dunn, B.D.; Dalgleish, T.; Lawrence, A.D. The somatic marker hypothesis: A critical evaluation. Neurosci. Biobehav. Rev. 2006, 30, 239–271. [Google Scholar] [CrossRef] [PubMed]
- Horoufchin, H.; Bzdok, D.; Buccino, G.; Borghi, A.M.; Binkofski, F. Action and object words are differentially anchored in the sensory motor system—A perspective on cognitive embodiment. Sci. Rep. 2018, 8, 6583. [Google Scholar] [CrossRef] [PubMed]
- Barbalet, J.M. William James’ Theory of Emotions: Filling in the Picture. J. Theory Soc. Behav. 1999, 29, 251–266. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, A.R. The somatic marker hypothesis: A neural theory of economic decision. Games Econ. Behav. 2005, 52, 336–372. [Google Scholar] [CrossRef]
- Eshafir, T.; Tsachor, R.P.; Welch, K.B. Emotion Regulation through Movement: Unique Sets of Movement Characteristics are Associated with and Enhance Basic Emotions. Front. Psychol. 2016, 6, 2030. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.B.; Bowler, P.J. Botulinum toxin cosmetic therapy correlates with a more positive mood. J. Cosmet. Dermatol. 2009, 8, 24–26. [Google Scholar] [CrossRef]
- Coles, N.A.; Larsen, J.T.; Lench, H.C. A meta-analysis of the facial feedback literature: Effects of facial feedback on emotional experience are small and variable. Psychol. Bull. 2019, 145, 610–651. [Google Scholar] [CrossRef]
- Ansfield, M.E. Smiling When Distressed: When a Smile Is a Frown Turned Upside Down. Pers. Soc. Psychol. Bull. 2007, 33, 763–775. [Google Scholar] [CrossRef]
- Kraft, T.L.; Pressman, S.D. Grin and bear it: The influence of manipulated facial expression on the stress response. Psychol. Sci. 2012, 23, 1372–1378. [Google Scholar] [CrossRef]
- Ekman, P.; Levenson, R.W.; Friesen, W.V. Autonomic nervous system activity distniguishes among emotions. Science 1983, 221, 1208–1210. [Google Scholar] [CrossRef] [Green Version]
- Harro, J.; Vasar, E. Cholecystokinin-induced anxiety: How is it reflected in studies on exploratory behavior? Neurosci. Biobehav. Rev. 1991, 15, 473–477. [Google Scholar] [CrossRef]
- Sears, R.M.; Fink, A.E.; Wigestrand, M.B.; Farb, C.R.; de Lecea, L.; Ledoux, J.E. Orexin/hypocretin system modulates amyg-dala-dependent threat learning through the locus coeruleus. Proc. Natl. Acad. Sci. USA 2013, 110, 20260–20265. [Google Scholar] [CrossRef] [Green Version]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Cannon, W.B.; Britton, S.W. Studies on the conditions of activity in endocrine glands: XV. Pseudaffective medulliadrenal se-cretion. Am. J. Physiol. 1925, 72, 283–294. [Google Scholar] [CrossRef]
- Bard, P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am. J. Physiol. Content 1928, 84, 490–515. [Google Scholar] [CrossRef]
- Bard, P. On emotional expression after decortication with some remarks on certain theoretical views: Part, I. Psychol. Rev. 1934, 41, 309–329. [Google Scholar] [CrossRef]
- Bard, P.; Rioch, D.M. A study of four cats deprived of neocortex and additional portions of the forebrain. Bull. Johns Hopkins Hosp. 1937, 60, 73–147. [Google Scholar]
- Breedlowe, S.; Watson, N.; Rosenzweig, M. Biological Psychology: An. Introduction to Behavioral, Cognitive, and Clinical Neuroscience, 7th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Akert, K. Walter Rudolf Hess (1881–1973) and His Contribution to Neuroscience. J. Hist. Neurosci. 1999, 8, 248–263. [Google Scholar] [CrossRef]
- Panksepp, J. Toward a general psychobiological theory of emotions. Behav. Brain Sci. 1982, 5, 407–422. [Google Scholar] [CrossRef]
- Panksepp, J.; Zellner, M.R. Towards a neurobiologically based unified theory of aggression. Rev. Int. Psychol. Soc. 2004, 17, 37–62. [Google Scholar]
- Mineka, S.; Keir, R.; Price, V. Fear of snakes in wild- and laboratory-reared rhesus monkeys (Macaca mulatta). Learn. Behav. 1980, 8, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Schachter, S.; Singer, J. Cognitive, social, and physiological determinants of emotional state. Psychol. Rev. 1962, 69, 379–399. [Google Scholar] [CrossRef]
- Young, P.T.; Arnold, M.B. Emotion and Personality. Am. J. Psychol. 1963, 76, 516. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.A.; Ellsworth, P.C. Patterns of cognitive appraisal in emotion. J. Pers. Soc. Psychol. 1985, 48, 813–838. [Google Scholar] [CrossRef]
- Ellsworth, P.C. Appraisal Theory: Old and New Questions. Emot. Rev. 2013, 5, 125–131. [Google Scholar] [CrossRef]
- Lazarus, R.S. Thoughts on the relations between emotion and cognition. Am. Psychol. 1982, 37, 1019–1024. [Google Scholar] [CrossRef]
- Unsplashed.com: Free Picture. Available online: http://ww1.unsplashed.com/ (accessed on 25 April 2021).
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Bechara, A.; Tranel, D.; Damasio, H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000, 123, 2189–2202. [Google Scholar] [CrossRef] [Green Version]
- Lebel, C.; Walker, L.; Leemans, A.; Phillips, L.; Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage 2008, 40, 1044–1055. [Google Scholar] [CrossRef]
- Burnett, S.; Blakemore, S.-J. The Development of Adolescent Social Cognition. Ann. N. Y. Acad. Sci. 2009, 1167, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Hiser, J.; Koenigs, M. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol. Psychiatry 2018, 83, 638–647. [Google Scholar] [CrossRef]
- Reimann, M.; Bechara, A. The somatic marker framework as a neurological thory of decision-making: Review, conceptual comparisons, and future. J. Econ. Psychol. 2010, 31, 767–776. [Google Scholar] [CrossRef]
- Damasio, A.R. Descartes’ Error: Emotion, Reason, and the Human Brain; Grosset/Putnam: New York, NY, USA, 1994. [Google Scholar]
- Damasio, A.R. William James and the modern neurobiology of emotion. In Emotion, Evolution, and Rationality; Evans, D., Cruse, P., Eds.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Damasio, A.R.; Carvalho, G.B. The nature of feelings: Evolutionary and neurobiological origins. Nat. Rev. Neurosci. 2013, 14, 143–152. [Google Scholar] [CrossRef]
- Gu, X.; Hof, P.R.; Friston, K.J.; Fan, J. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 2013, 521, 3371–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagan, S.E.; Kofler, L.; Riccio, S.; Gao, Y. Somatic marker production deficits do not explain the relationship between psy-chopathic traits and utilitarian moral decision making. Brain Sci. 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Feldman Barrett, L. The theory of constructed emotion: An active interference account of interoception and categorization. Soc. Cogn. Affect. Neurosci. 2017, 12, 1–23. [Google Scholar]
- Barrett, L.F.; Satpute, A.B. Historical pitfalls and new directions in the neuroscience of emotion. Neurosci. Lett. 2019, 693, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Feldman Barrett, L. How Emotions are Made. The Secret Life of the Brain; Houghton Mifflin Harcourt: Boston, MA, USA, 2017. [Google Scholar]
- Barrett, L.F. Emotions as natural kinds? Perspect. Psychol. Sci. 2006, 1, 28–58. [Google Scholar] [CrossRef]
- Adolphs, R.; Gosselin, F.; Buchanan, T.W.; Tranel, D.; Schyns, P.; Damasio, A.R. A mechanism for impaired fear recognition after amygdala damage. Nat. Cell Biol. 2005, 433, 68–72. [Google Scholar] [CrossRef]
- Feinstein, J.S.; Buzza, C.; Hurlemann, R.; Follmer, R.L.; Dahdaleh, N.S.; Coryell, W.; Welsh, M.; Tranel, D.; Wemmie, J.A. Fear and panic in humans with bilateral amygdala damage. Nat. Neurosci. 2013, 16, 270–272. [Google Scholar] [CrossRef] [Green Version]
- Celeghin, A.; Diano, M.; Bagnis, A.; Viola, M.; Tamietto, M. Basic Emotions in Human Neuroscience: Neuroimaging and Beyond. Front. Psychol. 2017, 8, 1432. [Google Scholar] [CrossRef] [Green Version]
- Sterling, P. Allostasis: A model of predictive regulation. Physiol. Behav. 2012, 106, 5–15. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Ohira, H. Predictive processing of interoception, decision-making, and allostasis: A computational framework and implications for emotional intelligence. Psychol. Top. 2020, 29, 1–16. [Google Scholar] [CrossRef]
- LeDoux, J. Rethinking the Emotional Brain. Neuron 2012, 73, 653–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeDoux, J.E. Anxious: Using the Brain to Understand and Treat. Fear and Anxiety; Penguin Boks: New York, NY, USA, 2015. [Google Scholar]
- LeDoux, J.E. Semantics, Surplus Meaning, and the Science of Fear. Trends Cogn. Sci. 2017, 21, 303–306. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Synaptic Self; Penguin Books: New York, NY, USA, 2002. [Google Scholar]
- LeDoux, J.E. The Emotional Brain; Simon and Schuster: New York, NY, USA, 1996. [Google Scholar]
- Brown, R.; Lau, H.; LeDoux, J.E. Understanding the Higher-Order Approach to Consciousness. Trends Cogn. Sci. 2019, 23, 754–768. [Google Scholar] [CrossRef]
- Schlitz, K.; Witzel, J.; Northoff, G.; Zierhut, K.; Gubka, U.; Fellmann, H.; Kaufmann, J.; Tempelmann, C.; Wiebking, C.; Bogerts, B. Brain pathology in pedophilic offenders: Evidence of volume reduction in the right amygdala and related diencephalic structures. Arch. Gen. Psychiatry. 2007, 64, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Pitkänen, A.; Savander, V.; LeDoux, J.E. Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997, 20, 517–523. [Google Scholar] [CrossRef]
- Luo, Q.; Holroyd, T.; Majestic, C.; Cheng, X.; Schechter, J.; Blair, R.J. Emotional automaticity is a matter of timing. J. Neurosci. 2010, 30, 5825–5829. [Google Scholar] [CrossRef] [Green Version]
- Stock, J.V.D.; Tamietto, M.; Sorger, B.; Pichon, S.; Grezes, J.; de Gelder, B. Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Proc. Natl. Acad. Sci. USA 2011, 108, 16188–16193. [Google Scholar] [CrossRef] [Green Version]
- Pourtois, G.; Schettino, A.; Vuilleumier, P. Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biol. Psychol. 2013, 92, 492–512. [Google Scholar] [CrossRef] [Green Version]
- Shackman, A.J.; Fox, A.S. Contributions of the Central Extended Amygdala to Fear and Anxiety. J. Neurosci. 2016, 36, 8050–8063. [Google Scholar] [CrossRef]
- Tamietto, M.; de Gelder, B. Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 2010, 11, 697–709. [Google Scholar] [CrossRef]
- Öhman, A. Automaticity and the Amygdala: Nonconscious Responses to Emotional Faces. Curr. Dir. Psychol. Sci. 2002, 11, 62–66. [Google Scholar] [CrossRef]
- Bornemann, B.; Winkielman, P.; Van Der Meer, E. Can you feel what you do not see? Using internal feedback to detect briefly presented emotional stimuli. Int. J. Psychophysiol. 2012, 85, 116–124. [Google Scholar] [CrossRef]
- Inman, C.S.; Bijanki, K.R.; Bass, D.I.; Gross, R.E.; Hamann, S.; Willie, J.T. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychol. 2020, 145, 106722. [Google Scholar] [CrossRef]
- Anderson, A.K.; Phelps, E.A. Is the Human Amygdala Critical for the Subjective Experience of Emotion? Evidence of Intact Dispositional Affect in Patients with Amygdala Lesions. J. Cogn. Neurosci. 2002, 14, 709–720. [Google Scholar] [CrossRef]
- Swanson, L.W.; Petrovich, G.D. What is the amygdala? Trends Neurosci. 1998, 21, 323–331. [Google Scholar] [CrossRef]
- Heimer, L.; De Olmos, J.; Alheid, G.; Pearson, J.; Sakamoto, N.; Shinoda, K.; Marksteiner, J.; Switzer, R. The human basal forebrain. Part II. In Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 1999; pp. 57–226. [Google Scholar]
- Amaral, D.G.; Price, J.L.; Pitkänen, A.; Carmichael, S.T. Anatomical organization of the primate amygdaloid complex. In The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction; Aggleton, J.P., Ed.; Wiley-Liss: New York, NY, USA, 1992; pp. 1–66. [Google Scholar]
- Price, J.L.; Russchen, F.T.; Amaral, D.G. The limbic region: II. The amygdaloid complex. In Handbook of Chemical Neuroanatomy; Vol. Integrated Systems of the CNS (Part, I); Bjorklund, A., Hokfelt, T., Swanson, L.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 279–388. [Google Scholar]
- Gloor, P. The amygdaloid system. In The Temporal Lobe and Limbic System; Gloor, P., Ed.; Oxford University Press: New York, NY, USA, 1997; pp. 591–721. [Google Scholar]
- Barger, N.; Stefanacci, L.; Semendeferi, K. A comparative volumetric analysis of the amygdaloid complex and basolateral division in the human and ape brain. Am. J. Phys. Anthr. 2007, 134, 392–403. [Google Scholar] [CrossRef]
- Schumann, C.M.; Amaral, D.G. Stereological estimation of the number of neurons in the human amygdaloid complex. J. Comp. Neurol. 2005, 491, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Pitkänen, A.; Amaral, D.G. Demonstration of projections from the lateral nucleus to the basal nucleus of the amygdala: A PHA-L study in the monkey. Exp. Brain Res. 1991, 83, 465–470. [Google Scholar] [CrossRef]
- Aggleton, J.P. A description of intra-amygdaloid connections in old world monkeys. Exp. Brain Res. 1985, 57, 390–399. [Google Scholar] [CrossRef]
- Pitkänen, A.; Kemppainen, S. Comparison of the distribution of calcium-binding proteins and intrinsic connectivity in the lateral nucleus of the rat, monkey, and human amygdala. Pharmacol. Biochem. Behav. 2002, 71, 369–377. [Google Scholar] [CrossRef]
- Smith, Y.; Paré, D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J. Comp. Neurol. 1994, 342, 232–248. [Google Scholar] [CrossRef]
- Agoglia, A.E.; Herman, M.A. The center of the emotional universe: Alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018, 72, 61–73. [Google Scholar] [CrossRef]
- AbuHasan, Q.; Reddy, V.; Siddiqui, W. Neuroanatomy, Amygdala; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Braak, H.; Braak, E. Neuronal types in the basolateral amygdaloid nuclei of man. Brain Res. Bull. 1983, 11, 349–365. [Google Scholar] [CrossRef]
- Spampanato, J.; Polepalli, J.; Sah, P. Interneurons in the basolateral amygdala. Neuropharmacology 2011, 60, 765–773. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nat. Cell Biol. 2015, 517, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Sangha, S.; Diehl, M.M.; Bergstrom, H.C.; Drew, M.R. Know safety, no fear. Neurosci. Biobehav. Rev. 2020, 108, 218–230. [Google Scholar] [CrossRef]
- Stefanacci, L.; Amaral, D.G. Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. J. Comp. Neurol. 2002, 451, 301–323. [Google Scholar] [CrossRef]
- Cho, Y.T.; Ernst, M.; Fudge, J.L. Cortico-Amygdala-Striatal Circuits Are Organized as Hierarchical Subsystems through the Primate Amygdala. J. Neurosci. 2013, 33, 14017–14030. [Google Scholar] [CrossRef] [Green Version]
- Ressler, R.L.; Maren, S. Synaptic encoding of fear memories in the amygdala. Curr. Opin. Neurobiol. 2019, 54, 54–59. [Google Scholar] [CrossRef]
- Lee, S.-C.; Amir, A.; Haufler, D.; Pare, D. Differential Recruitment of Competing Valence-Related Amygdala Networks during Anxiety. Neuron 2017, 96, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Sah, P. Fear, anxiety and amygdala. Neuron 2017, 96, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, J.-Z. From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits. Front. Neural Circuits 2017, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, J.; Amaral, D. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1981, 1, 1242–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsini, C.A.; Maren, S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev. 2012, 36, 1773–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitkänen, A.; Amaral, D.G. Organization of the intrinsic connections of the monkey amygdaloid complex: Projections origi-nating in the lateral nucleus. J. Comp. Neurol. 1998, 398, 431–458. [Google Scholar] [CrossRef]
- Amaral, D.G.; Insausti, R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp. Brain Res. 1992, 88, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Aosaki, T.; Kubota, Y. Cholinergic and GABAergic interneurons in the striatum. Nihon Shinkei Seishin Yakurigaku-Zasshi (Jpn. J. Psychopharmacol.) 1997, 17, 87–90. [Google Scholar] [CrossRef]
- Bauman, M.D.; Amaral, D.G. The distribution of serotonergic fibers in the macaque monkey amygdala: An immunohisto-chemical study using antisera to 5-hydroxytryptamine. Neuroscience 2005, 136, 193–203. [Google Scholar] [CrossRef]
- Decampo, D.M.; Fudge, J.L. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci. Biobehav. Rev. 2012, 36, 520–535. [Google Scholar] [CrossRef] [Green Version]
- Millhouse, O.E. The intercalated cells of the amygdala. J. Comp. Neurol. 1986, 247, 246–271. [Google Scholar] [CrossRef]
- Jacobsen, K.X.; Höistad, M.; Staines, W.A.; Fuxe, K. The distribution of dopamine D1 receptor and m-opioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: Relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience 2006, 141, 2007–2018. [Google Scholar]
- Amano, T.; Unal, C.T.; Paré, D. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 2010, 13, 489–494. [Google Scholar] [CrossRef]
- Likhtik, E.; Popa, D.; Apergis-Schoute, J.; Fidacaro, G.A.; Paré, D. Amygdala intercalated neurons are required for expression of fear extinction. Nat. Cell Biol. 2008, 454, 642–645. [Google Scholar] [CrossRef] [Green Version]
- Paré, D.; Royer, S.; Smith, Y.; Lang, E.J. Contextual Inhibitory Gating of Impulse Traffic in the Intra-amygdaloid Network. Ann. N. Y. Acad. Sci. 2006, 985, 78–91. [Google Scholar] [CrossRef]
- Adhikari, A.; Lerner, T.N.; Finkelstein, J.; Pak, S.; Jennings, J.H.; Davidson, T.J.; Ferenczi, E.A.; Gunaydin, L.A.; Mirzabekov, J.J.; Ye, L.; et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nat. Cell Biol. 2015, 527, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Adolphs, R.; Tranel, D.; Damasio, H.; Damasio, A.R. Fear and the human amygdala. J. Neurosci. 1995, 15, 5879–5891. [Google Scholar] [CrossRef]
- Roberto, M.; Kirson, D.; Khom, S. The Role of the Central Amygdala in Alcohol Dependence. Cold Spring Harb. Perspect. Med. 2021, 11, a039339. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.J. Cytoarchitecture of the central amygdaloid nucleus of the rat. J. Comp. Neurol. 1982, 208, 401–418. [Google Scholar] [CrossRef]
- Pitkanen, A.; Amaral, D. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: An immunohistochemical and in situ hybridization study. J. Neurosci. 1994, 14, 2200–2224. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.; Augustine, J. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience 1993, 52, 281–294. [Google Scholar] [CrossRef]
- Fudge, J.; Tucker, T. Amygdala projections to central amygdaloid nucleus subdivisions and transition zones in the primate. Neuroscience 2009, 159, 819–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, I.; Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Aouad, M.; Charlet, A.; Rodeau, J.-L.; Poisbeau, P. Reduction and prevention of vincristine-induced neuropathic pain symptoms by the non-benzodiazepine anxiolytic etifoxine are mediated by 3α-reduced neurosteroids. Pain 2009, 147, 54–59. [Google Scholar] [CrossRef]
- Purdy, R.H.; Morrow, A.L.; Moore, P.H., Jr.; Paul, S.M. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. USA 1991, 88, 4553–4557. [Google Scholar] [CrossRef] [Green Version]
- Navratilova, E.; Nation, K.; Remeniuk, B.; Neugebauer, V.; Bannister, K.; Dickenson, A.H.; Porreca, F. Selective modulation of tonic aversive qualities of neuropathic pain by morphine in the central nucleus of the amygdala requires endogenous opioid signaling in the anterior cingulate cortex. Pain 2020, 161, 609–618. [Google Scholar] [CrossRef]
- Nasagawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sukuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Social evolution. Oxyto-cin-gaze positive loop and the coevolution of human-dog bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef]
- Gottschalk, M.G.; Domschke, K. Oxytocin and Anxiety Disorders. Curr. Top. Behav. Neurosci. 2017, 35, 467–498. [Google Scholar]
- Neugebauer, V.; Mazzitelli, M.; Cragg, B.; Ji, G.; Navratilova, E.; Porreca, F. Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 2020, 170, 108052. [Google Scholar] [CrossRef]
- Gross, C.T.; Canteras, N.S. The many paths to fear. Nat. Rev. Neurosci. 2012, 13, 651–658. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Iwata, J.; Cicchetti, P.; Reis, D.J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988, 8, 2517–2529. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, F.V.; Hamani, C.; Fonoff, E.T.; Brentani, H.; Alho, E.J.L.; De Morais, R.M.C.B.; De Souza, A.L.; Rigonatti, S.P.; Martinez, R.C.R. Amygdala and Hypothalamus: Historical Overview With Focus on Aggression. Neurosurgery 2019, 85, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, S.T.; Clugnet, M.-C.; Price, J.L. Central olfactory connections in the macaque monkey. J. Comp. Neurol. 1994, 346, 403–434. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Sullivan, R.K.; Ianno, D.J.; Sah, P. Functional Properties and Projections of Neurons in the Medial Amygdala. J. Neurosci. 2014, 34, 8699–8715. [Google Scholar] [CrossRef] [Green Version]
- Millhouse, O.E.; Uemura-Sumi, M. The structure of the nucleus of the lateral olfactory tract. J. Comp. Neurol. 1985, 233, 517–552. [Google Scholar] [CrossRef]
- Vaz, R.P.; Cardoso, A.; Sá, S.I.; Pereira, P.; Madeira, M.D. The integrity of the nucleus of the lateral olfactory tract is essential for the normal functioning of the olfactory system. Brain Struct. Funct. 2017, 222, 3615–3637. [Google Scholar] [CrossRef] [Green Version]
- Zald, D.H.; Pardo, J.V. Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proc. Natl. Acad. Sci. USA 1997, 94, 4119–4124. [Google Scholar] [CrossRef] [Green Version]
- Johnston, J.B. Further contributions to the study of the evolution of the forebrain. J. Comp. Neurol. 1923, 35, 337–481. [Google Scholar] [CrossRef]
- Jimenez-Castellanos, J. The amygdaloid complex in monkey studied by reconstructional methods. J. Comp. Neurol. 1949, 91, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Van Hoesen, G. The differential distribution, diversity and sprouting of cortical projections to the amygdala in the rhesus monkey. In The Amygdaloid Complex; Ben-Ari, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 77–90. [Google Scholar]
- Turner, B.H.; Gupta, K.C.; Mishkin, M. The locus and cytoarchitecture of the projection areas of the olfactory bulb inMacaca mulatta. J. Comp. Neurol. 1978, 177, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Wierońska, J.M.; Nowak, G.; Pilc, A. Metabotropic Approaches to Anxiety. In Glutamate-Based Therapies for Psychiatric Disorders; Springer: Berlin, Germany, 2010; pp. 157–173. [Google Scholar]
- Benarroch, E.E. The amygdala: Functional organization and involvement in neurologic disorders. Neurology 2014, 84, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Partridge, J.G.; Forcelli, P.A.; Luo, R.; Cashdan, J.M.; Schulkin, J.; Valentino, R.J.; Vicini, S. Stress increases GABAergic neu-rotransmission in CRF neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology 2016, 107, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Duvarci, S.; Pare, D. Amygdala Microcircuits Controlling Learned Fear. Neuron 2014, 82, 966–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Ribas, E.C.; Wei, P.; Li, M.; Zhang, H.; Guo, Q. The ansa peduncularis in the human brain: A tractography and fiber dissection study. Brain Res. 2020, 1746, 146978. [Google Scholar] [CrossRef]
- Stefanacci, L.; Amaral, D.G. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. J. Comp. Neurol. 2000, 421, 52–79. [Google Scholar] [CrossRef]
- Herry, C.; Ferraguti, F.; Singewald, N.; Letzkus, J.; Ehrlich, I.; Lüthi, A. Neuronal circuits of fear extinction. Eur. J. Neurosci. 2010, 31, 599–612. [Google Scholar] [CrossRef]
- Nader, K.; Schafe, G.E.; Le Doux, J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nat. Cell Biol. 2000, 406, 722–726. [Google Scholar] [CrossRef]
- Adolphs, R. The Biology of Fear. Curr. Biol. 2013, 23, R79–R93. [Google Scholar] [CrossRef] [Green Version]
- Romanski, L.M.; Clugnet, M.C.; Bordi, F.; LeDoux, J.E. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav. Neurosci. 1993, 107, 444–450. [Google Scholar] [CrossRef]
- Halsell, C.B. Differential distribution of amygdaloid input across rostral solitary nucleus subdivisions in rat. Ann. N. Y. Acad. Sci. 1998, 855, 482–485. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Herman, M.A.; Roberto, M. The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders. Biol. Psychiatry 2015, 77, 859–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russchen, F.T.; Lohman, A.H.M. Afferent connections of the amygdala in the cat. Folia Anat. Iugosl. 1979, 9 (Suppl. S1), 57–63. [Google Scholar]
- Veening, J. Cortical afferents of the amygdaloid complex in the rat: An HRP study. Neurosci. Lett. 1978, 8, 191–195. [Google Scholar] [CrossRef]
- Asami, T.; Nakamura, R.; Takaishi, M.; Yoshida, H.; Yoshimi, A.; Whitford, T.J.; Hirayasu, Y. Smaller volumes in the lateral and basal nuclei of the amygdala in patients with panic disorder. PLoS ONE 2018, 13, e0207163. [Google Scholar] [CrossRef] [Green Version]
- Saunders, R.C.; Rosene, D.L.; Van Hoesen, G.W. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J. Comp. Neurol. 1988, 271, 185–207. [Google Scholar] [CrossRef]
- Insausti, R.; Amaral, D.G.; Cowan, W.M. The entorhinal cortex of the monkey: III. Subcortical afferents. J. Comp. Neurol. 1987, 264, 396–408. [Google Scholar] [CrossRef]
- Pitkänen, A.; Kelly, J.L.; Amaral, D.G. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus 2002, 12, 186–205. [Google Scholar] [CrossRef]
- Miller, L.A.; Taber, K.H.; Gabbard, G.O.; Hurley, R.A. Neural Underpinnings of Fear and Its Modulation: Implications for Anxiety Disorders. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 1–6. [Google Scholar] [CrossRef]
- Kim, M.J.; Loucks, R.A.; Palmer, A.L.; Brown, A.C.; Solomon, K.M.; Marchante, A.N.; Whalen, P.J. The structural and func-tional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav. Brain Res. 2011, 223, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Besteher, B.; Gaser, C.; Nenadić, I. Brain Structure and Subclinical Symptoms: A Dimensional Perspective of Psychopathology in the Depression and Anxiety Spectrum. Neuropsychobiology 2019, 79, 270–283. [Google Scholar] [CrossRef]
- Šešo-Šimić, Đ.; Sedmak, G.; Hof, P.R.; Šimić, G. Recent advances in the neurobiology of attachment behavior. Transl. Neurosci. 2010, 1, 148–159. [Google Scholar] [CrossRef]
- Apps, M.; Rushworth, M.F.; Chang, S.W. The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron 2016, 90, 692–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, H.; Pripfl, J.; Lamm, C.; Prainsack, C.; Taylor, N. Functional neuroanatomy of learned helplessness. NeuroImage 2003, 20, 927–939. [Google Scholar] [CrossRef]
- Seligman, M.E.P. Erlernte Hilflosigkeit. Erweitert um Franz Petermann: Neue Konzepte und Anwendungen; Psycholo-gie-Verlags-Union: Winheim, Germany, 1995. [Google Scholar]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 24, 155–184. [Google Scholar] [CrossRef]
- Lane, R.D.; Reiman, E.M.; Axelrod, B.; Yun, L.S.; Holmes, A.; Schwartz, G.E. Neural correlates of emotional awareness. Evi-dence of an interaction between emotion and attention in the anterior cingulate cortex. J. Cogn. Neurosci. 1998, 10, 525–535. [Google Scholar] [CrossRef]
- Papez, J.W. A proposed mechanism of emotion. Arch. Neurol. Psychiatry 1937, 38, 725. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Vogt, B.A.; Morrison, J.H.; Hof, P.R. Spindle neurons of the human anterior cingul. Ate cortex. J. Comp. Neurol. 1995, 355, 27–37. [Google Scholar] [CrossRef]
- Hyman, S.E.; Malenka, R.C.; Nestler, E.J. Neural mechanisms of addiction: The Role of Reward-Related Learning and Memory. Annu. Rev. Neurosci. 2006, 29, 565–598. [Google Scholar] [CrossRef] [Green Version]
- Koob, G.F.; Le Moal, M. Neurobiology of Addiction; Elsevier: Berlin, Germany, 2006. [Google Scholar]
- Nauta, W.J. The problem of the frontal lobe: A reinterpretation. Princ. Pract. Posit. Neuropsychiatry Res. 1972, 3-4, 167–187. [Google Scholar] [CrossRef]
- Walker, D.L.; Davis, M. Role of extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Struct. Funct. 2008, 213, 29–42. [Google Scholar] [CrossRef]
- Olucha-Bordonau, F.E.; Fortes-Marco, L.; Otero-García, M.; Lanuza, E.; Martínez-García, F. Amygdala: Structure and function. In The Rat Nervous System, 4th ed.; Paxinos, G., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 441–490. [Google Scholar]
- Tomer, R.; Slagter, H.A.; Christian, B.T.; Fox, A.S.; King, C.R.; Murali, D.; Gluck, M.A.; Davidson, R.J. Low to win or hate to lose? Asymmetry of dopamine D2 receptor binding predicts sensitivity to reward versus punishment. J. Cogn. Neurosci. 2014, 26, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damasio, A.; Damasio, H.; Tranel, D. Persistence of Feelings and Sentience after Bilateral Damage of the Insula. Cereb. Cortex 2012, 23, 833–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinstein, J.S.; Khalsa, S.S.; Salomons, T.V.; Prkachin, K.M.; Frey-Law, L.A.; Lee, J.E.; Tranel, D.; Rudrauf, D. Preserved emo-tional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct. Funct. 2016, 221, 1499–1511. [Google Scholar] [CrossRef] [Green Version]
- Salas, C.E. “No man is an island”: Recent findings on the emotional consequences of insula damage. Neuropsychoanalysis 2015, 17, 1–6. [Google Scholar] [CrossRef]
- Terasawa, Y.; Kurosaki, Y.; Ibata, Y.; Moriguchi, Y.; Umeda, S. Attenuated sensitivity to the emotions of others by insular lesion. Front. Psychol. 2015, 6, 1314. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Liu, X.; Van Dam, N.T.; Hof, P.R.; Fan, J. Cognition–Emotion Integration in the Anterior Insular Cortex. Cereb. Cortex 2013, 23, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Spagna, A.; Dufford, A.J.; Wu, Q.; Wu, T.; Zheng, W.; Coons, E.E.; Hof, P.R.; Hu, B.; Wu, Y.; Fan, J. Gray matter volume of the anterior insular cortex and social networking. J. Comp. Neurol. 2018, 526, 1183–1194. [Google Scholar] [CrossRef]
- Craig, A.D. (Bud) Significance of the insula for the evolution of human awareness of feelings from the body. Ann. N. Y. Acad. Sci. 2011, 1225, 72–82. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel. In An Interoceptive Moment with Your Neurobiological Self; Princeton University Press: Princeton, NJ, USA, 2015. [Google Scholar]
- Gasquoine, P.G. Contributions of the Insula to Cognition and Emotion. Neuropsychol. Rev. 2014, 24, 77–87. [Google Scholar] [CrossRef]
- Šimić, G.; Hof, P.R. In search of the definitive Brodmann’s map of cortical areas in human. J. Comp. Neurol. 2015, 523, 5–14. [Google Scholar] [CrossRef]
- Mesulam, M.M. (Ed.) Principles of Behavioral and Cognitive Neurology, 2nd ed.; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Wicker, B.; Keysers, C.; Plailly, J.; Royet, J.P.; Gallese, V.; Rizzolatti, G. Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron 2003, 40, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Weller, J.A.; Levin, I.P.; Shiv, B.; Bechara, A. The effects of insula damage on decision-making for risky gains and losses. Soc. Neurosci. 2009, 4, 347–358. [Google Scholar] [CrossRef]
- Humphrey, T. The development of the human amygdala during early embryonic life. J. Comp. Neurol. 1968, 132, 135–165. [Google Scholar] [CrossRef]
- Macchi, G. The ontogenic development of the olfactory telencephalon in man. J. Comp. Neurol. 1951, 95, 245–305. [Google Scholar] [CrossRef]
- Muller, F.; O’Rahilly, R. The amygdaloid complex and the medial and lateral ventricular eminences in staged human embryos. J. Anat. 2006, 208, 547–564. [Google Scholar] [CrossRef]
- Crosby, E.C.; Humphrey, T. Studies of the vertebrate telencephalon. II. The nuclear pattern of the anterior olfactory nucleus, tuberculum olfactorium and the amygdaloid complex in adult man. J. Comp. Neurol. 1941, 74, 309–352. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, I.; Kostović, I. Development of the lateral amygdaloid nucleus in the human fetus: Transient presence of discrete cy-toarchitectonic units. Anat. Embryol. 1986, 174, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Vasung, L.; Huang, H.; Jovanov-Milošević, N.; Pletikos, M.; Mori, S.; Kostović, I. Development of axonal pathways in the human fetal fronto-limbic brain: Histochemical characterization and diffusion tensor imaging. J. Anat. 2010, 217, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Saygin, Z.M.; Osher, D.E.; Koldewyn, K.; Martin, R.E.; Finn, A.; Saxe, R.; Gabrieli, J.D.; Sheridan, M. Structural Connectivity of the Developing Human Amygdala. PLoS ONE 2015, 10, e0125170. [Google Scholar] [CrossRef] [Green Version]
- Uematsu, A.; Matsui, M.; Tanaka, C.; Takahashi, T.; Noguchi, K.; Suzuki, M.; Nishijo, H. Developmental Trajectories of Amygdala and Hippocampus from Infancy to Early Adulthood in Healthy Individuals. PLoS ONE 2012, 7, e46970. [Google Scholar] [CrossRef] [Green Version]
- Ostby, Y.; Tamnes, C.K.; Fjell, A.M.; Westlye, L.T.; Due-Tønnessen, P.; Walhovd, K.B. Heterogeneity in subcortical brain de-velopment: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009, 29, 11772–11782. [Google Scholar] [CrossRef]
- Gabard-Durnam, L.J.; Flannery, J.; Goff, B.; Gee, D.G.; Humphreys, K.L.; Telzer, E.; Hare, T.; Tottenham, N. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. NeuroImage 2014, 95, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Banham Bridges, K.M. Emotional Development in Early Infancy. Child. Dev. 1932, 3, 324. [Google Scholar] [CrossRef]
- Liew, J. Effortful Control, Executive Functions, and Education: Bringing Self-Regulatory and Social-Emotional Competencies to the Table. Child. Dev. Perspect. 2012, 6, 105–111. [Google Scholar] [CrossRef]
- Lewis, M.D.; Granic, I. Phases of social-emotional development from birth to school age. In The Developmental Relations Among Mind, Brain and Education: Essays in Honor of Robbie Case; Ferrari, M., Vuletic, Lj., Eds.; Springer: New York, NY, USA, 2010; pp. 179–212. [Google Scholar]
- Klüver, H.; Bucy, P.C. An Analysis of Certain Effects of Bilateral Temporal Lobectomy in the Rhesus Monkey, with Special Reference to “Psychic Blindness”. J. Psychol. 1938, 5, 33–54. [Google Scholar] [CrossRef]
- Weiskrantz, L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J. Comp. Physiol. Psychol. 1956, 49, 381–391. [Google Scholar] [CrossRef]
- Das, J.M.; Siddiqui, W. Klüver Bucy Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bartels, A.; Zeki, S. The neural basis of romantic love. NeuroReport 2000, 11, 3829–3834. [Google Scholar] [CrossRef]
- Haller, J. The role of central and medial amygdala in normal and abnormal aggression: A review of classical approaches. Neurosci. Biobehav. Rev. 2018, 85, 34–43. [Google Scholar] [CrossRef]
- Blair, R.J. Neuroimaging of psychopathology and antisocial behavior: A targeted review. Curr. Psychiatry Rep. 2010, 12, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Begić, D. Psychopathology; Medicinska Naklada: Zagreb, Croatia, 2014. (in Croatian) [Google Scholar]
- Bogerts, B.; Schöne, M.; Breitschuh, S. Brain alterations potentially associated with aggression and terrorism. CNS Spectrums 2017, 23, 129–140. [Google Scholar] [CrossRef]
- Farah, T.; Ling, S.; Raine, A.; Yang, Y.; Schug, R. Alexithymia and reactive aggression: The role of the amygdala. Psychiatry Res. Neuroimaging 2018, 281, 85–91. [Google Scholar] [CrossRef]
- Wang, Y.; He, Z.; Zhao, C.; Li, L. Medial amygdala lesions modify aggressive behavior and immediate early gene expression in oxytocin and vasopressin neurons during intermale exposure. Behav. Brain Res. 2013, 245, 42–49. [Google Scholar] [CrossRef]
- Adebimpe, A.; Bassett, D.S.; Jamieson, P.E.; Romer, D. Intersubject Synchronization of Late Adolescent Brain Responses to Violent Movies: A Virtue-Ethics Approach. Front. Behav. Neurosci. 2019, 13, 260. [Google Scholar] [CrossRef]
- Sun, Y.; Gooch, H.; Sah, P. Fear conditioning and the basolateral amygdala. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [Green Version]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar]
- Molosh, A.I.; Dustrude, E.T.; Lukkes, J.L.; Fitz, S.D.; Caliman, I.F.; Abreu, A.R.R.; Dietrich, A.D.; Truitt, W.A.; Donck, L.V.; Ceusters, M.; et al. Panic results in unique molecular and network changes in the amygdala that facilitate fear responses. Mol. Psychiatry 2018, 25, 442–460. [Google Scholar] [CrossRef]
- Madonna, D.; DelVecchio, G.; Soares, J.C.; Brambilla, P. Structural and functional neuroimaging studies in generalized anxiety disorder: A systematic review. Rev. Bras. Psiquiatr. 2019, 41, 336–362. [Google Scholar] [CrossRef] [Green Version]
- Janiri, D.; Moser, D.A.; Doucet, G.E.; Luber, M.J.; Rasgon, A.; Lee, W.H.; Murrough, J.W.; Sani, G.; Eickhoff, S.B.; Frangou, S. Shared neural phenotypes for mood and anxiety disorders: A meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry 2020, 77, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Récamier-Carballo, S.; Estrada-Camarena, E.; López-Rubalcava, C. Maternal separation induces long-term effect on mono-amines and brain-derived neurotropic factor levels on the frontal cortex, amygdala, and hippocampus: Differential effects after a stress challenge. Behav. Pharmacol. 2017, 28, 545–557. [Google Scholar] [CrossRef]
- Kolesar, T.A.; Bilevicius, E.; Wilson, A.D.; Kornelsen, J. Systematic review and meta-analysis of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin. 2019, 24, 102016. [Google Scholar] [CrossRef] [PubMed]
- Fonzo, G.A.; Etkin, A. Affective neuroimaging in generalized anxiety disorder: An integrated review. Dialog Clin. Neurosci. 2017, 19, 169–179. [Google Scholar]
- Wahis, J.; Baudon, A.; Althammer, F.; Kerspern, D.; Goyon, S.; Hagiwara, D.; Lefevre, A.; Barteczko, L.; Boury-Jamot, B.; Bellanger, B.; et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 2021, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Malikowska-Racia, N.; Salat, K. Recent advances in the neurobiology of posttraumatic stress disorder: A review of possible mechanisms underlying an effective pharmacotherapy. Pharmacol. Res. 2019, 142, 30–49. [Google Scholar] [CrossRef]
- Kunimatsu, A.; Yasaka, K.; Akai, H.; Kunimatsu, N.; Abe, O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imaging 2020, 52, 380–396. [Google Scholar] [CrossRef]
- Duvarci, S.; Pare, D. Glucocorticoids Enhance the Excitability of Principal Basolateral Amygdala Neurons. J. Neurosci. 2007, 27, 4482–4491. [Google Scholar] [CrossRef]
- Preter, M.; Klein, D.F. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Sobanski, T.; Wagner, G. Functional neuroanatomy in panic disorder: Status quo of the research. World J. Psychiatry 2017, 7, 12–33. [Google Scholar] [CrossRef]
- Kaldewaij, R.; Reinecke, A.; Harmer, C.J. A lack of differentiation in amygdala responses to fearful expression intensity in panic disorder patients. Psychiatry Res. Neuroimaging 2019, 291, 18–25. [Google Scholar] [CrossRef]
- Carrigan, M.; Uryasev, O.; Frye, C.B.; Eckman, B.L.; Myers, C.R.; Hurley, T.; Benner, S.A. Hominids adapted to metabolize ethanol long before human-directed fermentation. Proc. Natl. Acad. Sci. USA 2015, 112, 458–463. [Google Scholar] [CrossRef] [Green Version]
- El-Guebaly, N.; El-Guebaly, A. Alcohol Abuse in Ancient Egypt: The Recorded Evidence. Int. J. Addict. 1981, 16, 1207–1221. [Google Scholar] [CrossRef]
- Steele, C.M.; Josephs, R.A. Alcohol myopia: Its prized and dangerous effects. Am. Psychol. 1990, 45, 921–933. [Google Scholar] [CrossRef]
- Darke, S. The toxicology of homicide offenders and victims: A review. Drug Alcohol Rev. 2009, 29, 202–215. [Google Scholar] [CrossRef]
- Darvishi, N.; Farhadi, M.; Haghtalab, T.; Poorolajal, J. Alcohol-Related Risk of Suicidal Ideation, Suicide Attempt, and Completed Suicide: A Meta-Analysis. PLoS ONE 2015, 10, e0126870. [Google Scholar] [CrossRef] [Green Version]
- Gilman, J.M.; Ramchandani, V.A.; Crouss, T.; Hommer, D.W. Subjective and Neural Responses to Intravenous Alcohol in Young Adults with Light and Heavy Drinking Patterns. Neuropsychopharmacology 2011, 37, 467–477. [Google Scholar] [CrossRef] [Green Version]
- McDaid, J.; McElvain, M.A.; Brodie, M.S. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ib: Involvement of barium-sensitive potassium currents. J. Neurophysiol. 2008, 100, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, H.; Morrisett, R.A. Ethanol action on dopaminergic neurons in the ventral tegmental area: Interaction with intrinsic ion channels and neurotransmitter inputs. Int. Rev. Neurobiol. 2010, 91, 235–288. [Google Scholar]
- Di Volo, M.; Morozova, E.O.; Lapish, C.C.; Kuznetsov, A.; Gutkin, B. Dynamical ventral tegmental area circuit mechanisms of alcohol-dependent dopamine release. Eur. J. Neurosci. 2019, 50, 2282–2296. [Google Scholar] [CrossRef] [Green Version]
- Rau, A.R.; Chappell, A.M.; Butler, T.R.; Ariwodola, O.J.; Weiner, J.L. Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress. J. Neurosci. 2015, 35, 9730–9740. [Google Scholar] [CrossRef] [Green Version]
- Ramchandani, V.A.; Stangl, B.L.; Blaine, S.K.; Plawecki, M.H.; Schwandt, M.L.; Kwako, L.E.; Sinha, R.; Cyders, M.A.; O’Connor, S.; Zakhari, S. Stress vulnerability and alcohol use and consequences: From human laboratory studies to clinical outcomes. Alcohol 2018, 72, 75–88. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Sell, L.A.; Morris, J.; Bearn, J.; Frackowiak, R.; Friston, K.J.; Dolan, R.J. Activation of reward circuitry in human opiate addicts. Eur. J. Neurosci. 1999, 11, 1042–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwood, A.S.; Munafo, M.R. Effects of acute alcohol consumption and processing of emotion in faces: Implications for un-derstanding alcohol-related aggression. J. Psychopharmacol. 2014, 28, 719–732. [Google Scholar] [CrossRef] [Green Version]
- Crane, C.A.; Godleski, S.A.; Przybyla, S.M.; Schlauch, R.C.; Testa, M. The proximal effects of acute alcohol consumption on male-to-female aggression: A meta-analytic review of the experimental literature. Trauma Violence Abuse 2016, 17, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Diener, E.; Chan, M.Y. Happy People Live Longer: Subjective Well-Being Contributes to Health and Longevity. Appl. Psychol. Heal. Well Being 2011, 3, 1–43. [Google Scholar] [CrossRef]
- Lyubomirsky, S.; King, L.; Diener, E. The benefits of frequent positive affect: Does happiness lead to success? Psychol. Bull. 2005, 131, 803–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, K.C.; Kringelbach, M.L. Pleasure Systems in the Brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [Green Version]
- Schultz, W. Reward prediction error. Curr. Biol. 2017, 27, R369–R371. [Google Scholar] [CrossRef] [Green Version]
- Berridge, K.C.; Robinson, T.E. Parsing reward. Trends Neurosci. 2003, 26, 507–513. [Google Scholar] [CrossRef]
- Shizgal, P. Neural basis of utility elimination. Curr. Opin. Neurobiol. 1997, 7, 198–208. [Google Scholar]
- Berridge, K.C.; Aldridge, J.W. Decision utility, incentive salience, and cue-triggered „wanting“. Oxf. Ser. Soc. Cogn. Soc. Neurosci. 2009, 2009, 509–533. [Google Scholar]
- Schultz, W.; Dayan, P.; Montague, P.R. A Neural Substrate of Prediction and Reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Lisman, J.E.; Grace, A.A. The Hippocampal-VTA Loop: Controlling the Entry of Information into Long-Term Memory. Neuron 2005, 46, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, H.; Itoh, H.; Kawagoe, R.; Takikawa, Y.; Hikosaka, O. Dopamine neurons can represent context-dependent pre-diction error. Neuron 2004, 41, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Bissonette, G.B.; Roesch, M.R. Development and function of the midbrain dopamine system: What we know and what we need to. Genes Brain Behav. 2016, 15, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Schultz, W. Dopamine reward prediction-error signalling: A two-component response. Nat. Rev. Neurosci. 2016, 17, 183–195. [Google Scholar] [CrossRef]
- Schelp, S.A.; Pultorak, K.J.; Rakowski, D.R.; Gomez, D.M.; Krzystyniak, G.; Das, R.; Oleson, E.B. A transient dopamine signal encodes subjective value and causally influences demand in an economic context. Proc. Natl. Acad. Sci. USA 2017, 114, E11303–E11312. [Google Scholar] [CrossRef] [Green Version]
- Schultz, W. Recent advances in understanding the role of phasic dopamine activity. F1000Research 2019, 8, 1680. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J.; Hyman, S.E.; Holtzman, D.M.; Malenka, R.C. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 3rd ed.; McGraw-Hill Medical: New York, NY, USA, 2015. [Google Scholar]
- Everitt, B.J.; Heberlein, U. Addiction. Curr. Opin. Neurobiol. 2013, 23, 463. [Google Scholar]
- Camerer, C.F.; Fehr, E. When does “economic man” dominate social behavior? Science 2006, 311, 47–52. [Google Scholar]
- Calipari, E.S.; Godino, A.; Salery, M.; Damez-Werno, D.M.; Cahill, M.E.; Werner, C.T.; Gancarz, A.M.; Peck, E.G.; Jlayer, Z.; Rabkin, J.; et al. Synaptic microtubule-associated protein EB3 and SRC phosphorylation mediate structural and behavioral adaptations during withdrawal from cocaine self-administration. J. Neurosci. 2019, 39, 5634–5646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brake, W.G.; Zhang, T.Y.; Diorio, J.; Meaney, M.J.; Gratton, A. Influence of early postnatal rearing condition on mesocortico-limbic dopamine and behavioral responses to psychostimulants and stressors in adult rats. Eur. J. Neurosci. 2004, 19, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Harlow, J.M. Recovery from the passage of an iron bar through the head. Publ. Mass. Med. Soc. 1868, 2, 327–347. [Google Scholar]
- Damasio, H.; Grabowski, T.; Frank, R.; Galaburda, A.; Damasio, A. The return of Phineas Gage: Clues about the brain from the skull of a famous patient. Science 1994, 264, 1102–1105. [Google Scholar] [CrossRef]
- Hänsel, A.; von Känel, R. The ventro-medial prefrontal cortex: A major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc. Med. 2008, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Van Horn, J.D.; Irimia, A.; Torgerson, C.M.; Chambers, M.C.; Kikinis, R.; Toga, A.W. Mapping Connectivity Damage in the Case of Phineas Gage. PLoS ONE 2012, 7, e37454. [Google Scholar] [CrossRef]
- Staut, C.C.; Naidich, T.P. Urbach-Wiethe disease (Lipoid proteinosis). Pediatr. Neurosurg. 1998, 28, 212–214. [Google Scholar] [CrossRef]
- Adolphs, R.; Tranel, D.; Damasio, H. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nat. Cell Biol. 1994, 372, 669–672. [Google Scholar] [CrossRef]
- Boes, A.D.; Grafft, A.H.; Joshi, C.; Chuang, N.A.; Nopoulos, P.; Anderson, S.W. Behavioral effects of congenital ventromedial prefrontal cortex malformation. BMC Neurol. 2011, 11, 151. [Google Scholar] [CrossRef] [Green Version]
- Tranel, D.; Hyman, B.T. Neuropsychological Correlates of Bilateral Amygdala Damage. Arch. Neurol. 1990, 47, 349–355. [Google Scholar] [CrossRef]
- De Martino, B.; Camerer, C.F.; Adolphs, R. Amygdala damage eliminates monetary loss aversion. Proc. Natl. Acad. Sci. USA 2010, 107, 3788–3792. [Google Scholar] [CrossRef] [Green Version]
- Colloca, L.; Sigaudo, M.; Benedetti, F. The role of learning in nocebo and placebo effects. Pain 2008, 136, 211–218. [Google Scholar] [CrossRef]
- Bär, K.-J.; Brehm, S.; Boettger, M.; Boettger, S.; Wagner, G.; Sauer, H. Pain perception in major depression depends on pain modality. Pain 2005, 117, 97–103. [Google Scholar] [CrossRef]
- Strigo, I.A.; Simmons, A.N.; Matthews, S.C.; Craig, A.D. (Bud); Paulus, M.P. Association of Major Depressive Disorder With Altered Functional Brain Response During Anticipation and Processing of Heat Pain. Arch. Gen. Psychiatry 2008, 65, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Harrison, L.A.; Hurlemann, R.; Adolphs, R. An Enhanced Default Approach Bias Following Amygdala Lesions in Humans. Psychol. Sci. 2015, 26, 1543–1555. [Google Scholar] [CrossRef] [Green Version]
- Weymar, M.; Schwabe, L. Amygdala and emotion: The bright side of it. Front. Neurosci. 2016, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Adolphs, R.; Baron-Cohen, S.; Tranel, D. Impaired Recognition of Social Emotions following Amygdala Damage. J. Cogn. Neurosci. 2002, 14, 1264–1274. [Google Scholar] [CrossRef] [Green Version]
- Han, K.-M.; De Berardis, D.; Fornaro, M.; Kim, Y.-K. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 20–27. [Google Scholar] [CrossRef]
- Sepede, G.; Spano, M.C.; Lorusso, M.; De Berardis, D.; Salerno, R.M.; Di Giannantonio, M.; Gambi, F. Sustained attention in psychosis: Neuroimaging findings. World J. Radiol. 2014, 6, 261–273. [Google Scholar] [CrossRef]
- Nolan, M.; Roman, E.; Nasa, A.; Levins, K.J.; O’Hanlon, E.; O’Keane, V.; Roddy, D.W. Hippocampal and Amygdalar Volume Changes in Major Depressive Disorder: A Targeted Review and Focus on Stress. Chronic Stress 2020, 4, 1–19. [Google Scholar] [CrossRef]
- Ho, N.F.; Chong, P.L.H.; Lee, D.R.; Chew, Q.H.; Chen, G.; Sim, K. The Amygdala in Schizophrenia and Bipolar Disorder: A Synthesis of Structural MRI, Diffusion Tensor Imaging, and Resting-State Functional Connectivity Findings. Harv. Rev. Psychiatry 2019, 27, 150–164. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. Abnormal neural activities in adults and youths with major depressive disorder during emotional processing: A meta-analysis. Brain Imaging Behav. 2021, 15, 1134–1154. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Liu, T.; Ma, L.; Wang, W.; Shi, S.; Wang, Y.; Gong, Q.; Wang, M. Altered Resting-State Functional Activity in Medication-Naive Patients With First-Episode Major Depression Disorder vs. Healthy Control: A Quantitative Meta-Analysis. Front. Behav. Neurosci. 2019, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Dannlowski, U.; Ohrmann, P.; Bauer, J.; Kugel, H.; Arolt, V.; Heindel, W.; Suslow, T. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res. Neuroimaging 2007, 154, 13–20. [Google Scholar] [CrossRef]
- Young, K.D.; Zotev, V.; Phillips, R.; Misaki, M.; Drevets, W.C.; Bodurka, J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry Clin. Neurosci. 2018, 72, 466–481. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.-H.; Han, P.-L. Reciprocal interactions across and within multiple levels of monoamine and cortico-limbic systems in stress-induced depression: A systematic review. Neurosci. Biobehav. Rev. 2019, 101, 13–31. [Google Scholar] [CrossRef]
- Larøi, F.; Thomas, N.; Aleman, A.; Fernyhough, C.; Wilkinson, S.; Deamer, F.; McCarthy-Jones, S. The ice in voices: Under-standing negative content in auditory-verbal hallucinations. Clin. Psychol. Rev. 2019, 67, 1–10. [Google Scholar] [CrossRef]
- Barch, D.M.; Pagliaco, D.; Luking, K. Mechanisms underlying motivational deficits in psychopathology: Similarities and differences in depression and schizophrenia. Curr. Top. Behav. Neurosci. 2016, 27, 411–449. [Google Scholar]
- Mujica-Parodi, L.R.; Cha, J.; Gao, J. From Anxious to Reckless: A Control Systems Approach Unifies Prefrontal-Limbic Regulation Across the Spectrum of Threat Detection. Front. Syst. Neurosci. 2017, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia León, I.; Kruse, O.; Stark, R.; Klucken, T. Relationship of sensation seeking with the neural correlates of appetitive con-ditioning. Soc. Cogn. Affect. Neurosci. 2019, 14, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.; Canli, T. The Endophenotype of Impulsivity: Reaching Consilience Through Behavioral, Genetic, and Neuroimaging Approaches. Behav. Cogn. Neurosci. Rev. 2005, 4, 262–281. [Google Scholar] [CrossRef]
- Weiland, B.J.; Heitzeg, M.M.; Zald, D.; Cummiford, C.; Love, T.; Zucker, R.A.; Zubieta, J.-K. Relationship between impulsivity, prefrontal anticipatory activation, and striatal dopamine release during rewarded task performance. Psychiatry Res. Neuroimaging 2014, 223, 244–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, B.J.; Del Giudice, M.; Dishion, T.J.; Figueredo, A.J.; Gray, P.B.; Griskevicius, V.; Hawley, P.H.; Jacobs, W.J.; James, J.; Volk, A.A.; et al. The evolutionary basis of risky adolescent behavior: Implications for science, policy, and practice. Dev. Psychol. 2012, 48, 598–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauffman, E.; Shulman, E.P.; Steinberg, L.; Claus, E.; Banich, M.T.; Graham, S.; Woolard, J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Dev. Psychol. 2010, 46, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.; McCrae, R.R.; De Fruyt, F.; Jussim, L.; Löckenhoff, C.E.; De Bolle, M.; Costa, P.T.; Sutin, A.R.; Realo, A.; Allik, J.; et al. Stereotypes of age differences in personality traits: Universal and accurate? J. Person. Soc. Psychol. 2012, 103, 1050–1066. [Google Scholar] [CrossRef]
- Steinberg, L.; Albert, D.; Cauffman, E.; Banich, M.; Graham, S.; Woolard, J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev. Psychol. 2008, 44, 1764–1778. [Google Scholar] [CrossRef] [Green Version]
- Figner, B.; Mackinlay, R.J.; Wilkening, F.; Weber, E.U. Affective and deliberative processes in risky choice: Age differences in risk taking in the Columbia Card Task. J. Exp. Psychol. Learn. Mem. Cogn. 2009, 35, 709–730. [Google Scholar] [CrossRef] [Green Version]
- Casey, B.J.; Caudle, K. The teenage brain: Self control. Curr. Dir. Psychol. Sci. 2013, 22, 82–87. [Google Scholar] [CrossRef]
- Simons-Morton, B.; Lerner, N.; Singer, J. The observed effects of teenage passengers on the risky driving behavior of teenage drivers. Accid. Anal. Prev. 2005, 37, 973–982. [Google Scholar] [CrossRef]
- Zimring, F.E. American Youth Violence; NYU Press: New York, NY, USA, 2014; pp. 7–36. [Google Scholar]
- Sommerville, L.H. Emotional Development in Adolescence. In Handbook of Emotions, 4th ed.; Feldman Barrett, L., Lewis, M., Haviland-Jones, J.M., Eds.; The Guilford Press: New York, NY, USA, 2016; pp. 350–365. [Google Scholar]
- Posner, M.I.; Rothbart, M.K.; Sheese, B.E.; Voelker, P. Control networks and neuromodulators of early development. Dev. Psychol. 2012, 48, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Gothelf, R.; Law, A.J.; Frisch, A.; Chen, J.; Zarchi, O.; Michaelovsky, E.; Ren-Patterson, R.; Lipska, B.K.; Carmel, M.; Kolachana, B.; et al. Biological Effects of COMT Haplotypes and Psychosis Risk in 22q11.2 Deletion Syndrome. Biol. Psychiatry 2014, 75, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Sheese, B.E.; Voelker, P.M.; Rothbart, M.K.; Posner, M.I. Parenting quality interacts with genetic variation in dopamine receptor DRD4 to influence temperament in early childhood. Dev. Psychopathol. 2007, 19, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Belsky, J.; Pluess, M. Beyond diathesis stress: Differential susceptibility to environment stress. Psychol. Bull. 2009, 135, 895–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheese, B.E.; Rothbart, M.K.; Voelker, P.M.; Posner, M.I. The Dopamine Receptor D4 Gene 7-Repeat Allele Interacts with Parenting Quality to Predict Effortful Control in Four-Year-Old Children. Child. Dev. Res. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, H.; van der Zwaluw, C.S.; Overbeek, G.; Granic, I.; Franke, B.; Engels, R.C. A variable-number-of-tandem-repeats polmorphism in the dopamine D4 receptor gene affects social adaptation of alcohol use: Investigation of a gene—environment interaction. Psychol. Sci. 2010, 21, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, K.; Nemoda, Z.; Fearon, R.M.P.; Csibra, G.; Sasvari-Szekely, M.; Johnson, M.H. Polymorphisms in dopamine system genes are associated with individual differences in attention in infancy. Dev. Psychol. 2010, 46, 404–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, D.G.; Adolphs, R. (Eds.) Living without an Amygdala; The Guilford Press: New York, NY, USA, 2016; p. 12. [Google Scholar]
- Frick, P.J.; Barry, C.T.; Bodin, S.D. Applying the concept of psychopathy to children: Implication for the assessment of antisocial youth. In The Clinical and Forensic Assessment of Psychopathy; Gacono, C.B., Ed.; Erlbaum: Mahway, NJ, USA, 2000; pp. 3–25. [Google Scholar]
- Davidson, R.J.; Putnam, K.M.; Larson, C.L. Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence. Science 2000, 289, 591–594. [Google Scholar] [CrossRef] [Green Version]
- Blair, R.J.R. Neurological basis of psychopathy. Br. J. Psychiatry 2003, 182, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, P.V.; Capra, C.M.; Moore, S.; Noussair, C.; Berns, G.S. Neurobiological regret and rejoice functions for aversive outcomes. NeuroImage 2008, 39, 1472–1484. [Google Scholar] [CrossRef] [Green Version]
- Kanske, P.; Kotz, S.A. Emotion speeds up conflict resolution: A new role for the ventral anterior cingulate cortex? Cereb. Cortex 2011, 21, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Ochsner, K.N.; Phelps, E. Emerging perspectives on emotion–cognition interactions. Trends Cogn. Sci. 2007, 11, 317–318. [Google Scholar] [CrossRef]
- Storbeck, J.; Clore, G.L. On the interdependence of cognition and emotion. Cogn. Emot. 2007, 21, 1212–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelps, E.A. Emotion and Cognition: Insights from Studies of the Human Amygdala. Annu. Rev. Psychol. 2006, 57, 27–53. [Google Scholar] [CrossRef] [Green Version]
- Okon-Singer, H.; Hendler, T.; Pessoa, L.; Shackman, J. The neurobiology of emotion—Cognition interactions: Fundamental questions and strategies for future research. Front. Hum. Neurosci. 2015, 9, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shackman, A.J.; Fox, A.S.; Seminowicz, D.A. The cognitive-emotional brain: Opportunities [corrected] and challenges for understanding neuropsychiatric disorders. Behav. Brain Sci. 2015, 38, e86. [Google Scholar] [CrossRef] [PubMed]
- LaBar, K.S.; Gatenby, J.C.; Gore, J.C.; LeDoux, J.E.; Phelps, E.A. Human amygdala activation during conditioned fear acqui-sition and extinction: A mixed-trial fMRI study. Neuron 1998, 20, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Bechara, A.; Tranel, D.; Damasio, H.; Adolphs, R.; Rockland, C.; Damasio, A.R. Double dissociation of conditioning and de-clarative knowledge relative to the amygdala and hippocampus in humans. Science 1995, 269, 1115–1118. [Google Scholar] [CrossRef]
- Indovina, I.; Robbins, T.W.; Núñez-Elizalde, A.O.; Dunn, B.D.; Bishop, S.J. Fear-Conditioning Mechanisms Associated with Trait Vulnerability to Anxiety in Humans. Neuron 2011, 69, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Phelps, E.A.; LeDoux, J.E. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, L. Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done”? Neuropsychologia 2010, 48, 3416–3429. [Google Scholar] [CrossRef] [Green Version]
- Dolcos, F.; Iordan, A.D.; Kragel, J.; Stokes, J.; Campbell, R.; McCarthy, G.; Cabeza, R. Neural Correlates of Opposing Effects of Emotional Distraction on Working Memory and Episodic Memory: An Event-Related fMRI Investigation. Front. Psychol. 2013, 4, 293. [Google Scholar] [CrossRef] [Green Version]
- Omura, K.; Constable, R.T.; Canli, T. Amygdala gray matter concentration is associated with extraversion and neuroticism. NeuroReport 2005, 16, 1905–1908. [Google Scholar] [CrossRef]
- Gray, J.R.; Braver, T.S.; Raichle, M.E. Integration of emotion and cognition in the lateral prefrontal cortex. Proc. Natl. Acad. Sci. USA 2002, 99, 4115–4120. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008, 9, 148–158. [Google Scholar] [CrossRef]
- Pessoa, L. The Cognitive-Emotional Brain: From Interactions to Integration; MIT Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Pessoa, L. Précis on the cognitive-emotional brain. Behav. Brain Sci. 2015, 38, e71. [Google Scholar] [CrossRef]
- Frank, D.; Dewitt, M.; Hudgens-Haney, M.; Schaeffer, D.; Ball, B.; Schwarz, N.; Hussein, A.; Smart, L.; Sabatinelli, D. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 2014, 45, 202–211. [Google Scholar] [CrossRef]
- Pessoa, L. A Network Model of the Emotional Brain. Trends Cogn. Sci. 2017, 21, 357–371. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Wang, X.; Wu, Q.; Spagna, A.; Yang, J.; Yuan, C.; Wu, Y.; Gao, Z.; Hof, P.R.; Fan, J. Anterior insular cortex is a bot-tleneck of cognitive control. Neuroimage 2019, 195, 490–504. [Google Scholar] [CrossRef]
- Pessoa, L. Understanding emotion with brain networks. Curr. Opin. Behav. Sci. 2018, 19, 19–25. [Google Scholar] [CrossRef]
- Pessoa, L.; Medina, L.; Hof, P.R.; Desfilis, E. Neural architecture of the vertebrate brain: Implications for the interaction between emotion and cognition. Neurosci. Biobehav. Rev. 2019, 107, 296–312. [Google Scholar] [CrossRef]
- Šimić, G. (Ed.) Introduction to Neuroscience of Emotions and Feelings; Naklada Ljevak: Zagreb, Croatia, 2020; pp. 11–137. (In Croatian) [Google Scholar]












| Aggression Type | Characteristics | Conditions in which It Occurs | The Role of the Amygdala |
|---|---|---|---|
| Impulsive (reactive) | Unplanned, caused by increased arousal to a provocation or a threat, accompanied by a feeling of anger; primary intention is to destroy the victim (usually the provocateur) | Intermittent explosive disorder, autism, impulsive type of emotionally unstable personality, post-TBI disorders, PTSD | Increased activity, especially of the amygdala in the right hemisphere, with decreased control of the amygdala via PFC (decreased PFC activity); increased activity of the ANS, which includes increased reactivity of the “threat system” (medial part of the amygdala, hypothalamus, PAG) |
| Planned (proactive, instrumental) | Planned in advance, associated with a reduced degree of compassion (empathy); intention is to achieve a certain goal (usually some personal benefit) | Antisocial (DSM5)/dissocial (ICD-10) personality disorder | Decreased volume of amygdala and its activity, especially in tasks involving compassion; decreased amygdala functional connectivity with vmPFC, OFC, and posterior cingulate cerebral cortex, decreased OFC activation to provocation |
| Case | Basic Neuropathological Findings | Altered Behavior | Reference(s) No. |
|---|---|---|---|
| Phineas Gage | Bilateral damage of the frontal lobe, especially vmPFC, including the extensive damage to the white matter of the frontal lobe as well as the anterior parts of temporal lobe and amygdala (amygdala disconnected from the frontal lobes) | Careful and reliable person before the injury after the injury became emotionally unstable, impulsive, unpredictable, dishonest, capricious, reckless, having disturbed social skills and difficulties in making decisions (“no longer Gage”) | [270,271,272,273] |
| Patient S.M. | Bilateral calcification of the amygdala and periamygdaloid gyrus due to the Urbach–Wiethe disease | Patient S.M. had highly specialized impairment associated with the emotion of fear: she could not experience fear nor she could recognize facial expressions showing fear | [61,274,275] |
| Boy B.W. | Congenital ventromedial prefrontal cortex malformation involving Brodmann areas 11, 12, 25 and 32, clusters of dysplastic neurons in the left amygdaloid nucleus | Throughout his childhood, this boy with a relatively normal cognitive performance on standard neurophychological tests displayed incremental emotional instability, impulsivity, lack of empathy, hypersexuality, and had been manipulative and aggressive towards others, including his own parents | [276] |
| Patient B. | Bilateral destruction mainly of the insula due to Herpes simplex infection, but to a lesser extent also of the orbitofrontal and temporal cortex, anterior part of the ACC, hippocampus, EC, amygdala and a part of basal telencephalon | Severe global amnesia, dense impairment of retrograde memory and shallow mental content, but, except for taste and olfaction, all aspects of feeling were intact | [183] |
| Patient Roger | Bilateral damage to insula, ACC, and amygdala due to Herpes simplex infection | Major deficits included global amnesia, anosmia (the inability to percieve smell/odor), and ageusia (the inability to taste), while his experience of pain was intact, at times even excessive | [184] |
| Patient A.P. | Selective bilateral damage to the amygdala due to the Urbach–Wiethe disease | A pleasant, cheerful young woman notable for her tendency to be somewhat coquetting and disinhibited, e.g., she had been quick to become friendly with examiners, and had often made mildly innapropriate sexual remarks. She had also suffered from a significant defect in visual, nonverbal memory, executive control manifesting with innapropriate social behaviors, and had deficits on tests of category formation, cognitive flexibility, and abstract reasoning | [277,278] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; R. Hof, P. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823. https://doi.org/10.3390/biom11060823
Šimić G, Tkalčić M, Vukić V, Mulc D, Španić E, Šagud M, Olucha-Bordonau FE, Vukšić M, R. Hof P. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules. 2021; 11(6):823. https://doi.org/10.3390/biom11060823
Chicago/Turabian StyleŠimić, Goran, Mladenka Tkalčić, Vana Vukić, Damir Mulc, Ena Španić, Marina Šagud, Francisco E. Olucha-Bordonau, Mario Vukšić, and Patrick R. Hof. 2021. "Understanding Emotions: Origins and Roles of the Amygdala" Biomolecules 11, no. 6: 823. https://doi.org/10.3390/biom11060823
APA StyleŠimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., Šagud, M., Olucha-Bordonau, F. E., Vukšić, M., & R. Hof, P. (2021). Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules, 11(6), 823. https://doi.org/10.3390/biom11060823








