The Emerging Role of Long Non-Coding RNAs and MicroRNAs in Neurodegenerative Diseases: A Perspective of Machine Learning
Abstract
:1. Introduction
2. MicroRNAs and Long Non-Coding RNAs
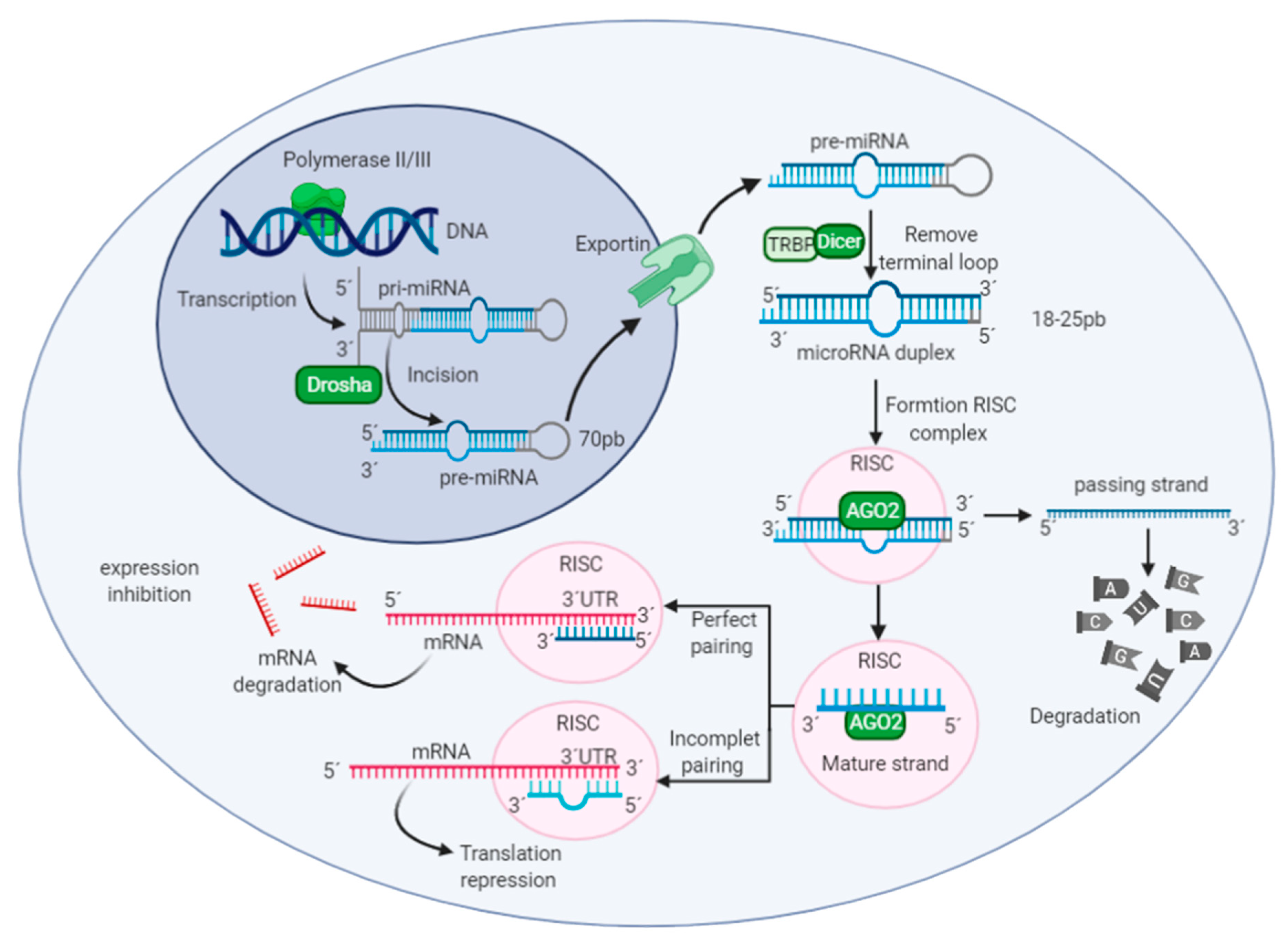
2.1. MicroRNAs
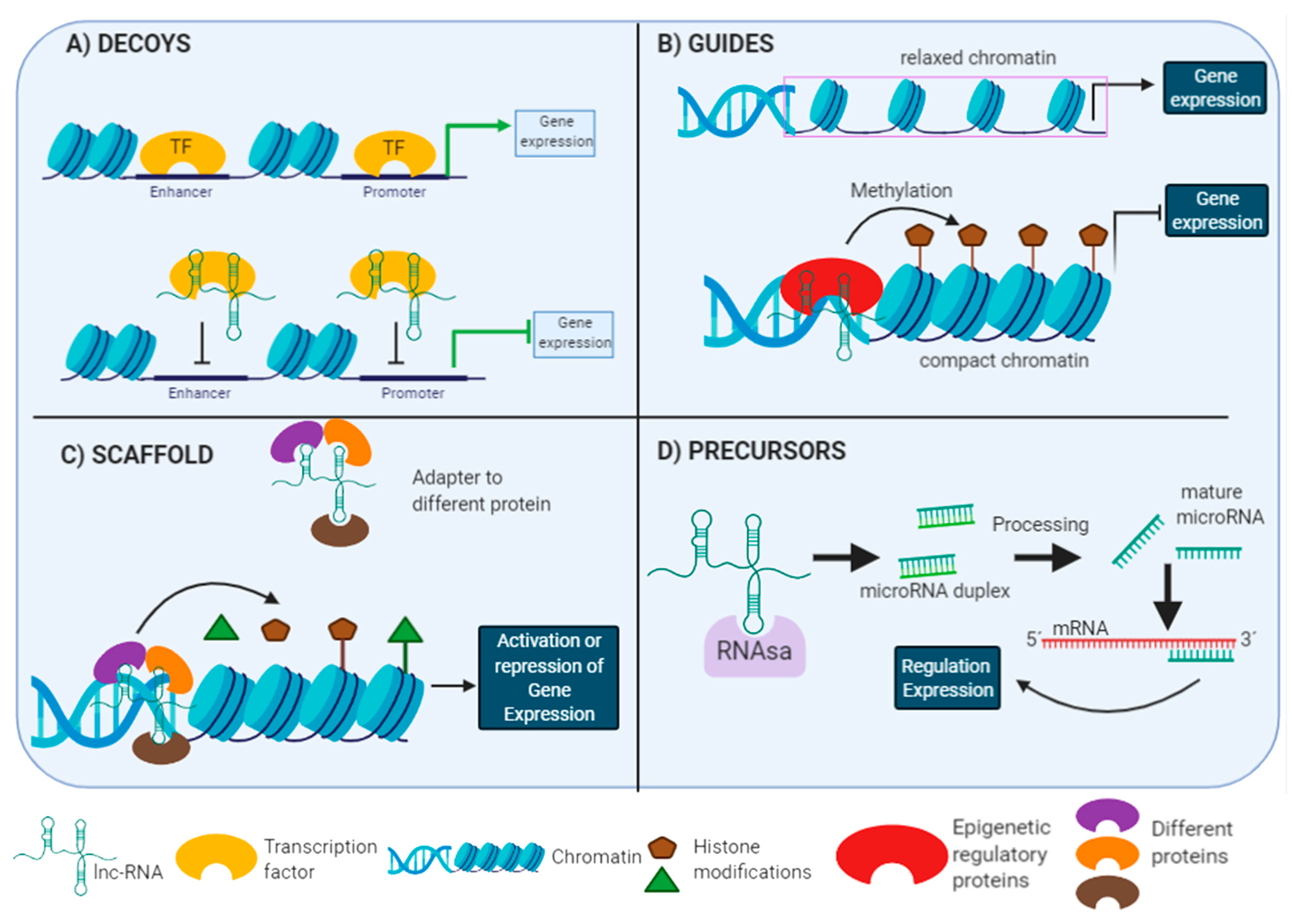
2.2. Long Non-Coding RNAs
Lnc-RNAs Functions
2.3. MicroRNAs and Long Non-Coding RNAs in Brain
3. Importance of MicroRNAs and Long Non-Coding RNAs in Brain Pathologies
3.1. MicroRNAs and Long Non-Coding RNAs as Biomarkers in Alzheimer’s Disease
3.2. MicroRNAs and Long Non-Coding RNAs as Biomarkers in Parkinson’s Disease
3.3. MicroRNAs and Long Non-Coding RNAs as Biomarkers in Huntington’s Disease
3.4. MicroRNAs and Long Non-Coding RNAs as Biomarkers in Amyotrophic Lateral Sclerosis’s Disease
4. Bioinformatic Tools for the Study of Non-Coding RNAs
5. Machine Learning Applied to Diagnosis of Neurodegenerative Diseases
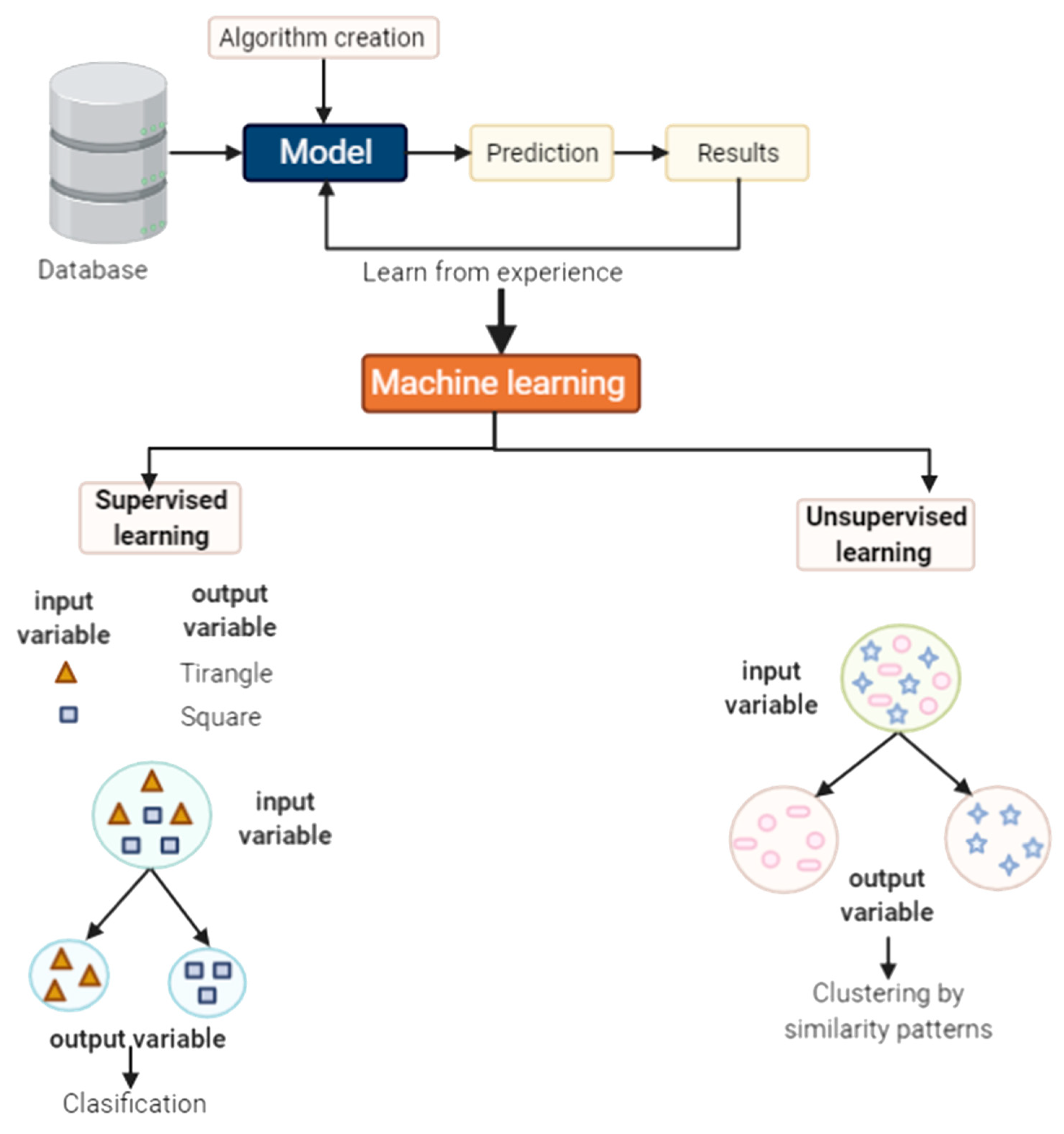
5.1. What Is Machine Learning?
5.2. Some Methods Used in Machine Learning
- (a)
- Data collection and processing: Data transformation, normalization and optimization.
- (b)
- Model selection: Selection of suitable algorithms and choice of a success indicator.
- (c)
- Model training: Precision, accuracy, sensitive and specificity.
- (d)
- Model validation: Parameter setting and reproducibility.
5.2.1. Supervised Learning
- Regression
- Classification
- (a)
- An input layer corresponds to independent variables. The model is trained by inputting information through this layer.
- (b)
- One or more intermediate layers or hidden layers communicated with each other through activation functions. The information is processed within this layer.
- (c)
- An output layer corresponds to dependent variables. The predictions are given through this layer.
5.2.2. Unsupervised Learning
- Clustering
- Association
5.3. Machine Learning in Brain
Machine Learning as a Diagnostic Tool of Neurodegenerative Diseases from MicroRNAs and Long Non-Coding RNAs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsagalioti, E.; Trifonos, C.; Morari, A.; Vadikolias, K.; Giaginis, C. Clinical value of nutritional status in neurodegenerative diseases: What is its impact and how it affects disease progression and management? Nutr. Neurosci. 2016, 21, 162–175. [Google Scholar] [CrossRef]
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, K.J. The genetics of neurodevelopmental disease. Curr. Opin. Neurobiol. 2011, 21, 197–203. [Google Scholar] [CrossRef]
- Gómez-Río, M.; Caballero, M.M.; Górriz Sáez, J.M.; Mínguez-Castellanos, A. Diagnosis of Neurodegenerative Diseases: The Clinical Approach. Curr. Alzheimer Res. 2016, 13, 469–474. [Google Scholar] [CrossRef]
- Yang, J.-H.; Li, J.-H.; Jiang, S.; Zhou, H.; Qu, L.-H. ChIPBase: A database for decoding the transcriptional regulation of long non-coding RNA and microRNA genes from ChIP-Seq data. Nucleic Acids Res. 2012, 41, D177–D187. [Google Scholar] [CrossRef]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Kumar, S.; Vijayan, M.; Bhatti, J.S.; Reddy, P. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017, 146, 47–94. [Google Scholar]
- Ehu, Y.-B.; Eli, C.-B.; Esong, N.; Ezou, Y.; Echen, S.-D.; Eren, R.-J.; Ewang, G. Diagnostic Value of microRNA for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2016, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Zuo, X.; Deng, H.; Liu, X.; Liu, L.; Ji, A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res. Bull. 2013, 97, 69–80. [Google Scholar] [CrossRef]
- Hubčík, L.; Galliková, D.; Pullmannová1, P.; Lacinová, L.; Sulová, Z.; Hanulová, M.; Funari, S.S.; Devínsky, F.; Uhríková, D. DNA–DOPE–gemini surfactants complexes at low surface charge density: From structure to transfection efficiency. Gen. Physiol. Biophys. 2018, 37, 57–69. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef]
- Quinlan, S.; Kenny, A.; Medina, M.; Engel, T.; Jimenez-Mateos, E.M. MicroRNAs in Neurodegenerative Diseases. Int. Rev. Cell Mol. Biol. 2017, 334, 309–343. [Google Scholar] [CrossRef]
- Riva, P.; Ratti, A.; Venturin, M. The Long Non-Coding RNAs in Neurodegenerative Diseases: Novel Mechanisms of Pathogen-esis. Curr. Alzheimer Res. 2016, 13. Available online: https://www.ingentaconnect.com/content/ben/car/2016/00000013/00000011/art00004 (accessed on 22 June 2021). [CrossRef]
- Ludwig, N.; Fehlmann, T.; Kern, F.; Gogol, M.; Maetzler, W.; Deutscher, S.; Gurlit, S.; Schulte, C.; von Thaler, A.-K.; Deuschle, C.; et al. Machine Learning to Detect Alzheimer’s Disease from Circulating Non-coding RNAs. Genom. Proteom. Bioinform. 2019, 17, 430–440. [Google Scholar] [CrossRef]
- Sonntag, K.-C. MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res. 2010, 1338, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Eacker, S.M.; Dawson, T.M.; Dawson, V. Understanding microRNAs in neurodegeneration. Nat. Rev. Neurosci. 2009, 10, 837–841. [Google Scholar] [CrossRef]
- Hooten, N.N.; Fitzpatrick, M.; Wood, W.H.; De, S.; Ejiogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.; Zonderman, A.B.; Evans, M. Age-related changes in microRNA levels in serum. Aging 2013, 5, 725–740. [Google Scholar] [CrossRef] [Green Version]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Machida, T.; Tomofuji, T.; Ekuni, D.; Maruyama, T.; Yoneda, T.; Kawabata, Y.; Mizuno, H.; Miyai, H.; Kunitomo, M.; Morita, M. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. Int. J. Mol. Sci. 2015, 16, 21294–21309. [Google Scholar] [CrossRef] [Green Version]
- El Naqa, I.; Murphy, M.J. What Is Machine Learning? In Machine Learning in Radiation Oncology; Springer: New York, NY, USA, 2015; pp. 3–11. [Google Scholar]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Cheng, Y.; Zhang, L. Tumor gene expression data classification via sample expansion-based deep learning. Oncotarget 2017, 8, 109646–109660. [Google Scholar] [CrossRef] [Green Version]
- Rahimy, E. Deep learning applications in ophthalmology. Curr. Opin. Ophthalmol. 2018, 29, 254–260. [Google Scholar] [CrossRef]
- Huang, C.; Mezencev, R.; McDonald, J.F.; Vannberg, F. Open source machine-learning algorithms for the prediction of optimal cancer drug therapies. PLoS ONE 2017, 12, e0186906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinbaum, R.; Ambros, V.; Lee, R. The C. elegans Heterochronic Gene lin-4 Encodes Small RNAs with Antisense Complemen-tarity to lin-4. Cell 2004, 116, 843–854. [Google Scholar]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Mattick, J.S. The State of Long Non-Coding RNA Biology. Non-Coding RNA 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, B.J.; Slack, F.J.; Basson, M.; Pasquinelli, A.E.; Bettinger, J.C.; Rougvie, A.E.; Horvitz, H.R.; Ruvkun, G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000, 403, 901–906. [Google Scholar] [CrossRef]
- Almeida, M.I.; Reis, R.; Calin, G. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. Mol. Mech. Mutagen. 2011, 717, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pasquinelli, A.E.; Reinhart, B.J.; Slack, F.; Martindale, M.Q.; Kuroda, M.I.; Maller, B.; Hayward, D.C.; Ball, E.E.; Degnan, B.; Müller, P.; et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000, 408, 86–89. [Google Scholar] [CrossRef]
- Su, J.-L.; Chen, P.-S.; Johansson, G.; Kuo, M.-L. Function and Regulation of Let-7 Family microRNAs. MicroRNA 2012, 1, 34–39. [Google Scholar] [CrossRef]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [Green Version]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Johnson, R.; Noble, W.; Tartaglia, G.G.; Buckley, N.J. Neurodegeneration as an RNA disorder. Prog. Neurobiol. 2012, 99, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Teo, S.; Lam, D.H.; Jeyaseelan, K.; Wang, S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012, 3, e398. [Google Scholar] [CrossRef] [PubMed]
- Heravi-Moussavi, A.; Anglesio, M.S.; Cheng, S.-W.G.; Senz, J.; Yang, W.; Prentice, L.; Fejes, A.P.; Chow, C.; Tone, A.; Kalloger, S.E.; et al. Recurrent SomaticDICER1Mutations in Nonepithelial Ovarian Cancers. N. Engl. J. Med. 2012, 366, 234–242. [Google Scholar] [CrossRef]
- Rakheja, D.; Chen, K.; Liu, Y.; Shukla, A.A.; Schmid, V.; Chang, T.-C.; Khokhar, S.; Wickiser, J.E.; Karandikar, N.; Malter, J.S.; et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat. Commun. 2014, 5, 4802. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef]
- Kim, V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005, 6, 376–385. [Google Scholar] [CrossRef]
- Guo, L.; Lu, Z. The Fate of miRNA* Strand through Evolutionary Analysis: Implication for Degradation as Merely Carrier Strand or Potential Regulatory Molecule? PLoS ONE 2010, 5, e11387. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Huang, X.; Ma, M.; Chang, Q.; Tu, Y.; Li, Q.; Zhang, K.; Hong, Y. Analysis of flash flood disaster characteristics in China from 2011 to 2015. Nat. Hazards 2018, 90, 407–420. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Chen, L.-L. The Diversity of Long Noncoding RNAs and Their Generation. Trends Genet. 2017, 33, 540–552. [Google Scholar] [CrossRef]
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 1990, 10, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Lyon, M.F. Gene Action in the X-chromosome of the Mouse (Mus musculus L.). Nat. Cell Biol. 1961, 190, 372–373. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The Sequence of the Human Genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef] [Green Version]
- Jathar, S.; Kumar, V.; Srivastava, J.; Tripathi, V. Technological Developments in lncRNA Biology. Adv. Exp. Med. Biol. 2017, 1008, 283–323. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Xu, M.; Zhang, Y.; Zhang, X.; Guo, W. Focusing on long noncoding RNA dysregulation in gastric cancer. Tumor Biol. 2014, 36, 129–141. [Google Scholar] [CrossRef]
- Uchida, S.; Dimmeler, S. Long Noncoding RNAs in Cardiovascular Diseases. Circ. Res. 2015, 116, 737–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; Horlings, H.M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinn, J.L.; Chang, H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [Green Version]
- Franco-Zorrilla, J.M.; Valli, A.; Todesco, M.; Mateos, I.; Puga, M.I.; Somoza, I.R.; Leyva, A.; Weigel, D.; Garcia, J.A.; Paz-Ares, J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 2007, 39, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, T.; Qu, Q.; Kang, T.; Yang, Q. Long Noncoding RNA SNHG1 Promotes Neuroinflammation in Parkinson’s Disease via Regulating miR-7/NLRP3 Pathway. Neuroscience 2018, 388, 118–127. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanly, D.J.; Esteller, M.; Berdasco, M. Interplay between long non-coding RNAs and epigenetic machinery: Emerging targets in cancer? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170074. [Google Scholar] [CrossRef]
- Kotake, Y.; Nakagawa, T.; Kitagawa, K.; Suzuki, S.; Liu, N.; Kitagawa, M.; Xiong, Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15INK4B tumor suppressor gene. Oncogene 2010, 30, 1956–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Ruscio, A.; Ebralidze, A.K.; Benoukraf, T.; Amabile, G.; Goff, L.; Terragni, J.; Figueroa, M.E.; Pontes, L.L.D.F.; Jorda, M.A.; Zhang, P.; et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nat. Cell Biol. 2013, 503, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Ge, S.; Gong, W.; Xu, J.; Guo, Z.; Liu, Z.; Gao, X.; Wei, X.; Ge, S. LncRNA ANRIL acts as a modular scaffold of WDR5 and HDAC3 complexes and promotes alteration of the vascular smooth muscle cell phenotype. Cell Death Dis. 2020, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Cullen, B.R. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA 2007, 13, 313–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, S.U.; Grote, P.; Herrmann, B.G. Mechanisms of long noncoding RNA function in development and disease. Cell. Mol. Life Sci. 2016, 73, 2491–2509. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pang, W.-J.; Wei, N.; Xiong, Y.; Wu, W.-J.; Zhao, C.-Z.; Shen, Q.-W.; Yang, G.-S. Identification, stability and expression of Sirt1 antisense long non-coding RNA. Gene 2014, 539, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Zander, S.; Gutschner, T. The Dark Side of the Epitranscriptome: Chemical Modifications in Long Non-Coding RNAs. Int. J. Mol. Sci. 2017, 18, 2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.-K.; Xu, X.-M. MicroRNA in central nervous system trauma and degenerative disorders. Physiol. Genom. 2011, 43, 571–580. [Google Scholar] [CrossRef]
- Clark, B.S.; Blackshaw, S. Understanding the Role of lncRNAs in Nervous System Development. Adv. Exp. Med. Biol. 2017, 1008, 253–282. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.-Y.; Johnson, R.; Stanton, L.W. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2011, 31, 522–533. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Zhang, L.; Pearce, W. MicroRNAs in brain development and cerebrovascular pathophysiology. Am. J. Physiol. Physiol. 2019, 317, C3–C19. [Google Scholar] [CrossRef]
- Ramos, A.D.; Andersen, R.; Liu, S.J.; Nowakowski, T.J.; Hong, S.J.; Gertz, C.C.; Salinas, R.D.; Zarabi, H.; Kriegstein, A.R.; Lim, D.A. The Long Noncoding RNA Pnky Regulates Neuronal Differentiation of Embryonic and Postnatal Neural Stem Cells. Cell Stem Cell 2015, 16, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Ponjavic, J.; Oliver, P.L.; Lunter, G.; Ponting, C.P. Genomic and Transcriptional Co-Localization of Protein-Coding and Long Non-Coding RNA Pairs in the Developing Brain. PLoS Genet. 2009, 5, e1000617. [Google Scholar] [CrossRef] [Green Version]
- Mercer, T.R.; Dinger, M.E.; Sunkin, S.M.; Mehler, M.F.; Mattick, J.S. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. USA 2008, 105, 716–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Pedrosa, E.; Shah, A.; Hrabovsky, A.; Maqbool, S.; Zheng, D.; Lachman, H.M. RNA-Seq of Human Neurons Derived from iPS Cells Reveals Candidate Long Non-Coding RNAs Involved in Neurogenesis and Neuropsychiatric Disorders. PLoS ONE 2011, 6, e23356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipovich, L.; Tarca, A.L.; Cai, J.; Jia, H.; Chugani, H.T.; Sterner, K.; Grossman, L.I.; Uddin, M.; Hof, P.R.; Sherwood, C.C.; et al. Developmental Changes in the Transcriptome of Human Cerebral Cortex Tissue: Long Noncoding RNA Transcripts. Cereb. Cortex 2013, 24, 1451–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muslimov, I.A.; Banker, G.; Brosius, J.; Tiedge, H. Activity-dependent Regulation of Dendritic BC1 RNA in Hippocampal Neurons in Culture. J. Cell Biol. 1998, 141, 1601–1611. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The Nuclear-Retained Noncoding RNA MALAT1 Regulates Alternative Splicing by Modulating SR Splicing Factor Phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Chuang, S.-C.; Bianchi, R.; Zhao, W.; Lee, H.; Fenton, A.; Wong, R.K.S.; Tiedge, H. BC1 Regulation of Metabotropic Glutamate Receptor-Mediated Neuronal Excitability. J. Neurosci. 2009, 29, 9977–9986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, D.; Prasanth, K.V.; Tripathi, V.; Colasse, S.; Nakamura, T.; Xuan, Z.; Zhang, M.Q.; Sedel, F.; Jourdren, L.; Coulpier, F.; et al. A long nuclear-retained non-coding RNA regulates synaptogenesis by modulating gene expression. EMBO J. 2010, 29, 3082–3093. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, L.; Gräfe, A.; Seiler, A.; Schumacher, S.; Nitsch, R.; Wulczyn, F.G. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005, 21, 1469–1477. [Google Scholar] [CrossRef]
- Meza-Sosa, K.F.; Epedraza-Alva, G.; Pérez-Martínez, L. microRNAs: Key triggers of neuronal cell fate. Front. Cell. Neurosci. 2014, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Szulwach, K.E.; Li, X.; Smrt, R.D.; Li, Y.; Luo, Y.; Lin, L.; Santistevan, N.; Li, W.; Zhao, X.; Jin, P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 2010, 189, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Zhao, J.; Hu, T.; Luo, Y.; Zhu, J.; Li, Z. miR-501-3p mediates the activity-dependent regulation of the expression of AMPA receptor subunit GluA1. J. Cell Biol. 2015, 208, 949–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, C.P.D.; Rhee, H.; Phee, B.; Kim, K.; Kim, H.; Lee, H.; Park, J.H.; Jung, J.H.; Kim, J.Y.; Kim, H.; et al. miR-204 downregulates EphB2 in aging mouse hippocampal neurons. Aging Cell 2016, 15, 380–388. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Dua, P.; Pogue, A.I.; Eicken, C.; Hill, J.M. Upregulation of Micro RNA-146a (miRNA-146a), A Marker for Inflammatory Neurodegeneration, in Sporadic Creutzfeldt–Jakob Disease (sCJD) and Gerstmann–Straussler–Scheinker (GSS) Syndrome. J. Toxicol. Environ. Health Part A 2011, 74, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.M.; Van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A Key Regulator of Astrocyte-Mediated Inflammatory Response. PLoS ONE 2012, 7, e44789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef] [Green Version]
- Eacker, S.M.; Dawson, T.M.; Dawson, V.L. The interplay of microRNA and neuronal activity in health and disease. Front. Cell. Neurosci. 2013, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Krol, J.; Busskamp, V.; Markiewicz, I.; Stadler, M.B.; Ribi, S.; Richter, J.; Duebel, J.; Bicker, S.; Fehling, H.J.; Schübeler, D.; et al. Characterizing Light-Regulated Retinal MicroRNAs Reveals Rapid Turnover as a Common Property of Neuronal MicroRNAs. Cell 2010, 141, 618–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierksma, A.; Lu, A.; Salta, E.; Eynden, E.V.; Callaerts-Vegh, Z.; D’Hooge, R.; Blum, D.; Buée, L.; Fiers, M.; De Strooper, B. Deregulation of neuronal miRNAs induced by amyloid-β or TAU pathology. Mol. Neurodegener. 2018, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Massone, S.; Vassallo, I.; Fiorino, G.; Castelnuovo, M.; Barbieri, F.; Borghi, R.; Tabaton, M.; Robello, M.; Gatta, E.; Russo, C.; et al. 17A, a novel non-coding RNA, regulates GABA B alternative splicing and signaling in response to inflammatory stimuli and in Alzheimer disease. Neurobiol. Dis. 2011, 41, 308–317. [Google Scholar] [CrossRef]
- Ke, S.; Yang, Z.; Yang, F.; Wang, X.; Tan, J.; Liao, B. Long Noncoding RNA NEAT1 Aggravates Aβ-Induced Neuronal Damage by Targeting miR-107 in Alzheimer’s Disease. Yonsei Med. J. 2019, 60, 640–650. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Guo, Y.; Rong, H.; Liu, T. The long noncoding RNA HOTAIR promotes Parkinson’s disease by upregulating LRRK2 expression. Oncotarget 2017, 8, 24449–24456. [Google Scholar] [CrossRef] [Green Version]
- Caggiu, E.; Paulus, K.; Mameli, G.; Arru, G.; Sechi, G.P.; Sechi, L.A. Differential expression of miRNA 155 and miRNA 146a in Parkinson’s disease patients. eNeurologicalSci 2018, 13, 1–4. [Google Scholar] [CrossRef]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [Green Version]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; Van Der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G. The impact of obesity on neurodegenerative diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef] [PubMed]
- De Guire, V.; Robitaille, R.; Tétreault, N.; Guérin, R.; Ménard, C.; Bambace, N.; Sapieha, P. Circulating miRNAs as sensitive and specific biomarkers for the diagnosis and monitoring of human diseases: Promises and challenges. Clin. Biochem. 2013, 46, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.-Y. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Durães, F.; Pinto, M.; Sousa, M.E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, V.; Richardson, R.M. Gene Therapy for Neurodegenerative Diseases. Neurotherapeutics 2019, 16, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciotta, S.; Emeregalli, M.; Etorrente, Y. The involvement of microRNAs in neurodegenerative diseases. Front. Cell. Neurosci. 2013, 7, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.H. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain Res. 2011, 1415, 136–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.-L.; Wang, N.; Sun, F.-R.; Cao, X.-P.; Zhang, W.; Yu, J.-T. Tau in neurodegenerative disease. Ann. Transl. Med. 2018, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016, 12, 459–509. [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Villa, C.; Fenoglio, C.; Serpente, M.; Ghezzi, L.; Cioffi, S.M.; Arighi, A.; Fumagalli, G.G.; Scarpini, E. Circulating miRNAs as Potential Biomarkers in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Ebhatnagar, S.; Echertkow, H.; Schipper, H.M.; Eyuan, Z.; Eshetty, V.; Ejenkins, S.; Ejones, T.; Ewang, E. Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma. Front. Mol. Neurosci. 2014, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Zovoilis, A.; Agbemenyah, H.Y.; Agís-Balboa, R.C.; Stilling, R.; Edbauer, D.; Rao, P.; Farinelli, L.; Delalle, I.; Schmitt, A.; Falkai, P.; et al. microRNA-34c is a novel target to treat dementias. EMBO J. 2011, 30, 4299–4308. [Google Scholar] [CrossRef]
- Faghihi, M.A.; Modarresi, F.; Khalil, A.M.; Wood, D.E.; Sahagan, B.G.; Morgan, T.E.; Finch, C.E.; Iii, G.S.L.; Kenny, P.J.; Wahlestedt, C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of β-secretase. Nat. Med. 2008, 14, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Laird, F.M.; Cai, H.; Savonenko, A.V.; Farah, M.H.; He, K.; Melnikova, T.; Wen, H.; Chiang, H.-C.; Xu, G.; Koliatsos, V.E.; et al. BACE1, a Major Determinant of Selective Vulnerability of the Brain to Amyloid- Amyloidogenesis, is Essential for Cognitive, Emotional, and Synaptic Functions. J. Neurosci. 2005, 25, 11693–11709. [Google Scholar] [CrossRef] [Green Version]
- Magistri, M.; Velmeshev, D.; Makhmutova, M.; Faghihi, M.A. Transcriptomics Profiling of Alzheimer’s Disease Reveal Neurovascular Defects, Altered Amyloid-β Homeostasis, and Deregulated Expression of Long Noncoding RNAs. J. Alzheimer’s Dis. 2015, 48, 647–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.Y.; Niu, Y.; Santiago, A.; Liu, J.; Albert, S.H.; Robertson, K.; Liao, D. An EBF3-Mediated Transcriptional Program That Induces Cell Cycle Arrest and Apoptosis. Cancer Res. 2006, 66, 9445–9452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, J.-I.; Kino, Y.; Niida, S. MicroRNA-Seq Data Analysis Pipeline to Identify Blood Biomarkers for Alzheimer’s Disease from Public Data. Biomark. Insights 2015, 10, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Fransquet, P.; Ryan, J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer’s disease. Clin. Biochem. 2018, 58, 5–14. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Wu, Q.; Xu, W.; Xia, M. Knockdown of BACE1-AS by siRNA improves memory and learning behaviors in Alzheimer’s disease animal model. Exp. Ther. Med. 2018, 16, 2080–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, H.M.; Maes, O.C.; Chertkow, H.M.; Wang, E. MicroRNA Expression in Alzheimer Blood Mononuclear Cells. Gene Regul. Syst. Biol. 2007, 1, GRSB-S361. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.-T.; Liu, Q.-Y.; Tan, M.-S.; Zhang, W.; Hu, N.; Wang, Y.-L.; Sun, L.; Jiang, T.; Tan, L. Circulating miR-125b as a biomarker of Alzheimer’s disease. J. Neurol. Sci. 2014, 336, 52–56. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.-T.; Tan, M.-S.; Liu, Q.-Y.; Wang, H.-F.; Zhang, W.; Jiang, T.; Tan, L. Genome-Wide Serum microRNA Expression Profiling Identifies Serum Biomarkers for Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 40, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Doecke, J.D.; Sharples, R.A.; Villemagne, V.L.; Fowler, C.J.; Rembach, A.; Martins, R.; Rowe, C.C.; Macaulay, S.L.; Masters, C.; et al. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol. Psychiatry 2015, 20, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Dezso, Z.; MacKenzie, C.; Oestreicher, J.; Agoulnik, S.; Byrne, M.; Bernier, F.; Yanagimachi, M.; Aoshima, K.; Oda, Y. Circulating miRNA Biomarkers for Alzheimer’s Disease. PLoS ONE 2013, 8, e69807. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.; Cohen, A.M.; Bennett, D.A.; Shah, R.; Fields, C.; Hernandez, A.G.; Smalheiser, N.R. Plasma Exosomal miRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS ONE 2015, 10, e0139233. [Google Scholar] [CrossRef] [Green Version]
- Denk, J.; Boelmans, K.; Siegismund, C.S.; Lassner, D.; Arlt, S.; Jahn, H. MicroRNA Profiling of CSF Reveals Potential Biomarkers to Detect Alzheimer‘s Disease. PLoS ONE 2015, 10, e0126423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA Changes in Alzheimer’s Disease Brain and CSF Yields Putative Biomarkers and Insights into Disease Pathways. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef]
- Alexandrov, P.N.; Dua, P.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. microRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int. J. Biochem. Mol. Boil. 2012, 3, 365–373. [Google Scholar]
- Lukiw, W.J. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. NeuroReport 2007, 18, 297–300. [Google Scholar] [CrossRef]
- Yao, J.; Wang, X.; Li, Y.; Shan, K.; Yang, H.; Wang, Y.; Yao, M.; Liu, C.; Li, X.; Shen, Y.; et al. Long non-coding RNA MALAT 1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol. Med. 2016, 8, 346–362. [Google Scholar] [CrossRef]
- Geekiyanage, H.; Chan, C. MicroRNA-137/181c Regulates Serine Palmitoyltransferase and In Turn Amyloid, Novel Targets in Sporadic Alzheimer’s Disease. J. Neurosci. 2011, 31, 14820–14830. [Google Scholar] [CrossRef] [Green Version]
- Jayadev, S.; Case, A.; Alajajian, B.; Eastman, A.J.; Möller, T.; Garden, G.A. Presenilin 2 influences miR146 level and activity in microglia. J. Neurochem. 2013, 127, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Hebert, S.; De Strooper, B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009, 32, 199–206. [Google Scholar] [CrossRef]
- Fang, M.; Wang, J.; Zhang, X.; Geng, Y.; Hu, Z.; Rudd, J.A.; Ling, S.; Chen, W.; Han, S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 2012, 209, 94–105. [Google Scholar] [CrossRef]
- Dickson, J.R.; Kruse, C.; Montagna, D.R.; Finsen, B.; Wolfe, M.S. Alternative polyadenylation and miR-34 family members regulate tau expression. J. Neurochem. 2013, 127, 739–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absalon, S.; Kochanek, D.M.; Raghavan, V.; Krichevsky, A.M. MiR-26b, Upregulated in Alzheimer’s Disease, Activates Cell Cycle Entry, Tau-Phosphorylation, and Apoptosis in Postmitotic Neurons. J. Neurosci. 2013, 33, 14645–14659. [Google Scholar] [CrossRef]
- Patel, N.; Hoang, D.; Miller, N.; Ansaloni, S.; Huang, Q.; Rogers, J.T.; Lee, J.C.; Saunders, A.J. MicroRNAs can regulate human APP levels. Mol. Neurodegener. 2008, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.; Lahiri, D.K. MicroRNA-101 downregulates Alzheimer’s amyloid-β precursor protein levels in human cell cultures and is differentially expressed. Biochem. Biophys. Res. Commun. 2011, 404, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Al Hashimi, A.; Girard, J.; DeLay, C.; Hebert, S. In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J. Neurochem. 2010, 116, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Hébert, S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-K.; Wang, X.; Li, L.; Du, Y.-H.; Ye, H.-T.; Li, C.-Y. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor. Neurosci. Bull. 2013, 29, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Li, H.; Zhao, J.; Cui, J.; Hu, G. Identification of age- and gender-associated long noncoding RNAs in the human brain with Alzheimer’s disease. Neurobiol. Aging 2019, 81, 116–126. [Google Scholar] [CrossRef]
- Spreafico, M.; Grillo, B.; Rusconi, F.; Battaglioli, E.; Venturin, M. Multiple Layers of CDK5R1 Regulation in Alzheimer’s Disease Implicate Long Non-Coding RNAs. Int. J. Mol. Sci. 2018, 19, 2022. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Chen, Y. Long noncoding RNAs and Alzheimer’s disease. Clin. Interv. Aging 2016, 11, 867–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, M.; Zhao, H.; Wang, X.; Sun, J.; Su, J. Analysis of long noncoding RNAs highlights region-specific altered expression patterns and diagnostic roles in Alzheimer’s disease. Briefings Bioinform. 2019, 20, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, J. Identification of Alzheimer’s disease–associated long noncoding RNAs. Neurobiol. Aging 2015, 36, 2925–2931. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson s disease: A review. Front. Biosci. 2014, S6, 65–74. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Inoue, K.; Ishii, J.; Vanti, W.; Voronov, S.V.; Murchison, E.; Hannon, G.; Abeliovich, A. A MicroRNA Feedback Circuit in Midbrain Dopamine Neurons. Science 2007, 317, 1220–1224. [Google Scholar] [CrossRef] [Green Version]
- Elkouris, M.; Kouroupi, G.; Vourvoukelis, A.; Papagiannakis, N.; Kaltezioti, V.; Matsas, R.; Stefanis, L.; Xilouri, M.; Politis, P.K. Long Non-coding RNAs Associated With Neurodegeneration-Linked Genes Are Reduced in Parkinson’s Disease Patients. Front. Cell. Neurosci. 2019, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; van der Walt, J.M.; Mayhew, G.; Li, Y.-J.; Züchner, S.; Scott, W.K.; Martin, E.R.; Vance, J.M. Variation in the miRNA-433 Binding Site of FGF20 Confers Risk for Parkinson Disease by Overexpression of α-Synuclein. Am. J. Hum. Genet. 2008, 82, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [Green Version]
- Junn, E.; Lee, K.-W.; Jeong, B.S.; Chan, T.W.; Im, J.-Y.; Mouradian, M.M. Repression of α-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-S.; Wang, Z.-H.; Zhang, J.-L.; Duan, Y.-L.; Li, G.-F.; Zheng, D.-L. Beta-asarone protects against MPTP-induced Parkinson’s disease via regulating long non-coding RNA MALAT1 and inhibiting α-synuclein protein expression. Biomed. Pharmacother. 2016, 83, 153–159. [Google Scholar] [CrossRef]
- Margis, R.; Margis, R.; Rieder, C.R. Identification of blood microRNAs associated to Parkinsońs disease. J. Biotechnol. 2011, 152, 96–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, M.C.T.; Bell, R.; Da Costa, A.N. Recent developments in circulating biomarkers in Parkinson’s disease: The potential use of miRNAs in a clinical setting. Bioanalysis 2016, 8, 2497–2518. [Google Scholar] [CrossRef]
- Chi, L.-M.; Wang, L.-P.; Jiao, D. Identification of Differentially Expressed Genes and Long Noncoding RNAs Associated with Parkinson’s Disease. Park. Dis. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Li, J.; Yang, Q.; Gong, C.; Gao, H.; Mao, Z.; Yuan, X.; Zhu, S.; Xue, Z. Dysregulated Long Non-coding RNAs in Parkinson’s Disease Contribute to the Apoptosis of Human Neuroblastoma Cells. Front. Neurosci. 2019, 13, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Gu, C.; Li, J.; Zhu, L.; Huang, G.; Dai, J.; Huang, H. Aberrantly expressed long noncoding RNAs and genes in Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2018, 14, 3219–3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardo, L.F.; Coto, E.; de Mena, L.; Ribacoba, R.; Moris, G.; Menéndez, M.; Álvarez, L.D.M. Profile of microRNAs in the plasma of Parkinson’s disease patients and healthy controls. J. Neurol. 2013, 260, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, C.; Lu, S.; Yu, C.; Huang, L.; Feng, W.; Xu, H.; Chen, X.; Zen, K.; Yan, Q.; et al. A panel of four decreased serum microRNAs as a novel biomarker for early Parkinson’s disease. Biomarkers 2015, 21, 129–137. [Google Scholar] [CrossRef]
- Vallelunga, A.; Ragusa, M.; Di Mauro, S.; Iannitti, T.; Pilleri, M.; Biundo, R.; Weis, L.; Di Pietro, C.S.; De Iuliis, A.; Nicoletti, A.; et al. Identification of circulating microRNAs for the differential diagnosis of Parkinson’s disease and Multiple System Atrophy. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta-Orfila, T.; Morató, X.; Compta, Y.; Lozano, J.J.; Falgàs, N.; Valldeoriola, F.; Pont-Sunyer, C.; Vilas, D.; Mengual, L.; Fernández, M.; et al. Identification of blood serum micro-RNAs associated with idiopathic andLRRK2Parkinson’s disease. J. Neurosci. Res. 2014, 92, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Doxakis, E. Post-transcriptional Regulation of α-Synuclein Expression by mir-7 and mir-153. J. Biol. Chem. 2010, 285, 12726–12734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.J.; Liu, G.; Jin, S.M.; Parisiadou, L.; Xie, C.; Yu, J.; Sun, L.; Ma, B.; Ding, J.; Vancraenenbroeck, R.; et al. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Hum. Mol. Genet. 2013, 22, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Gehrke, S.; Imai, Y.; Sokol, N.; Lu, B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nat. Cell Biol. 2010, 466, 637–641. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.-C.; Lu, H.-F.; Ma, N.; Wang, S.; Mao, H.-Q.; Leong, K.W. New polyphosphoramidate with a spermidine side chain as a gene carrier. J. Control. Release 2002, 83, 157–168. [Google Scholar] [CrossRef]
- Davis, E.; Caiment, F.; Tordoir, X.; Cavaillé, J.; Ferguson-Smith, A.; Cockett, N.; Georges, M.; Charlier, C. RNAi-Mediated Allelic trans-Interaction at the Imprinted Rtl1/Peg11 Locus. Curr. Biol. 2005, 15, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.-W.; Luo, T.; Zou, S.-S.; Wu, A.-S. The Role of Long Noncoding RNAs in Central Nervous System and Neurodegenerative Diseases. Front. Behav. Neurosci. 2018, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, E.; Zuccato, C.; Tartari, M. Normal huntingtin function: An alternative approach to Huntington’s disease. Nat. Rev. Neurosci. 2005, 6, 919–930. [Google Scholar] [CrossRef]
- Zuccato, C.; Valenza, M.; Cattaneo, E. Molecular Mechanisms and Potential Therapeutical Targets in Huntington’s Disease. Physiol. Rev. 2010, 90, 905–981. [Google Scholar] [CrossRef]
- Ghosh, R.; Tabrizi, S.J. Clinical Features of Huntington’s Disease. In Polyglutamine Disorders. Advances in Experimental Medicine and Biology; Nóbrega, C., Pereira de Almeida, L., Eds.; Springer: Cham, Switzerland, 2018; Volume 1049. [Google Scholar] [CrossRef]
- Snowden, J.S. The Neuropsychology of Huntington’s Disease. Arch. Clin. Neuropsychol. 2017, 32, 876–887. [Google Scholar] [CrossRef] [Green Version]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Wyant, K.J.; Ridder, A.J.; Dayalu, P. Huntington’s Disease—Update on Treatments. Curr. Neurol. Neurosci. Rep. 2017, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Zuccato, C.; Belyaev, N.D.; Guest, D.; Cattaneo, E.; Buckley, N. A microRNA-based gene dysregulation pathway in Huntington’s disease. Neurobiol. Dis. 2008, 29, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ma, Q.; Goswami, D.; Shang, J.; Miller, G.M. Modulation of nuclear REST by alternative splicing: A potential therapeutic target for Huntington’s disease. J. Cell. Mol. Med. 2017, 21, 2974–2984. [Google Scholar] [CrossRef] [Green Version]
- Baldelli, P.; Meldolesi, J. The Transcription Repressor REST in Adult Neurons: Physiology, Pathology, and Diseases. Eneuro 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Conaco, C.; Otto, S.; Han, J.-J.; Mandel, G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc. Natl. Acad. Sci. USA 2006, 103, 2422–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, N.; Klein, M.E.; Varlamova, O.; Keller, D.M.; Yamamoto, T.; Goodman, R.H.; Impey, S. From The Cover: A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16426–16431. [Google Scholar] [CrossRef] [Green Version]
- Packer, A.N.; Xing, Y.; Harper, S.Q.; Jones, L.; Davidson, B.L. The Bifunctional microRNA miR-9/miR-9* Regulates REST and CoREST and Is Downregulated in Huntington’s Disease. J. Neurosci. 2008, 28, 14341–14346. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Richter, N.; Jauch, R.; Gaughwin, P.M.; Zuccato, C.; Cattaneo, E.; Stanton, L.W. Human accelerated region 1 noncoding RNA is repressed by REST in Huntington’s disease. Physiol. Genom. 2010, 41, 269–274. [Google Scholar] [CrossRef]
- Díez-Planelles, C.; Sánchez-Lozano, P.; Crespo, M.D.C.; Gil Zamorano, J.; Ribacoba, R.; González, N.; Suárez, E.; Martínez-Descals, A.; Camblor, P.M.; Álvarez, V.; et al. Circulating microRNAs in Huntington’s disease: Emerging mediators in metabolic impairment. Pharmacol. Res. 2016, 108, 102–110. [Google Scholar] [CrossRef]
- Martí, E.; Pantano, L.; Bañez-Coronel, M.; Llorens, F.; Miñones-Moyano, E.; Porta, S.; Sumoy, L.; Ferrer, I.; Estivill, X. A myriad of miRNA variants in control and Huntington’s disease brain regions detected by massively parallel sequencing. Nucleic Acids Res. 2010, 38, 7219–7235. [Google Scholar] [CrossRef]
- Hu, G.; Niu, F.; Humburg, B.A.; Liao, K.; Bendi, V.S.; Callen, S.; Fox, H.S.; Buch, S. Molecular mechanisms of long noncoding RNAs and their role in disease pathogenesis. Oncotarget 2018, 9, 18648–18663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunwoo, J.-S.; Lee, S.-T.; Im, W.; Lee, M.; Byun, J.-I.; Jung, K.-H.; Park, K.-I.; Jung, K.-Y.; Lee, S.K.; Chu, K.; et al. Altered Expression of the Long Noncoding RNA NEAT1 in Huntington’s Disease. Mol. Neurobiol. 2017, 54, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Long non-coding RNAs in Huntington’s disease neurodegeneration. Neurobiol. Dis. 2012, 46, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sparber, P.; Filatova, A.; Khantemirova, M.; Skoblov, M. The role of long non-coding RNAs in the pathogenesis of hereditary diseases. BMC Med. Genom. 2019, 12, 63–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, D.W.; Rudnicki, D.D.; Yu, L.; Margolis, R.L. A natural antisense transcript at the Huntington’s disease repeat locus regulates HTT expression. Hum. Mol. Genet. 2011, 20, 3467–3477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toivonen, J.M.; Manzano, R.; Oliván, S.; Zaragoza, P.; García-Redondo, A.; Osta, R. MicroRNA-206: A Potential Circulating Biomarker Candidate for Amyotrophic Lateral Sclerosis. PLoS ONE 2014, 9, e89065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oskarsson, B.; Gendron, T.F.; Staff, N.P. Amyotrophic Lateral Sclerosis: An Update for 2018. Mayo Clin. Proc. 2018, 93, 1617–1628. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.; Eisen, A.; Hardiman, O.; Burrell, J.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Dardiotis, E.; Aloizou, A.-M.; Siokas, V.; Patrinos, G.P.; Deretzi, G.; Mitsias, P.; Aschner, M.; Tsatsakis, A. The Role of MicroRNAs in Patients with Amyotrophic Lateral Sclerosis. J. Mol. Neurosci. 2018, 66, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Waller, R.; Goodall, E.; Milo, M.; Cooper-Knock, J.; Da Costa, M.; Hobson, E.; Kazoka, M.; Wollff, H.; Heath, P.R.; Shaw, P.; et al. Serum miRNAs miR-206, 143-3p and 374b-5p as potential biomarkers for amyotrophic lateral sclerosis (ALS). Neurobiol. Aging 2017, 55, 123–131. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, H.M.; de Albuquerque, M.; Avansini, S.; Rocha, C.D.S.; Dogini, D.; Nucci, A.; Carvalho, B.; Lopes-Cendes, I.; França, M.C. MicroRNAs-424 and 206 are potential prognostic markers in spinal onset amyotrophic lateral sclerosis. J. Neurol. Sci. 2016, 368, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a Key Modulator of Skeletal Muscle Development and Disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 Delays ALS Progression and Promotes Regeneration of Neuromuscular Synapses in Mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Haramati, S.; Chapnik, E.; Sztainberg, Y.; Eilam, R.; Zwang, R.; Gershoni, N.; McGlinn, E.; Heiser, P.; Wills, A.-M.; Wirguin, I.; et al. miRNA malfunction causes spinal motor neuron disease. Proc. Natl. Acad. Sci. USA 2010, 107, 13111–13116. [Google Scholar] [CrossRef] [Green Version]
- Shioya, M.; Obayashi, S.; Tabunoki, H.; Arima, K.; Saito, Y.; Ishida, T.; Satoh, J. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator. Neuropathol. Appl. Neurobiol. 2010, 36, 320–330. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Mori, F.; Kakita, A.; Takahashi, H.; Utsumi, J.; Sasaki, H. Analysis of microRNA from archived formalin-fixed paraffin-embedded specimens of amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2014, 2, 173. [Google Scholar] [CrossRef] [Green Version]
- De Felice, B.; Annunziata, A.; Fiorentino, G.; Borra, M.; Biffali, E.; Coppola, C.; Cotrufo, R.; Brettschneider, J.; Giordana, M.L.; Dalmay, T.; et al. miR-338-3p is over-expressed in blood, CFS, serum and spinal cord from sporadic amyotrophic lateral sclerosis patients. Neurogenetics 2014, 15, 243–253. [Google Scholar] [CrossRef]
- Barik, S. An intronic microRNA silences genes that are functionally antagonistic to its host gene. Nucleic Acids Res. 2008, 36, 5232–5241. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nakagawa, S.; Hirose, T.; Okano, H.J.; Takao, M.; Shibata, S.; Suyama, S.; Kuwako, K.-I.; Imai, T.; Murayama, S.; et al. The long non-coding RNA nuclear-enriched abundant transcript 1_2 induces paraspeckle formation in the motor neuron during the early phase of amyotrophic lateral sclerosis. Mol. Brain 2013, 6, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardi, S.; Zucca, S.; Pandini, C.; Diamanti, L.; Bordoni, M.; Sproviero, D.; Arigoni, M.; Olivero, M.; Pansarasa, O.; Ceroni, M.; et al. Long non-coding and coding RNAs characterization in Peripheral Blood Mononuclear Cells and Spinal Cord from Amyotrophic Lateral Sclerosis patients. Sci. Rep. 2018, 8, 2378. [Google Scholar] [CrossRef]
- Gagliardi, S.; Pandini, C.; Garofalo, M.; Bordoni, M.; Pansarasa, O.; Cereda, C. Long non coding RNAs and ALS: Still much to do. Non-Coding RNA Res. 2018, 3, 226–231. [Google Scholar] [CrossRef]
- Freischmidt, A.; Müller, K.; Zondler, L.; Weydt, P.; Volk, A.E.; Božič, A.L.; Walter, M.; Bonin, M.; Mayer, B.; Von Arnim, C.A.F.; et al. Serum microRNAs in patients with genetic amyotrophic lateral sclerosis and pre-manifest mutation carriers. Brain 2014, 137, 2938–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freischmidt, A.; Müller, K.; Ludolph, A.C.; Weishaupt, J.H. Systemic dysregulation of TDP-43 binding microRNAs in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2013, 1, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freischmidt, A.; Müller, K.; Zondler, L.; Weydt, P.; Mayer, B.; von Arnim, C.A.; Hübers, A.; Dorst, J.; Otto, M.; Holzmann, K.; et al. Serum microRNAs in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 2015, 36, 2660.e15–2660.e20. [Google Scholar] [CrossRef]
- Matamala, J.M.; Arias-Carrasco, R.; Sanchez, C.; Uhrig, M.; Bargsted, L.; Matus, S.; Maracaja-Coutinho, V.; Abarzua, S.; van Zundert, B.; Verdugo, R.; et al. Genome-wide circulating microRNA expression profiling reveals potential biomarkers for amyotrophic lateral sclerosis. Neurobiol. Aging 2018, 64, 123–138. [Google Scholar] [CrossRef]
- Takahashi, I.; Hama, Y.; Matsushima, M.; Hirotani, M.; Kano, T.; Hohzen, H.; Yabe, I.; Utsumi, J.; Sasaki, H. Identification of plasma microRNAs as a biomarker of sporadic Amyotrophic Lateral Sclerosis. Mol. Brain 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Sheinerman, K.S.; Toledo, J.; Tsivinsky, V.G.; Irwin, D.; Grossman, M.; Weintraub, D.; Hurtig, H.I.; Chen-Plotkin, A.; Wolk, D.A.; McCluskey, L.F.; et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimer’s Res. Ther. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- De Felice, B.; Guida, M.; Guida, M.; Coppola, C.; De Mieri, G.; Cotrufo, R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene 2012, 508, 35–40. [Google Scholar] [CrossRef]
- Liguori, M.; Nuzziello, N.; Introna, A.; Consiglio, A.; Licciulli, F.; D’Errico, E.; Scarafino, A.; Distaso, E.; Simone, I.L. Dysregulation of MicroRNAs and Target Genes Networks in Peripheral Blood of Patients with Sporadic Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2018, 11, 288. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, Q.; Chen, X.; Li, C.; Cao, B.; Ou, R.; Hadano, S.; Shang, H.-F. Aberration of miRNAs Expression in Leukocytes from Sporadic Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2016, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benigni, M.; Ricci, C.; Jones, A.R.; Giannini, F.; Al-Chalabi, A.; Battistini, S. Identification of miRNAs as Potential Biomarkers in Cerebrospinal Fluid from Amyotrophic Lateral Sclerosis Patients. NeuroMol. Med. 2016, 18, 551–560. [Google Scholar] [CrossRef]
- Russell, A.P.; Wada, S.; Vergani, L.; Hock, M.B.; Lamon, S.; Léger, B.; Ushida, T.; Cartoni, R.; Wadley, G.; Hespel, P.; et al. Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 49, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Campos-Melo, D.; Droppelmann, C.A.; He, Z.; Volkening, K.; Strong, M.J. Altered microRNA expression profile in amyotrophic lateral sclerosis: A role in the regulation of NFL mRNA levels. Mol. Brain 2013, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Koval, E.D.; Shaner, C.; Zhang, P.; Du Maine, X.; Fischer, K.; Tay, J.; Chau, B.N.; Wu, G.F.; Miller, T.M. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet. 2013, 22, 4127–4135. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Romero, C.; Hur, J.; Lunn, J.S.; Paez-Colasante, X.; Bender, D.E.; Yung, R.; Sakowski, S.A.; Feldman, E.L. Expression of microRNAs in human post-mortem amyotrophic lateral sclerosis spinal cords provides insight into disease mechanisms. Mol. Cell. Neurosci. 2016, 71, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Ishtiaq, M.; Campos-Melo, D.; Volkening, K.; Strong, M.J. Analysis of Novel NEFL mRNA Targeting microRNAs in Amyotrophic Lateral Sclerosis. PLoS ONE 2014, 9, e85653. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A. Science, medicine, and the future: Bioinformatics. BMJ 2002, 324, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Z.; Wang, D.; Qiu, C.; Liu, M.; Chen, X.; Zhang, Q.; Yan, G.; Cui, Q. LncRNADisease: A database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2012, 41, D983–D986. [Google Scholar] [CrossRef] [Green Version]
- Bao, Z.; Yang, Z.; Huang, Z.; Zhou, Y.; Cui, Q.; Dong, D. LncRNADisease 2.0: An updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019, 47, D1034–D1037. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, J.; Zhang, Y.; Xie, A.; Yu, L.; Zhang, C.; Lei, J.; Xu, H.; Leng, Z.; Li, T.; et al. LncTarD: A manually-curated database of experimentally-supported functional lncRNA–target regulations in human diseases. Nucleic Acids Res. 2019, 48, D118–D126. [Google Scholar] [CrossRef]
- Ma, L.; Cao, J.; Liu, L.; Du, Q.; Li, Z.; Zou, D.; Bajic, V.B.; Zhang, Z. LncBook: A curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res. 2019, 47, D128–D134. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Shi, J.; Gao, Y.; Cui, C.; Zhang, S.; Li, J.; Zhou, Y.; Cui, Q. HMDD v3.0: A database for experimentally supported human microRNA–disease associations. Nucleic Acids Res. 2019, 47, D1013–D1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, M.; Zhou, D.; Zhi, H.; Wang, P.; Zhang, Y.; Gao, Y.; Guo, M.; Li, X.; Wang, Y.; Zhang, Y.; et al. MSDD: A manually curated database of experimentally supported associations among miRNAs, SNPs and human diseases. Nucleic Acids Res. 2018, 46, D181–D185. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Saha, P.; Chakravorty, N. miRwayDB: A database for experimentally validated microRNA-pathway associations in pathophysiological conditions. Database 2018, 2018. [Google Scholar] [CrossRef]
- Jakhar, D.; Kaur, I. Artificial intelligence, machine learning and deep learning: Definitions and differences. Clin. Exp. Dermatol. 2020, 45, 131–132. [Google Scholar] [CrossRef]
- Luxton, D.D. An Introduction to Artificial Intelligence in Behavioral and Mental Health Care; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar]
- Handelman, G.S.; Kok, H.K.; Chandra, R.V.; Razavi, A.H.; Lee, M.J.; Asadi, H. eDoctor: Machine learning and the future of medicine. J. Intern. Med. 2018, 284, 603–619. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Bi, Q.; Goodman, K.E.; Kaminsky, J.; Lessler, J. What is Machine Learning? A Primer for the Epidemiologist. Am. J. Epidemiol. 2019, 188, 2222–2239. [Google Scholar] [CrossRef] [PubMed]
- Senders, J.T.; Staples, P.C.; Karhade, A.V.; Zaki, M.M.; Gormley, W.B.; Broekman, M.L.; Smith, T.R.; Arnaout, O. Machine Learning and Neurosurgical Outcome Prediction: A Systematic Review. World Neurosurg. 2018, 109, 476–486.e1. [Google Scholar] [CrossRef]
- Carpenter, K. Machine Learning-based Virtual Screening and Its Applications to Alzheimer’s Drug Discovery: A Review. Curr. Pharm. Des. 2018, 24, 3347–3358. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; Sprivulis, P.; Dwivedi, G. Artificial intelligence and machine learning in emergency medicine. Emerg. Med. Australas. 2018, 30, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.D.; Matias-Guiu, J.A.; Cabrera-Martín, M.N.; Risco-Martín, J.L.; Ayala, J.L. An application of machine learning with feature selection to improve diagnosis and classification of neurodegenerative disorders. BMC Bioinform. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shew, M.; New, J.; Wichova, H.; Koestler, D.C.; Staecker, H. Using Machine Learning to Predict Sensorineural Hearing Loss Based on Perilymph Micro RNA Expression Profile. Sci. Rep. 2019, 9, 3393. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, H.H.; Tran, N.K.; Betts, E.V.; Howell, L.P.; Green, R. Artificial Intelligence and Machine Learning in Pathology: The Present Landscape of Supervised Methods. Acad. Pathol. 2019, 6, 2374289519873088. [Google Scholar] [CrossRef] [PubMed]
- DeGregory, K.W.; Kuiper, P.; DeSilvio, T.; Pleuss, J.D.; Miller, R.; Roginski, J.W.; Fisher, C.B.; Harness, D.; Viswanath, S.; Heymsfield, S.B.; et al. A review of machine learning in obesity. Obes. Rev. 2018, 19, 668–685. [Google Scholar] [CrossRef]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine learning in medicine: A practical introduction. BMC Med. Res. Methodol. 2019, 19, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, J.; Kantarcıoğlu, M.; Clifton, C. Privacy-preserving Naïve Bayes classification. VLDB J. 2008, 17, 879–898. [Google Scholar] [CrossRef]
- Renganathan, V. Overview of artifi cial neural network models in the biomedical domain. Bratisl. Med. J. 2015, 116, 296–301. [Google Scholar] [CrossRef]
- Kriegeskorte, N.; Golan, T. Neural network models and deep learning. Curr. Biol. 2019, 29, R231–R236. [Google Scholar] [CrossRef] [PubMed]
- Kodinariya, T.M.; Makwana, P.R. Review on determining number of Cluster in K-Means Clustering. Int. J. Adv. Res. Comput. Sci. Manag. Stud. 2013, 1, 2321–7782. [Google Scholar]
- Lin, Y.; Zhu, X.; Zheng, Z.; Dou, Z.; Zhou, R. The individual identification method of wireless device based on dimensionality reduction and machine learning. J. Supercomput. 2019, 75, 3010–3027. [Google Scholar] [CrossRef]
- Computational Toxicology. Methods Mol. Biol. 2013, 27, 258–284. [CrossRef]
- Smith, L.I. A Tutorial on Principal Components Analysis (Computer Science Technical Report No. OUCS-2002-12). 2002. Available online: http://hdl.handle.net/10523/7534 (accessed on 10 March 2021).
- Dos Santos, M.C.T.; Barreto-Sanz, M.A.; Correia, B.R.S.; Bell, R.; Widnall, C.; Perez, L.T.; Berteau, C.; Schulte, C.; Scheller, D.; Berg, D.; et al. miRNA-based signatures in cerebrospinal fluid as potential diagnostic tools for early stage Parkinson’s disease. Oncotarget 2018, 9, 17455–17465. [Google Scholar] [CrossRef]
- Zhang, Z. Long non-coding RNAs in Alzheimer’s disease. Curr. Top. Med. Chem. 2015, 16, 511–519. [Google Scholar] [CrossRef]
- Leidinger, P.; Backes, C.; Deutscher, S.; Schmitt, K.; Mueller, S.C.; Frese, K.; Haas, J.; Ruprecht, K.; Paul, F.; Stähler, C.; et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol. 2013, 14, R78. [Google Scholar] [CrossRef] [Green Version]
| Source | Target Genes | Condition | Dysregulated nc-RNAs | Study |
|---|---|---|---|---|
| Whole-blood | 1 | miR-26b-3p, miR-28-3p, miR-30c-5p, miR-30d-5p, miR-148b-5p, miR-151a-3p, miR-186-5p, miR-425-5p, miR-550a-5p, miR-1468, miR-4781-3p, miR-5001-3p, and miR-6513-3p. | [115] | |
| 2 | let-7a-5p, let-7e-5p, let-7f-5p, let-7g-5p, miR-15a-5p, miR-17-3p, miR-29b-3p, miR-98–5p, miR-144-5p, miR-148a-3p, miR-502-3p, miR-660-5p, miR-1294, and miR-3200-3p. | |||
| 2 | miR-29b, miR-107, miR-125b, miR-146a, miR-181c, and miR-342 | [116] | ||
| 1 | BACE1-AS | [117] | ||
| PBMCs | 1 | miR-34a, miR-34b, miR-34c and miR-181b | [118] | |
| Serum | 2 1 | miR-23a, miR-26b, miR-125b and miR-181c. miR-9 | [119] | |
| 2 | miR- 98-5p, miR-191-5p, miR-342-3p, miR-483-3p, miR-885-5p, and let-7d-5p | [120] | ||
| Serum exosomal | 1 | miR-15a-5p, miR18b-5b, miR-20a-5p, miR-30e-5p, miR-93-5p, miR-101-3p, miR106a-5p, miR-106b-5p, miR-143-3p, miR335-5p, miR-361-5p, miR-425-5p, miR-582-5p and miR-3065-5p | [121] | |
| 2 | miR-15b-3p, miR-342-3p and miR-1306-5p | |||
| Plasma | BCL2, SIRT1 | 1 | miR-34c | [122] |
| 2 | let-7d-5p, let-7g-5p, miR-15b-5p, miR-142-3p, miR-191- 5p, miR-301a-3p, and miR-545-3p | [122] | ||
| Plasma exosomal | 3 | miR-23b-3p, miR-24-3p, miR-29b-3p, miR-125b-5p, miR-138-5p, miR-139-5p, miR-141-3p, miR-150-5p, miR-152-3p, miR-185-5p, miR-338-3p, miR342-3p, miR-342-5p, miR-548at-5p, miR-659-5p, miR-3065-5p, miR-3613-3p, miR-3916, miR-4772-3p and miR-5001-3p | [123] | |
| Cerebrospinal fluid (CSF) | 3 | miR-100, miR-103, miR-146a, miR-219, miR-296, miR-335, miR-375, miR-449, miR-505, miR-708, miR-766, miR-1274a, miR-3622b-3p, miR-4467 and miR-4674 | [124] | |
| 2 | miR-10a, miR-10b, miR-15b, miR-99a, miR-124, miR-125, miR-126, miR-127, miR-142-5p, miR-143, miR-146b, miR-154, miR-181a, miR-181c, miR-194, miR-195, miR-199a, miR-221, miR-328, miR-422b, miR-451, miR-455 and miR-497 | [125] | ||
| 1 | miR-9, miR-125b, miR-146a and miR-155 | [126,127] | ||
| 1 | Let-7f, miR-30a-3p, miR-30a-5p, miR-30b, miR-30c, miR-30d, miR-32, miR-105, miR-125a, miR-135a, miR-138, miR-141, miR-151, miR-186, miR-191, miR-197, miR-204, miR-205, miR-216, miR-302b miR-345, miR-362, miR-371, miR-374, miR-375, miR-380-3p, miR-429, miR-448, miR-449, miR-494, miR-501, miR-517, miR-517b, miR-518b, miR-518f, miR-520a and miR-526a | [125] | ||
| 2 | MALAT1 | [128] | ||
| Brain | SPT | 1 | miR-9, miR-29a, miR-29b-1, miR-15, miR-137 and miR-181c | [129] |
| SPTLC1 | 1 | (miR-181c y miR-137) * | ||
| Secretases | 1 | miR-146 | [130] | |
| BACE1/ SPTLC2 | 2 | miR-9, miR-29a, miR-29b-1 and miR-124 * | [131,132] | |
| TAU | 1 | miR-26b and miR-34a | [133,134] | |
| APP | 4 | miR-101, miR-106a, and miR-520c | [135,136] | |
| APP | 2 | miR-124 * | [137] | |
| BACE1 APP | 3 | miR-15a, miR-29b-1, miR-9, and miR-19b, let-7, miR-101, miR-15a, and miR-106b | [138] | |
| IGF-1 | 4 | miR-98 * | [139] | |
| 1 | XIST, LNC01094, NEAT1, VAC14-A81, lnc-SERF1B-1, RP11-274-H2-5, AF001548-5, LINC00844, lnc-VS1G10-1, lnc-POTEG-4, EMX2OS, lnc-INADL-2, lncXRN2-2, RP11-953820-1, lnc-ADAM30-1, LINC00320 and lnc-TAF9-2 | [140] | ||
| miR-15/107 | 1 | NEAT1 (In temporal cortex and hippocampus), HOTAIR (In hippocampus and cerebellum) | [141] | |
| BACE1 | 1 | BACE1-AS | [142] | |
| SORL1 | 51A | |||
| GABA B | 17A | |||
| BACE and γ-secretase | NDM29 | |||
| eIF4A | BC200 | |||
| BDNF-AS SOX2OT | ||||
| Rad18 | NAD-RAT 18 | |||
| 3 | MIR7-3HG, AL109615.3, NEBL-AS1, ATP6V0E2-AS1, PDXDC2P-NPIPB14P, LOC441204, A2M-AS1, TGFB2-OT1, LINC00672 and LncSigAD9 | [143] | ||
| 1 | n336934 | [144] | ||
| 2 | n341006 |
| Source | Target Genes | Condition | Dysregulated nc-RNAs | Study |
|---|---|---|---|---|
| Blood | 2 | miR-1, miR-22p and miR-29a | [155] | |
| 1 | miR-16-2-3p, miR-26a-2-3p and miR-30a | |||
| 1 | miR-18b, miR-20a miR-21, miR-30b, miR-103a, miR-150, miR-199b, miR-378c, miR-1274b, miR-671, miR-1249. miR-4293 | [156] | ||
| 2 | miR-1, miR-16, miR 22, miR 29a, miR-92b, miR-320a, miR-320b, miR-320c, miR-769 | |||
| 1 | LINC00302 and LINC00328 | [157] | ||
| 2 | FAM215A, MCF2L-AS1, NOP14-AS1, PART1, XIST | |||
| 1 | AC131056.3-001, HOTAIRM1, lnc-MOK-6:1, and RF01976.1-201 | [158] | ||
| 1 | TM4SF19-TCTEXID2, LOC101927369, LOCI102724104, LINC01871, LOC105373420, LOC105371464, LINC00943, LOC105370060, LOC101927012, LOC105372055 | [159] | ||
| 2 | LOC102724765, LOC105369772, KRT73-AS1, LOC105379392, JHDM1D-ASI, LOC105372185, LOC105377225, LOC105378701, LOC105375056 and LOC105373204 | |||
| PMBCs | 2 | miR-19b, miR-26a, miR-28-5p, miR-29b, miR-29c, miR-30b, miR-30c, miR-126, miR-151-3p, miR-147, miR-151-5p, miR-199a-3p, miR-199a-5p, miR-199b-3p, miR-301a, miR-335, miR-374a, miR-374b | [156] | |
| Plasma | 1 | miR-181c, miR-331-5p, miR-193a-5p, miR196b, miR-454, miR-125a-3p and miR-137 | [160] | |
| 2 | miR-222, miR-505, miR 626 | [156] | ||
| Serum | 2 | miR141, miR-214, miR-146b-5p and miR193a-3p | [161] | |
| 1 | miR-233, miR324-3p and miR-24 | [162] | ||
| 2 | miR-339-5p, miR-30c and miR-148-b | |||
| 1 | miR-24, miR 30a-3p, miR-30e-3p, miR-195, miR-223, miR-324-3p, miR-338-3p | [156] | ||
| 2 | miR-15b, miR-16-2-3p, miR 19b, miR-29a, miR 29c, miR-30c, miR 148b, miR-181a, miR-185, miR-221, miR 1294 | |||
| CSF | 1 | miR 10a-5p, miR19a-3p, miR-16-2, miR19b-3p, miR-26 miR-30b, miR-103a, miR-127-3p, miR-132-5p, miR 136-3p, miR-153, miR-331-5p, miR-370, miR 485-5p, let-7g-3p, miR-409-3p, miR-433, miR 873-3p | [156] | |
| 2 | miR 1, miR 19b-3p, miR 19c, miR 22, miR-28, miR 29, miR 119a miR-128, miR 132-5p, miR-126, miR-127-3p, miR-151, miR-212-3p, miR-301a, miR-370, miR 374, miR-409-3p, miR-485-5p, miR-873-3p, miR-1224-5p, miR-4448 | |||
| Brain | 2 | miR 19a, miR-19b, miR-29a and miR-29c | [163] | |
| SNCA | 1 | miR-7 and miR-153 | [126] | |
| E2F1 | 1 | miR-184 | [164] | |
| LRKK2 | 1 | miR-205 | [165,166] | |
| DP | 1 | let-7 | [162] | |
| FGF20 | 1 | miR-433 | [167,168] | |
| 1 | miR-16-5p, miR 21, miR26b, miR-29a-3p, miR 106a, miR 127-5p, miR 224, miR 301b, miR 373, miR 548d | [143] | ||
| 2 | miR 10b-5p, miR 22, mir 29a, miR 29b, miR 29c, miR 127-3p, miR 135b, miR 181a, miR 181b, miR181c, miR 181d, miR 184, miR 198, miR 205, miR 485-5p, let-7i-3p/5p, miR 1224 | |||
| Sox2 | 1 | SoX2OT | [169] | |
| PINK 1 | naPINK1 | |||
| PINK1 UCHL1 | 3 | BC200 PINK1-AS UCHL1-AS | ||
| SNCA | 2 | SNCA-AS1 | [150] | |
| LRRK2 | AK127687 | |||
| UCHL1 | UCHL1-AS1 | |||
| PINK1 | PINK1-AS1 | |||
| DJ1 | AX747125 | |||
| MAPT | MAPT-AS1 |
| Source | Target Genes | Condition | Dysregulated miRNAs | Study |
|---|---|---|---|---|
| Plasma | 1 | miR-22-5p, miR-30d-5p, miR-128, miR-130b-3p, miR-223-3p, miR-223-5p, miR-222-3p, miR-338-3p, miR-361-5, miR-425-5p, miR-628-3p, miR-877-5p and miR-942 | [183] | |
| Parietal cortical tissue | REST | 1 | miR-29a and miR-135b | [176] |
| 2 | miR-132 | |||
| Brodmann’s area 4 (BA4) | REST | 2 | miR-9, miR-29b and miR-124a | [181] |
| 1 | miR-132, miR-486 and miR-196a | |||
| Frontal cortex and striatum | 1 | miR 15a, miR-15b, miR-16, miR-17, miR-19b, miR 20a, miR 27b, miR-30a, miR-30b, miR-30c, miR-30e, miR-33a, miR-33b, miR-92a, miR-93, miR-99b, miR-100, miR 101, miR 106b, miR-126, miR-145, miR-146a, miR-148b, miR-151-5p miR-151-3p, miR-181a, miR-193b, miR-199b-3p, miR-204, miR-219-2-3p, miR-219-5p, miR-338-3p, miR-363, miR-451, miR-486-5p, miR-887, miR1250, miR-1974 | [184] | |
| 2 | miR-95, miR-103, miR-107, miR-124, miR-127-3p, miR-128, miR-139-3p, miR-181d, miR-221, miR-222, miR-323-3p, miR-330-3p, miR-369-5p, miR-382, miR-383, miR-409-5p, miR-423-5p, miR-432, miR-433 and miR-483-3p, miR-485-3p, miR-485-5p, miR-495, miR-543, miR-598, miR-708, miR-1224-5p, miR-1301, miR-1307 | |||
| Brain | HTTA PCR2 | 2 | HTT-AS MEG3 HAR1R HAR1F | [169,185] |
| 1 | TUG1, LINC00341, RPS20P22 and NEAT1 | [186,187] | ||
| 2 | MEG3, DGCR5 and LINC00342 | |||
| BDNF HTT | 3 | BDNF-AS HTT-AS | [188,189] |
| Source | Target Genes | Condition | Dysregulated nc-RNAs | Study |
|---|---|---|---|---|
| Serum | HDAC4 | 1 | miR-206 miR 106b | [190] |
| 2 | miR-4747.5p, miR-3665, miR 1915-3p, miR 4530 | [206] | ||
| 2 | miR 132-5p, miR 132-3p, miR 143-5p, miR-143,3p, miR and LET7B-5p | [207] | ||
| 2 | miR-1234-3p and miR-1825 | [208] | ||
| 1 | miR-143-3p, miR-206 | [194] | ||
| 2 | miR-374b-5p | |||
| 1 | miR 142-3p | [209] | ||
| 2 | miR 1249-3p | |||
| Plasma | EPHA4 | 1 | miR-4649-5 | [210] |
| 2 | miR-4299 | |||
| 1 | miR-4258 and miR-663b. | |||
| 2 | miR-26b-5p, miR-4299, let-7f-5p, miR-4419a, miR-3187-5p and miR-4496 | |||
| 1 | miR-424 and miR 206 | [195] | ||
| 1 | miR-206, miR-338-3p, miR-9, miR-129-3p and miR-335-5p | [211] | ||
| Whole Blood | 1 | miR-338-3p | [212] | |
| 2 | miR-451, miR-1275, miR-328, miR-638, miR-149, miR-665, miR-583 | |||
| 2 | let-7a-5p, let-7d-5p, let-7f-5p, let-7g-5p, let-7i-5p, miR-103a-3p, miR-106b-3p, miR-128-3p, miR-130a-3p, miR-130b-3p, miR-144-5p, miR-148a-3p, miR-148b-3p, miR-15a-5p, miR-15b-5p, miR-151a-5p, miR-151b, miR-16-5p, miR-182-5p, miR-183-5p, miR-186-5p, miR-22-3p, miR-221-3p, miR-223-3p, miR-23a-3p, miR-26a-5p, miR-26b-5p, miR-27b-3p, miR-28-3p, miR-30b-5p, miR-30c-5p, miR-342-3p, miR-425-5p, miR-451a, miR-532-5p, miR-550a-3p, miR-584-5p, miR-93-5p | [213] | ||
| 1 | miR-34a, miR-100, miR-193b and miR 4485 | [214] | ||
| 2 | miR-124, miR-183, miR-193b, miR-451, miR- 3690, miR-3935, miR-4538, miR-4701 | |||
| Cerebrospinal Fluid (CSF) | 1 | miR-143-5p and miR-574-5p | [207] | |
| 2 | miR-132-3p, miR-132-5p and miR-143-3p | |||
| 1 | miR 181a-5p | [215] | ||
| 2 | LET7A-5p, LET7B-5p, LET7F-5p, miR-15b-5p, miR-21-5p, miR-195-5p, miR-148-3p | |||
| Skeletal muscle | PGC-1α | 1 | miRNAs-23a miR-29b, miR 31, miR-206 and miR-455 | [216] |
| Substanzia nigra | 1 | miR-26b | [134] | |
| Spinal cord | NFL | 1 | miR-16-2, miR-508-5p, miR 558 | [217] |
| 1 | miR-146 | |||
| 2 | miR-524-5 miR 582-3p | |||
| 2 | miR-23a, miR-23b, miR 30a, miR-30b, miR-192, miR-193a-5p, miR-215, miR 520e, 548a-5p, miR 556-5p, miR-606, miR-612, miR-624, miR-647 and | |||
| 1 | miR-24-2, miR-142-3p, miR-142-5p, miR-146a, miR-146b, miR-155 | [218] | ||
| 2 | miR-148b-5p, miR- 577, miR 133b and miR-140-3p | [219] | ||
| NEFL | 2 | miR-b1336 and miR-b2403 | [220] | |
| Brain | NAV3 | 1 | miR 29a miR 29b and miR-338-3p | [199] |
| NEFL | 1 | miR-9. | [198] |
| Database | Platform Functions | Website | Reference |
|---|---|---|---|
| LncRNA and disease database |
| http://www.cuilab.cn/lncrnadisease (Accessed on 18 February 2021). http://www.rnanut.net/lncrnadisease/ (Accessed on 22 June 2021) | [222,223] |
| LncTard |
| http://biocc.hrbmu.edu.cn/LncTarD/jsp/Browser.jsp (Accessed on 18 February 2021). | [224] |
| LncBook |
| http://bigd.big.ac.cn/lncbook/index (Accessed on 19 February 2021). | [225] |
| The Human microRNA Disease Database (HMDD v3.0) |
| http://www.cuilab.cn/hmdd (Accessed on 18 February 2021). | [226] |
| miRNA SNP Disease database (MSDD) |
| http://bio-bigdata.hrbmu.edu.cn/msdd/browse.jsp (Accessed on 19 February 2021). | [227] |
| miRwayDB |
| http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=mirpath/index (Accessed on 19 February 2021). | [228] |
| Method | Features | Advantage | Disadvantage |
|---|---|---|---|
| Supervised Learning | |||
| Linear regression |
|
|
|
| Method of classification |
|
|
|
| Bayesian Algorithms |
|
|
|
| Artificial neural networks (ANN) |
|
|
|
| Unsupervised learning | |||
| K-means clustering |
|
|
|
| Dimensionality reduction |
|
|
|
| Year | ML Method | nc-RNAs Included | Dysregulated nc-RNAs | Biofluid Sample | Technique Used | Patients | Higher Accuracy | Study |
|---|---|---|---|---|---|---|---|---|
| 2019 | Supervised learning: Decision trees (Boosted tree model) | 21 miRNAs | 18 dysregulated miRNAs | Blood | qPCR | Patients with AD in comparison with three groups: mild cognitive impairment, other neurological diseases, and healthy controls | 83.5% | [14] |
| 2018 | Supervised learning: Biomarker Optimization Software System (BOSS) | 15 miRNAs | 5 dysregulated miRNAs | Cerebrospinal Fluid | Next-generation sequencing | 40 patients with early-stage PD vs. 40 healthy controls | 82% | [250] |
| 2015 | Supervised learning:
| 20 miRNAs | 7 dysregulated miRNAs | Plasma sample | Deep sequencing | 35 patients with AD vs. 35 healthy people | 83–89% | [123] |
| 2013 | Supervised learning: Support Vector Machines (SVM) | 140 miRNAs | 12 dysregulated miRNAs | Blood sample | Next-generation sequencing | 48 patients with AD vs. 22 healthy controls | 93.3% | [252] |
| 2018 | Supervised learning: Support Vector Machines (SVM) | 47 Lnc-RNAs | 9 dysregulated Lnc-RNAs | Data available in GEO. | Microarray | 57 patients with AD vs. 57 healthy people (controls) from the platform | 87.7% | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Fonseca, Á.; Martin-Jimenez, C.; Barreto, G.E.; Pachón, A.F.A.; González, J. The Emerging Role of Long Non-Coding RNAs and MicroRNAs in Neurodegenerative Diseases: A Perspective of Machine Learning. Biomolecules 2021, 11, 1132. https://doi.org/10.3390/biom11081132
García-Fonseca Á, Martin-Jimenez C, Barreto GE, Pachón AFA, González J. The Emerging Role of Long Non-Coding RNAs and MicroRNAs in Neurodegenerative Diseases: A Perspective of Machine Learning. Biomolecules. 2021; 11(8):1132. https://doi.org/10.3390/biom11081132
Chicago/Turabian StyleGarcía-Fonseca, Ángela, Cynthia Martin-Jimenez, George E. Barreto, Andres Felipe Aristizábal Pachón, and Janneth González. 2021. "The Emerging Role of Long Non-Coding RNAs and MicroRNAs in Neurodegenerative Diseases: A Perspective of Machine Learning" Biomolecules 11, no. 8: 1132. https://doi.org/10.3390/biom11081132
APA StyleGarcía-Fonseca, Á., Martin-Jimenez, C., Barreto, G. E., Pachón, A. F. A., & González, J. (2021). The Emerging Role of Long Non-Coding RNAs and MicroRNAs in Neurodegenerative Diseases: A Perspective of Machine Learning. Biomolecules, 11(8), 1132. https://doi.org/10.3390/biom11081132








